Abstract
To determine whether the molecular configuration of vitamin B12 influences the attachment of intrinsic factor-vitamin B12 complex to ileal microvillous membrane receptor sites, we have examined the kinetics of uptake of intrinsic factor-bound cyanocobalamin by brush borders and microvillous membranes isolated from guinea pig ileum, and have compared this uptake with that of intrinsic factor alone and with that of intrinsic factor complexed with various analogs of cyanocobalamin.
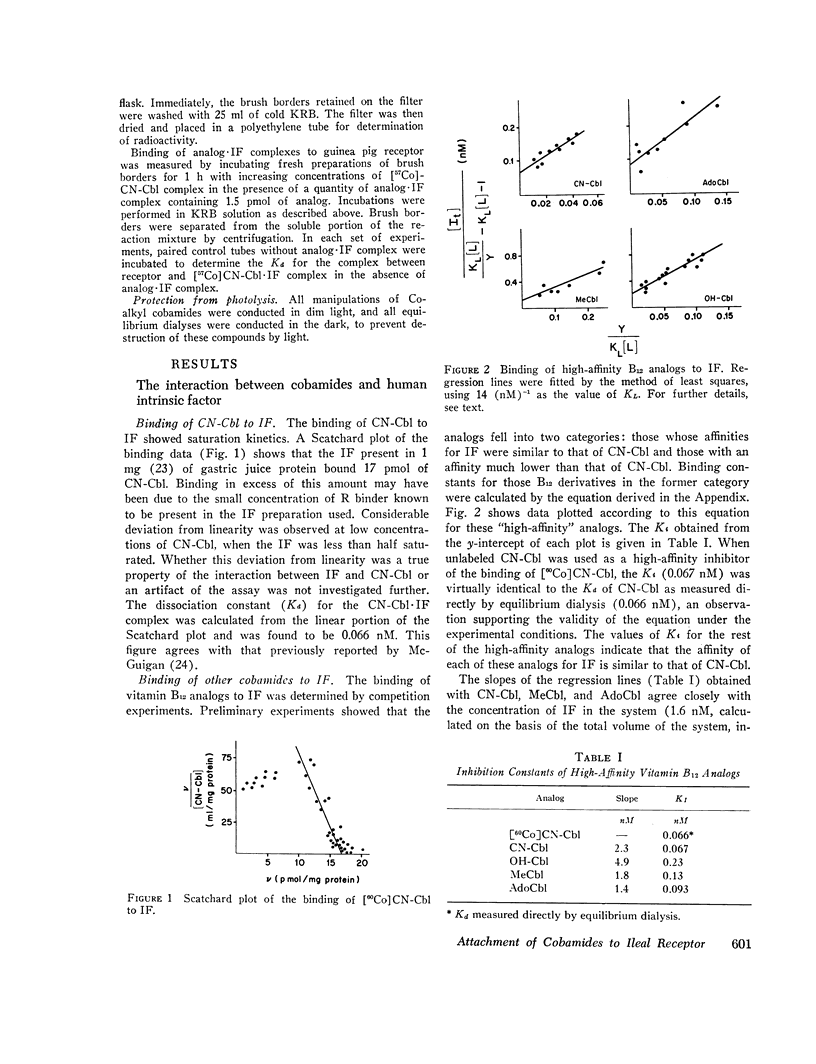
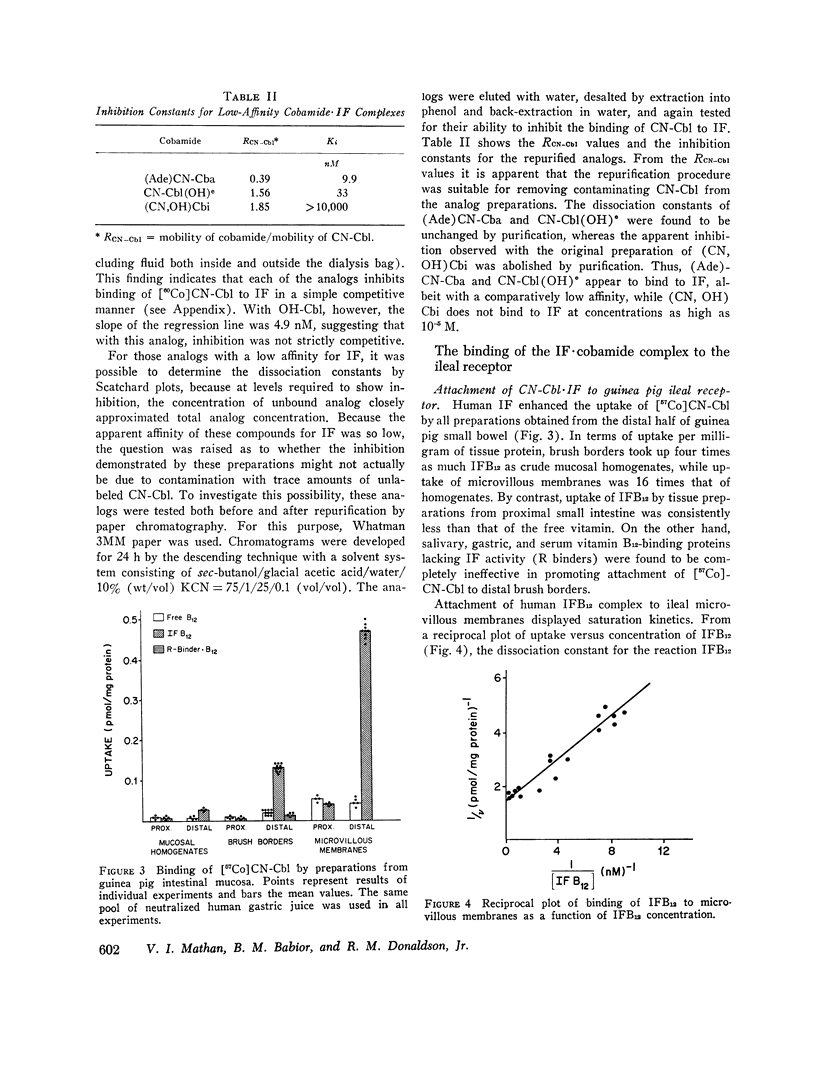
We first studied the kinetics of binding of cyanocobalamin and other cobamides to human gastric intrinsic factor. The binding of cyanocobalamin showed saturation kinetics and, at relatively high concentrations of cyanocobalamin, a Scatchard plot of binding was linear. The dissociation constant for the intrinsic factor-cyanocobalamin complex was 0.066 nM. When the binding of various vitamin B12 analogs to intrinsic factor was determined by competition experiments, the analogs could be separated into two categories: those with affinities similar to that of cyanocobalamin and those with affinities much lower than that of cyanocobalamin. The affinity of cyanocobalamin for intrinsic factor was not altered by various substitutions at the -CN position, while removal of a single amido group on the corrin ring of substitution of the dimethylbenzimidazole base greatly reduced affinity. Removal of the base totally abolished binding. These findings, confirming those reported by others, are consistent with the concept that the cyanocobalamin molecule fits into a “pocket” in the intrinsic factor molecule, with the nucleotide base facing inward and the -CN side of the planar corrin ring facing outward.
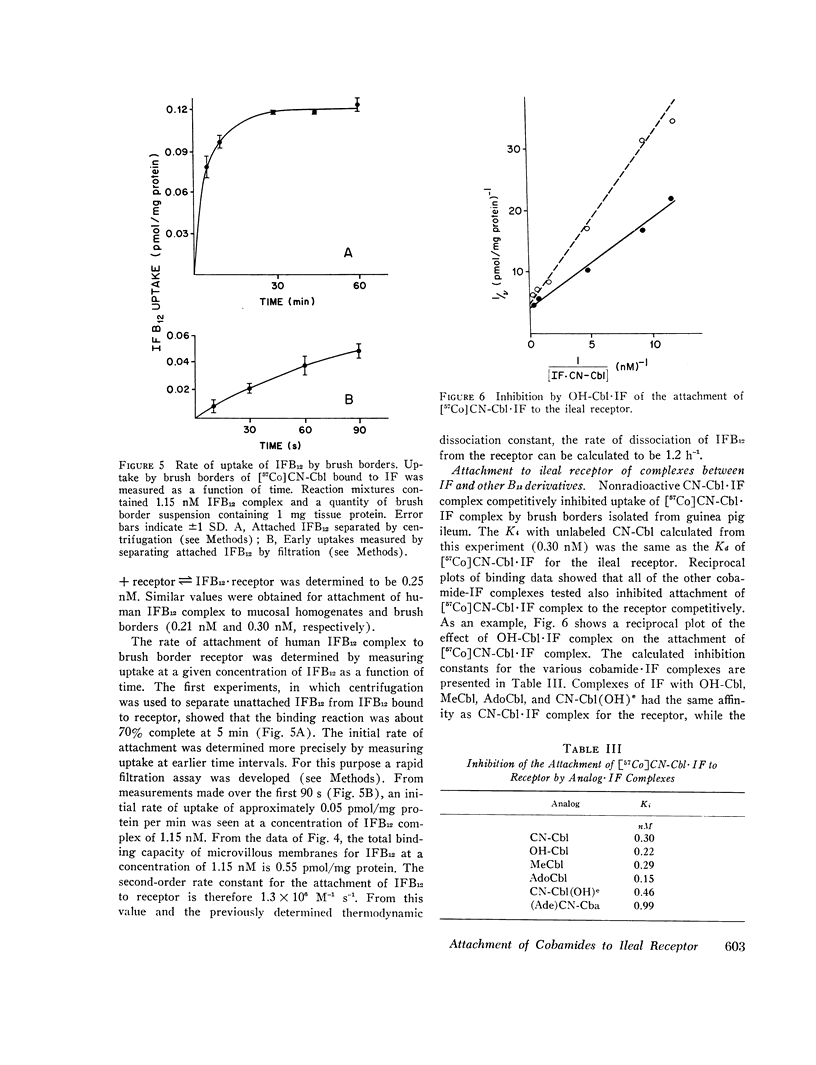
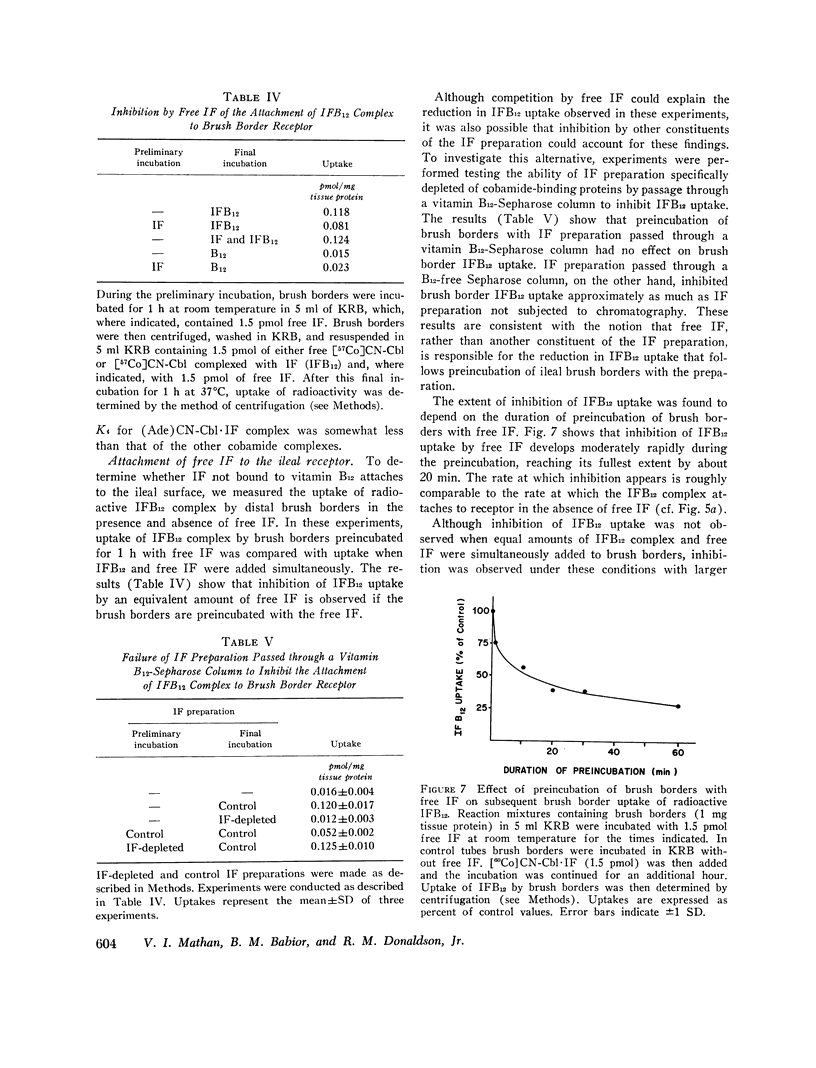
