Abstract
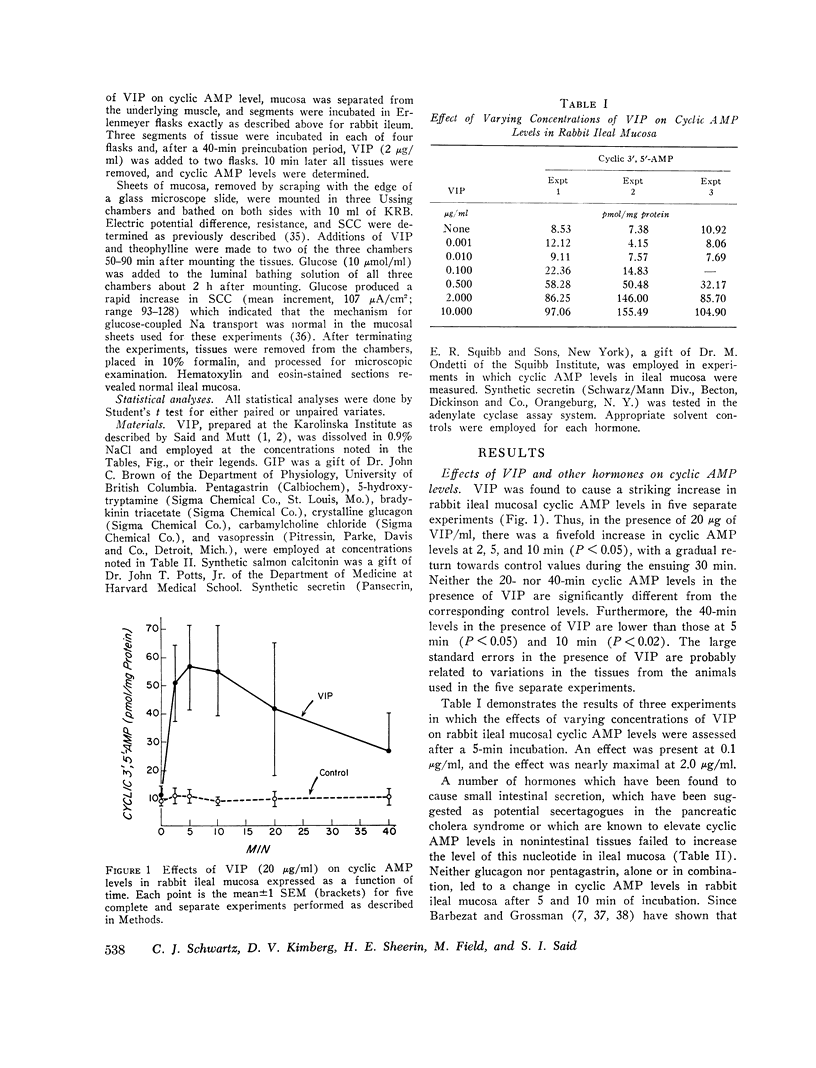
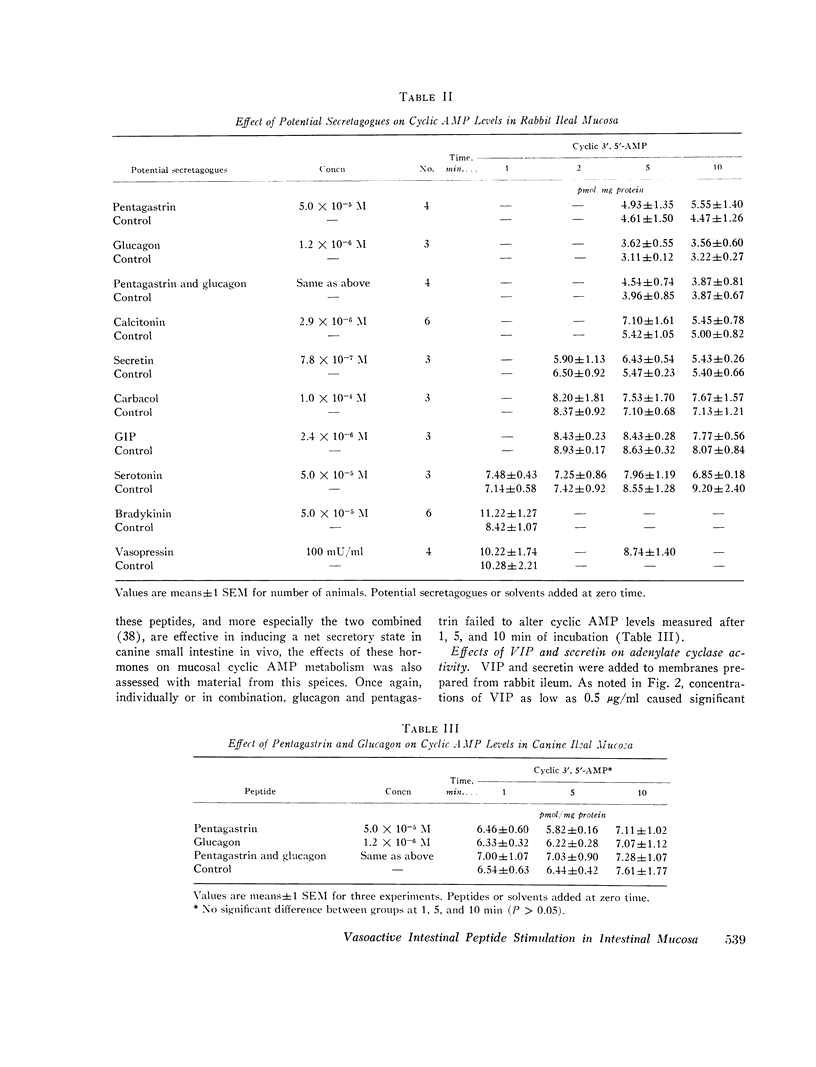
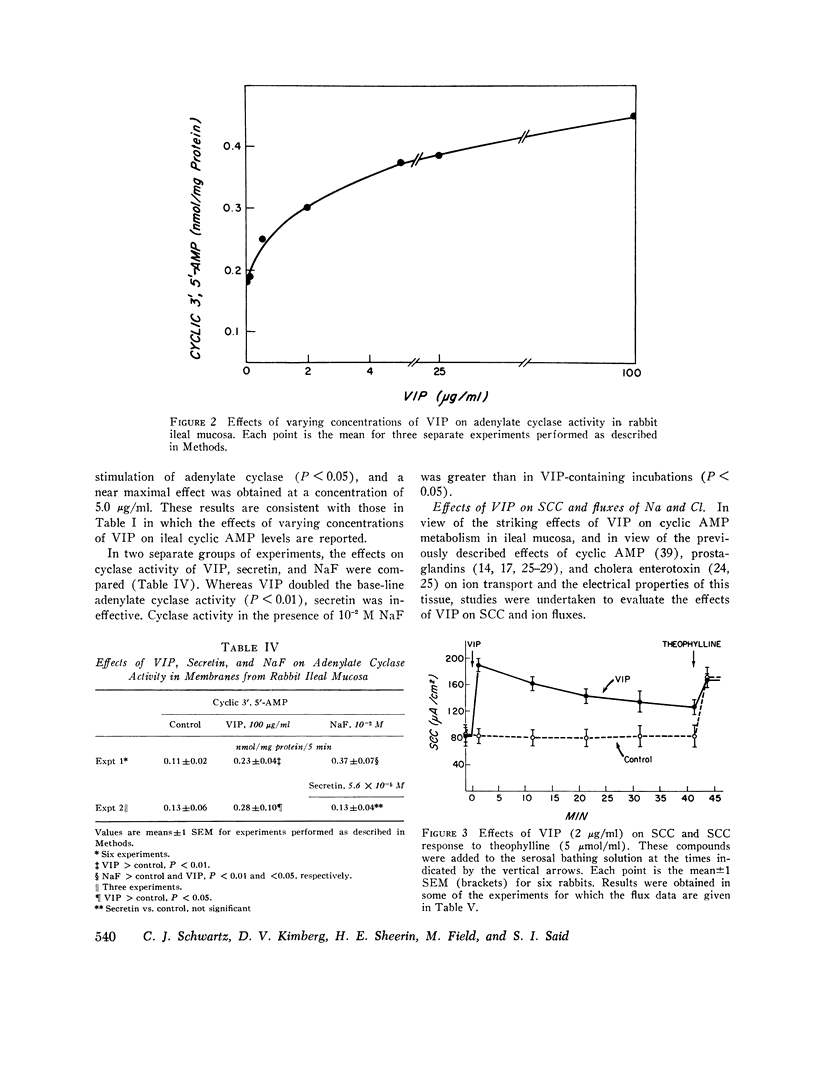
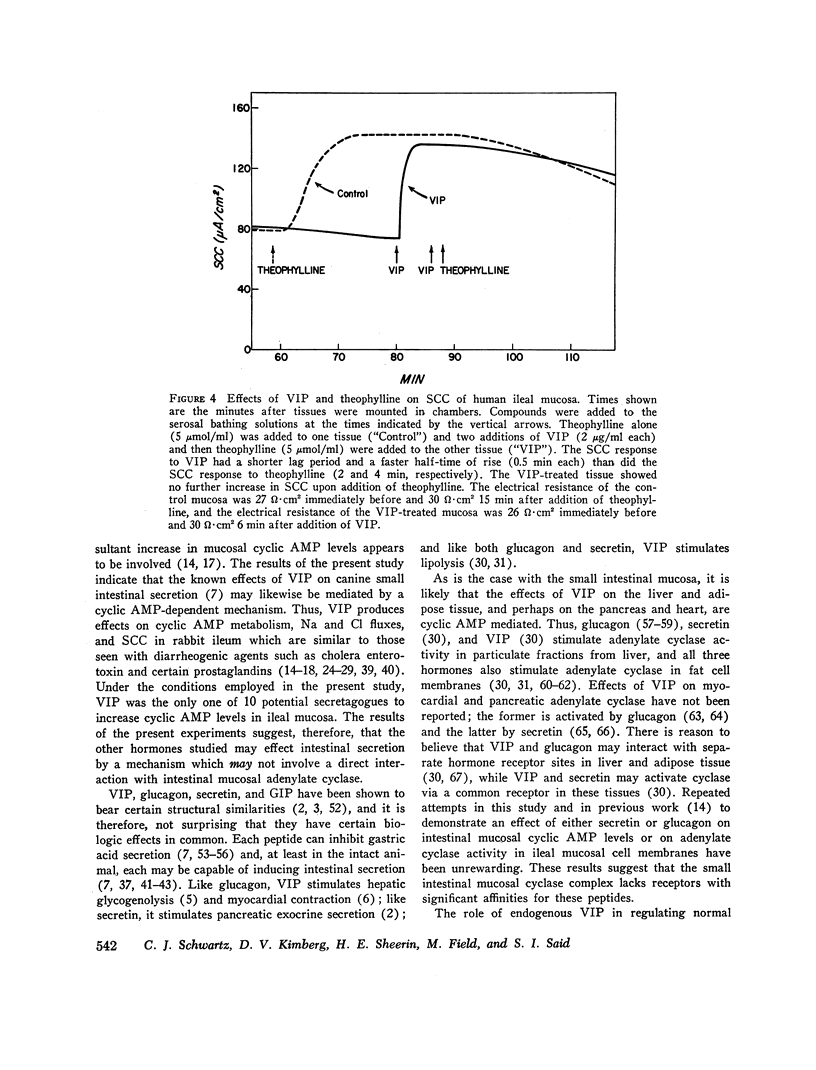
Vasoactive intestinal peptide (VIP), originally isolated from hog small intestinal mucosa, has been shown to cause small intestinal secretion. More recently, this peptide has been identified in the plasma and tumors of patients with the so-called “pancreatic cholera” syndrome. In order to explore the possible role of VIP in the pathogenesis of this syndrome, we examined the effects of this peptide and other hormones on the cyclic AMP levels, adenylate cyclase activity, and ion transport in in vitro preparations of ileal mucosa. In rabbit ileal mucosa, VIP (20 μg/ml) caused a prompt fivefold increase in cyclic AMP level, whereas nine other hormones, which have been postulated to cause intestinal secretion, failed to exert such an effect. Pentagastrin and glucagon also failed to increase cyclic AMP levels in canine ileal mucosa. An increase in mucosal cyclic AMP levels was observed at a VIP concentration of 0.1 μg/ml and appeared to be nearly maximal at 2.0 μg/ml. VIP (100 μg/ml) stimulated adenylate cyclase activity in a membrane preparation from rabbit ileal mucosa. Secretin (6.0 × 10-5 M) failed to do so. When added to the serosal side of isolated rabbit ileal mucosa clamped in an Ussing chamber, VIP (2 μg/ml) increased short-circuit current (SCC) and caused net secretion of both Cl and Na. Net Cl secretion exceeded net Na secretion. These effects of VIP on mucosal cyclic AMP metabolism and ion transport are similar to those observed with cholera enterotoxin and certain prostaglandins. VIP was also tested with normal human ileal mucosa. At a concentration of 2 μg/ml it caused a fivefold increase in cyclic AMP level and an increase in SCC of the same magnitude as that caused by 5 mM theophylline. Addition of a second 2-μg/ml dose of VIP and addition of theophylline after VIP produced no further change in SCC. We conclude the VIP stimulates adenylate cyclase and active ion secretion in both rabbit and human ileal mucosa. This may be related to the pathogenesis of diarrhea in patients with the pancreatic cholera syndrome.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbezat G. O., Grossman M. I. Cholera-like diarrhoea induced by glucagon plus gastrin. Lancet. 1971 May 15;1(7707):1025–1026. doi: 10.1016/s0140-6736(71)91428-0. [DOI] [PubMed] [Google Scholar]