We then investigated the attachment of intrinsic factor-bound cyanocobalamin to ileal receptor. Attachment to microvillous membranes showed saturation kinetics with a dissociation constant of 0.25 nM. Attachment was rapid and was 70% complete within 5 min; the second-order rate constant for attachment was 1.3 × 106 M-1 s-1. The half-time for dissociation of intrinsic factor-bound cyanocobalamin from the ileal receptor was approximately 35 min. Free intrinsic factor inhibited the attachment of intrinsic factor-bound cyanocobalamin, but the rate of attachment of free intrinsic factor was slower than that of intrinsic factor bound to cyanocobalamin. When intrinsic factor was complexed with various analogs of cyanocobalamin, the affinities of these complexes for ileal microvillous membranes were similar to that of intrinsic factor-bound cyanocobalamin. These findings suggest that the molecular configuration of vitamin B12 is not a major determinant in the interaction between intrinsic factor-bound vitamin B12 and its ileal receptor site.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H., Majerus P. W. Isolation of vitamin B12-binding proteins using affinity chromatography. I. Preparation and properties of vitamin B12-sepharose. J Biol Chem. 1972 Dec 10;247(23):7695–7701. [PubMed] [Google Scholar]
- Allen R. H., Mehlman C. S. Isolation of gastric vitamin B 12 -binding proteins using affinity chromatography. I. Purification and properties of human intrinsic factor. J Biol Chem. 1973 May 25;248(10):3660–3669. [PubMed] [Google Scholar]
- BUNGE M. B., SCHILLING R. F. Intrinsic factor studies. VI. Competition for vit. B12 binding sites offered by analogues of the vitamin. Proc Soc Exp Biol Med. 1957 Dec;96(3):587–592. doi: 10.3181/00379727-96-23548. [DOI] [PubMed] [Google Scholar]
- BUNGE M. B., SCHILLING R. F., SCHLOESSER L. L. Intrinsic factor studies. IV. Selective absorption and binding of cyanocobalamin by gastric juice in the presence of excess pseudovitamin B12 or 5, 6-dimethylbenzimidazole. J Lab Clin Med. 1956 Nov;48(5):735–744. [PubMed] [Google Scholar]
- Babior B. M. The mechanism of action of ethanolamine deaminase. VI. Ethylene glycol, a quasi-substrate for ethanolamine deaminase. J Biol Chem. 1970 Apr 10;245(7):1755–1766. [PubMed] [Google Scholar]
- Bernhauer K., Wagner F., Beisbarth H., Rietz P., Vogelmann H. Zur Chemie und Biochemie der Corrinoide. XXVI. Darstellung und Charakterisierung von Corrinoidcarbonsäuren. Biochem Z. 1966 Apr 27;344(3):289–309. [PubMed] [Google Scholar]
- DONALDSON R. M., Jr, KATZ J. H. Exchange between free and gastric juice-bound cyanocobalamin. J Clin Invest. 1963 Apr;42:534–545. doi: 10.1172/JCI104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOSCHERHOLMEN A., HAGEN P. S. Delay of absorption of radiolabeled cyanocobalamin in the intestinal wall in the presence of intrinsic factor. J Lab Clin Med. 1959 Sep;54:434–439. [PubMed] [Google Scholar]
- Donaldson R. M., Jr, Mackenzie I. L., Trier J. S. Intrinsic factor-mediated attachment of vitamin B12 to brush borders and microvillous membranes of hamster intestine. J Clin Invest. 1967 Jul;46(7):1215–1228. doi: 10.1172/JCI105615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- GREGORY M. E., HOLDSWORTH E. S. The binding of cyanocobalamin and its naturally occurring analogues by certain body fluids and tissue extracts. Biochim Biophys Acta. 1960 Aug 26;42:462–469. doi: 10.1016/0006-3002(60)90824-6. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. W., Retief F. P., Herbert V. Blockade of vitamin B12-binding sites in gastric juice, serum and saliva by analogues and derivatives of vitamin B12 and by antibody to intrinsic factor. Biochim Biophys Acta. 1967 Aug 29;141(3):560–572. doi: 10.1016/0304-4165(67)90185-7. [DOI] [PubMed] [Google Scholar]