- Barbezat G. O., Grossman M. I. Glucagon stimulates intestinal secretion. Lancet. 1971 May 1;1(7705):918–918. doi: 10.1016/s0140-6736(71)92487-1. [DOI] [PubMed] [Google Scholar]
- Bataille D. P., Freychet P., Kitabgi P. E., Rosselin G. E. Gut glucagon: A common receptor site with pancreatic glucagon in liver cell plasma membranes. FEBS Lett. 1973 Mar 1;30(2):215–218. doi: 10.1016/0014-5793(73)80654-4. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Bloom S. R., Polak J. M., Pearse A. G. Vasoactive intestinal peptide and watery-diarrhoea syndrome. Lancet. 1973 Jul 7;2(7819):14–16. doi: 10.1016/s0140-6736(73)91947-8. [DOI] [PubMed] [Google Scholar]
- Bodanszky M., Klausner Y. S., Said S. I. Biological activities of synthetic peptides corresponding to fragments of and to the entire sequence of the vasoactive intestinal peptide. Proc Natl Acad Sci U S A. 1973 Feb;70(2):382–384. doi: 10.1073/pnas.70.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C., Dryburgh J. R. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can J Biochem. 1971 Aug;49(8):867–872. doi: 10.1139/o71-122. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Mutt V., Pederson R. A. Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol. 1970 Jul;209(1):57–64. doi: 10.1113/jphysiol.1970.sp009155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussjaeger L. J., Johnson L. R. Evidence for hormonal regulation of intestinal absorption by cholecystokinin. Am J Physiol. 1973 Jun;224(6):1276–1279. doi: 10.1152/ajplegacy.1973.224.6.1276. [DOI] [PubMed] [Google Scholar]
- Bynum T. E., Jacobson E. D., Johnson L. R. Gastrin inhibition of intestinal absorption in dogs. Gastroenterology. 1971 Dec;61(6):858–862. [PubMed] [Google Scholar]
- Bär H. P., Hechter O. Adenyl cyclase and hormone action. I. Effects of adrenocorticotropic hormone, glucagon, and epinephrine on the plasma membrane of rat fat cells. Proc Natl Acad Sci U S A. 1969 Jun;63(2):350–356. doi: 10.1073/pnas.63.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Newman A., Misiewicz J. J., Milton-Thompson G. J., Billings J. A. Effect of intravenous prostaglandin F 2 on small intestinal function in man. Nature. 1973 May 18;243(5403):169–171. doi: 10.1038/243169a0. [DOI] [PubMed] [Google Scholar]
- Desbuguois B., Laudat M. H., Laudat P. Vasoactive intestinal polypeptide and glucagon: stimulation of adenylate cyclase activity via distinct receptors in liver and fat cell membranes. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1187–1194. doi: 10.1016/0006-291x(73)90590-1. [DOI] [PubMed] [Google Scholar]
- Dietz J., Field M. Ion transport in rabbit ileal mucosa. IV. Bicarbonate secretion. Am J Physiol. 1973 Oct;225(4):858–861. doi: 10.1152/ajplegacy.1973.225.4.858. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Intestinal secretion. Gastroenterology. 1974 May;66(5):1063–1084. [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K., Moody A. J. Lipolytic action of a newly isolated vasoactive intestinal polypeptide. Horm Metab Res. 1973 May;5(3):196–199. doi: 10.1055/s-0028-1093951. [DOI] [PubMed] [Google Scholar]
- Gardner J. D., Peskin G. W., Cerda J. J., Brooks F. P. Alterations of in vitro fluid and electrolyte absorption by gastrointestinal hormones. Am J Surg. 1967 Jan;113(1):57–64. doi: 10.1016/0002-9610(67)90257-7. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell J. C., Davies M. W., Shields R. Effect of a synthetic gastrin-like pentapeptide upon the intestinal transport of sodium, potassium, and water. Gut. 1968 Feb;9(1):111–116. doi: 10.1136/gut.9.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T. K., Bieberdorf F. A., Fordtran J. S. Thyrocalcitonin and the jejunal absorption of calcium, water, and electrolytes in normal subjects. J Clin Invest. 1973 Dec;52(12):3084–3088. doi: 10.1172/JCI107507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle P. T., Eggenton J. The effect of acetylcholine on the electrical activity of intestinal epithelial cells. Biochim Biophys Acta. 1973 Feb 27;298(1):95–100. doi: 10.1016/0005-2736(73)90013-8. [DOI] [PubMed] [Google Scholar]