- Gräsbeck R., Simons K., Sinkkonen I. Isolation of intrinsic factor and its probable degradation product, as their vitamin B12 complexes, from human gastric juice. Biochim Biophys Acta. 1966 Sep 26;127(1):47–58. doi: 10.1016/0304-4165(66)90474-0. [DOI] [PubMed] [Google Scholar]
- HERBERT V. Mechanism of intrinsic factor action in everted sacs of rat small intestine. J Clin Invest. 1959 Jan 1;38(1 Pt 1):102–109. doi: 10.1172/JCI103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe E., Haber E., Olesen H. Nature of vitamin B 12 binding. II. Steric orientation of vitamin B 12 on binding and number of combining sites of human intrinsic factor and the transcobalamins. Biochim Biophys Acta. 1971 Jul 25;243(1):75–82. [PubMed] [Google Scholar]
- Hippe E., Olesen H. Nature of vitamin B 12 binding. 3. Thermodynamics of binding to human intrinsic factor and transcobalamins. Biochim Biophys Acta. 1971 Jul 25;243(1):83–88. [PubMed] [Google Scholar]
- Kaplan B. H., Stadtman E. R. Ethanolamine deaminase, a cobamide coenzyme-dependent enzyme. II. Physical and chemical properties and interaction with cobamides and ethanolamine. J Biol Chem. 1968 Apr 25;243(8):1794–1803. [PubMed] [Google Scholar]
- LATNER A. L., RAINE L. Effect of analogues on the uptake of vitamin B12 by the intact rat. Nature. 1957 Nov 30;180(4596):1197–1198. doi: 10.1038/1801197a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackenzie I. L., Donaldson R. M., Jr Effect of divalent cations and pH on intrinsic factor-mediated attachment of vitamin B 12 to intestinal microvillous membranes. J Clin Invest. 1972 Sep;51(9):2465–2471. doi: 10.1172/JCI107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan J. E. Measurement of the affinity of human gastric intrinsic factor for cyanocobalamin. J Lab Clin Med. 1967 Oct;70(4):666–672. [PubMed] [Google Scholar]
- Okuda K., Yashima K., Takamatsu M. Intestinal absorption of cobamide coenzyme in relation to structural integrity and intrinsic factor. J Lab Clin Med. 1969 Aug;74(2):218–228. [PubMed] [Google Scholar]