- Hicks T., Turnberg L. A. The influence of secretin on ion transport in the human jejunum. Gut. 1973 Jun;14(6):485–490. doi: 10.1136/gut.14.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton E. W., Main I. H., Thompson C. J., Wright P. M. Effect of orally administered prostaglandin E1 on gastric secretion and gastrointestinal motility in man. Gut. 1968 Dec;9(6):655–658. doi: 10.1136/gut.9.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. R., Grossman M. I. Secretin: the enterogastrone released by acid in the duodenum. Am J Physiol. 1968 Oct;215(4):885–888. doi: 10.1152/ajplegacy.1968.215.4.885. [DOI] [PubMed] [Google Scholar]
- Karim S. M., Filshie G. M. Therapeutic abortion using prostaglandin F2alpha. Lancet. 1970 Jan 24;1(7639):157–159. doi: 10.1016/s0140-6736(70)90402-2. [DOI] [PubMed] [Google Scholar]
- Kerins C., Said S. I. Hyperglycemic and glycogenolytic effects of vasoactive intestinal polypeptide. Proc Soc Exp Biol Med. 1973 Mar;142(3):1014–1017. doi: 10.3181/00379727-142-37165. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Gershon E., Henderson A. Effects of prostaglandins and cholera enterotoxin on intestinal mucosal cyclic AMP accumulation. Evidence against an essential role for prostaglandins in the action of toxin. J Clin Invest. 1974 Mar;53(3):941–949. doi: 10.1172/JCI107635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft A. R., Tompkins R. K., Zollinger R. M. Recognition and management of the diarrheal syndrome caused by nonbeta islet cell tumors of the pancreas. Am J Surg. 1970 Feb;119(2):163–170. doi: 10.1016/0002-9610(70)90028-0. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levey G. S., Epstein S. E. Activation of adenyl cyclase by glucagon in cat and human heart. Circ Res. 1969 Feb;24(2):151–156. doi: 10.1161/01.res.24.2.151. [DOI] [PubMed] [Google Scholar]
- MAKMAN M. H., SUTHERLAND E. W., Jr USE OF LIVER ADENYL CYCLASE FOR ASSAY OF GLUCAGON IN HUMAN GASTRO-INTESTINAL TRACT AND PANCREAS. Endocrinology. 1964 Jul;75:127–134. doi: 10.1210/endo-75-1-127. [DOI] [PubMed] [Google Scholar]
- Marks I. N., Bank S., Louw J. H. Islet cell tumor of the pancreas with reversible watery diarrhea and achylorhydraia. Gastroenterology. 1967 Apr;52(4):695–708. [PubMed] [Google Scholar]
- Marois C., Morisset J., Dunnigan J. Presence and stimulation of adenyl cyclase in pancreas homogenate. Rev Can Biol. 1972 Dec;31(4):253–257. [PubMed] [Google Scholar]
- Matuchansky C., Bernier J. J. Effect of prostaglandin E 1 on glucose, water, and electrolyte absorption in the human jejunum. Gastroenterology. 1973 Jun;64(6):1111–1118. [PubMed] [Google Scholar]
- Misiewicz J. J., Waller S. L., Kiley N. Effect of oral prostaglandin E1 on intestinal transit in man. Lancet. 1969 Mar 29;1(7596):648–651. doi: 10.1016/s0140-6736(69)92012-1. [DOI] [PubMed] [Google Scholar]
- Murad F., Vaughan M. Effect of glucagon on rat heart adenyl cyclase. Biochem Pharmacol. 1969 May;18(5):1053–1059. doi: 10.1016/0006-2952(69)90109-9. [DOI] [PubMed] [Google Scholar]
- Pederson R. A., Brown J. C. Inhibition of histamine-, pentagastrin-, and insulin-stimulated canine gastric secretion by pure "gastric inhibitory polypeptide". Gastroenterology. 1972 Mar;62(3):393–400. [PubMed] [Google Scholar]
- Pierce N. F., Carpenter C. C., Jr, Elliott H. L., Greenough W. B., 3rd Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology. 1971 Jan;60(1):22–32. [PubMed] [Google Scholar]
- Piper P. J., Said S. I., Vane J. R. Effects on smooth muscle preparations of unidentified vasoactiv peptides from intestine and lung. Nature. 1970 Mar 21;225(5238):1144–1146. doi: 10.1038/2251144a0. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. Glucagon-sensitive adenyl cylase in plasma membrane of hepatic parenchymal cells. Science. 1969 May 2;164(3879):566–567. doi: 10.1126/science.164.3879.566. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L. Adenyl cyclase in fat cells. 3. Stimulation by secretin and the effects of trypsin on the receptors for lipolytic hormones. J Biol Chem. 1970 Feb 25;245(4):718–722. [PubMed] [Google Scholar]