- ROSENBLUM C., WOODBURY D. T., GILBERT J. P., OKUDA K., CHOW B. F. Comparative absorption of vitamin B12 analogues by normal humans. I. Chlorocobalamin vs. cyanocobalamin. Proc Soc Exp Biol Med. 1955 May;89(1):63–66. doi: 10.3181/00379727-89-21717. [DOI] [PubMed] [Google Scholar]
- ROSENBLUM C., YAMAMATO R. S., WOOD R., WOODBURY D. T., OKUDA K., CHOW B. F. Comparative absorption of vitamin B12 analogues by normal humans. II. Chloro-, sulfato-, nitro- and thiocyanato- vs. cyanocobalamin. Proc Soc Exp Biol Med. 1956 Mar;91(3):364–367. doi: 10.3181/00379727-91-22264. [DOI] [PubMed] [Google Scholar]
- Retief F. P., Gottlieb C. W., Kochwa S., Pratt P. W., Herbert V. Separation of vitamin B 12-binding proteins of serum, gastric juice and saliva by rapid DEAE cellulose chromatography. Blood. 1967 Apr;29(4):501–516. [PubMed] [Google Scholar]
- STRAUSS E. W., WILSON T. H. Factors controlling B12 uptake by intestinal sacs in vitro. Am J Physiol. 1960 Jan;198:103–107. doi: 10.1152/ajplegacy.1960.198.1.103. [DOI] [PubMed] [Google Scholar]
- SULLIVAN L. W., HERBERT V., CASTLE W. B. IN VITRO ASSAY FOR HUMAN INTRINSIC FACTOR. J Clin Invest. 1963 Sep;42:1443–1458. doi: 10.1172/JCI104829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOPOREK M. The relation of binding power to intrinsic factor activity. Effect of pseudovitamin B12 on absorption of vitamin B12. Am J Clin Nutr. 1960 May-Jun;8:297–300. doi: 10.1093/ajcn/8.3.297. [DOI] [PubMed] [Google Scholar]
- WEISSBACH H., LADD J. N., VOLCANI B. E., SMYTH R. D., BARKER H. A. Structure of the adenylcobamide coenzyme: degradation by cyanide, acid, and light. J Biol Chem. 1960 May;235:1462–1473. [PubMed] [Google Scholar]
- WILSON T. H., STRAUSS E. W. Some species differences in the intrinsic factor stimulation of B12 uptake by small intestine in vitro. Am J Physiol. 1959 Oct;197:926–928. doi: 10.1152/ajplegacy.1959.197.4.926. [DOI] [PubMed] [Google Scholar]