- Rutten W. J., de Pont J. J., Bonting S. L. Adenylate cyclase in the rat pancreas properties and stimulation by hormones. Biochim Biophys Acta. 1972 Jul 3;274(1):201–213. doi: 10.1016/0005-2736(72)90294-5. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Isolation from porcine-intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon. Eur J Biochem. 1972 Jul 13;28(2):199–204. doi: 10.1111/j.1432-1033.1972.tb01903.x. [DOI] [PubMed] [Google Scholar]
- Said S. I., Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970 Sep 18;169(3951):1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Sandler M., Karim S. M., Williams E. D. Prostaglandins in amine-peptide-secreting tumours. Lancet. 1968 Nov 16;2(7577):1053–1054. doi: 10.1016/s0140-6736(68)91528-6. [DOI] [PubMed] [Google Scholar]
- Sanzenbacher L. J., Mekhjian H. S., King D. R., Zollinger R. M. Studies on the potential role of secretin in the islet cell tumor diarrheogenic syndrome. Ann Surg. 1972 Sep;176(3):394–402. doi: 10.1097/00000658-197209000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein P. S. Islet cell tumors: current concepts and management. Ann Intern Med. 1973 Aug;79(2):239–257. doi: 10.7326/0003-4819-79-2-239. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Soergel K. H., Whalen G. E., Harris J. A., Geenen J. E. Effect of antidiuretic hormone on human small intestinal water and solute transport. J Clin Invest. 1968 May;47(5):1071–1082. doi: 10.1172/JCI105797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIDBALL C. S. Active chloride transport during intestinal secretion. Am J Physiol. 1961 Feb;200:309–312. doi: 10.1152/ajplegacy.1961.200.2.309. [DOI] [PubMed] [Google Scholar]
- VERNER J. V., MORRISON A. B. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med. 1958 Sep;25(3):374–380. doi: 10.1016/0002-9343(58)90075-5. [DOI] [PubMed] [Google Scholar]
- Williams E. D., Karim S. M., Sandler M. Prostaglandin secretion by medullary carcinoma of the thyroid. A possible cause of the associated idarrhoea. Lancet. 1968 Jan 6;1(7532):22–23. doi: 10.1016/s0140-6736(68)90010-x. [DOI] [PubMed] [Google Scholar]
- el-Awqati Q., Cameron J. L., Greenough W. B., 3rd Electrolyte transport in human ileum: effect of purified cholera exotoxin. Am J Physiol. 1973 Apr;224(4):818–823. doi: 10.1152/ajplegacy.1973.224.4.818. [DOI] [PubMed] [Google Scholar]


