Abstract
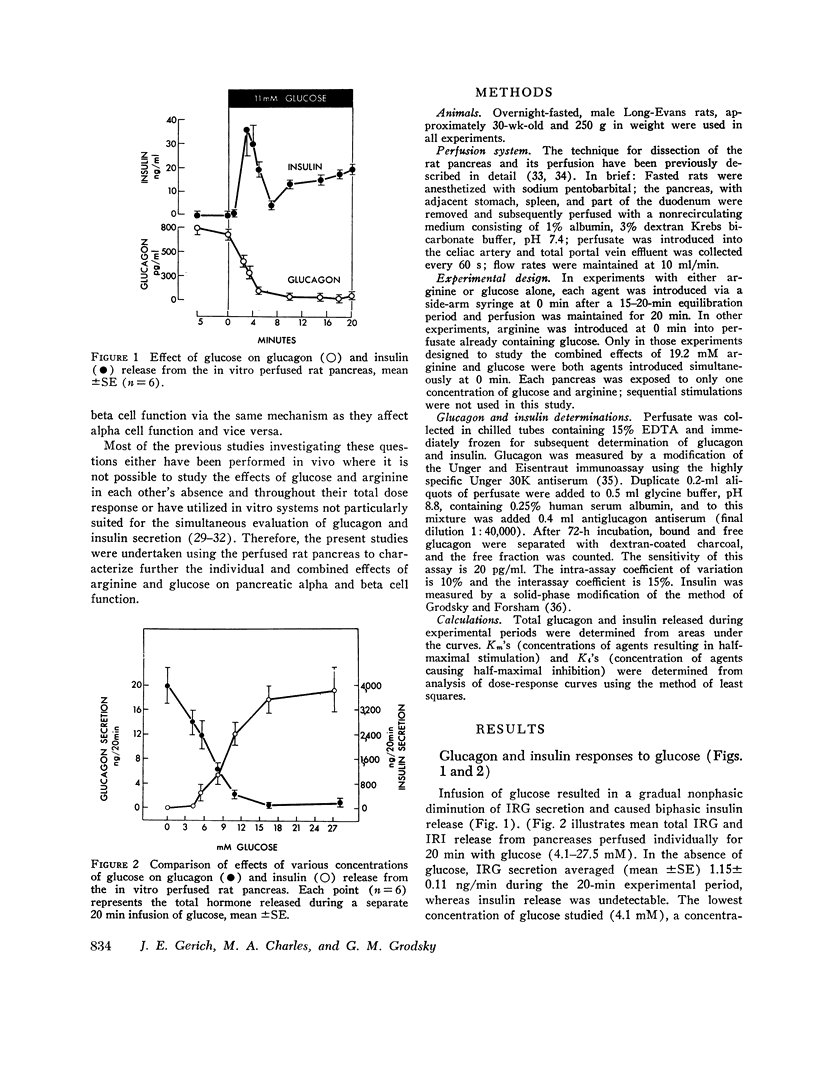
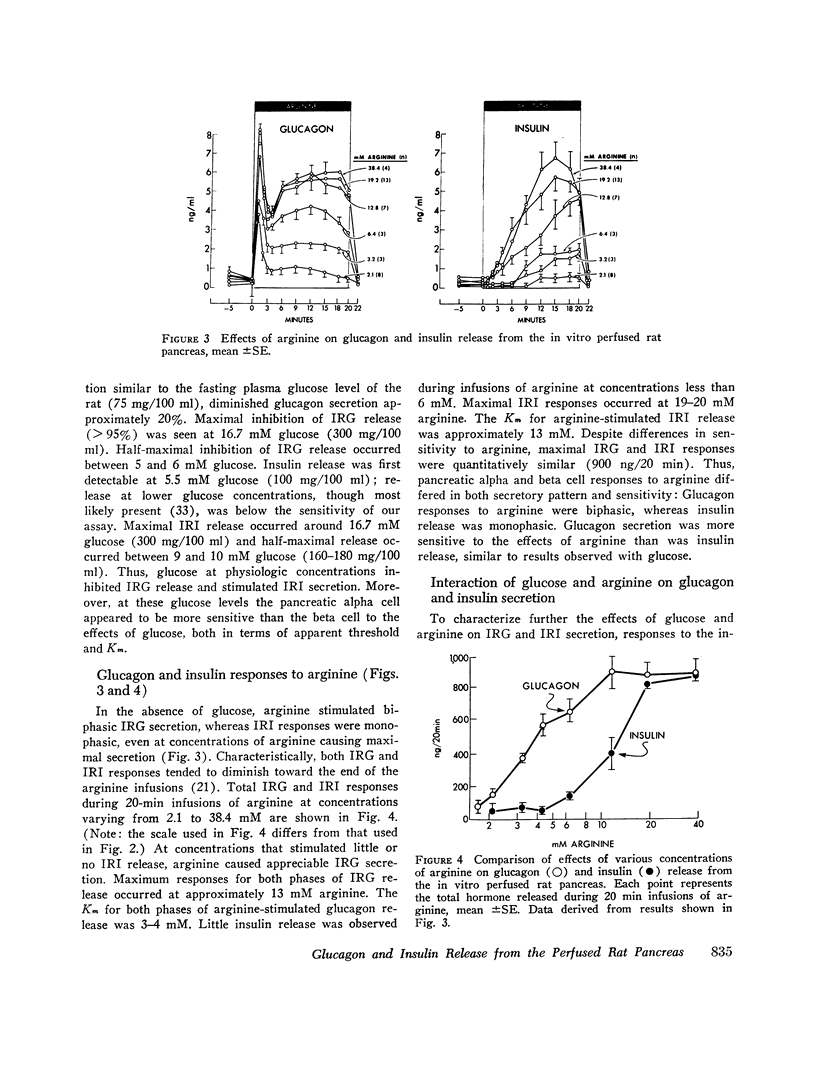
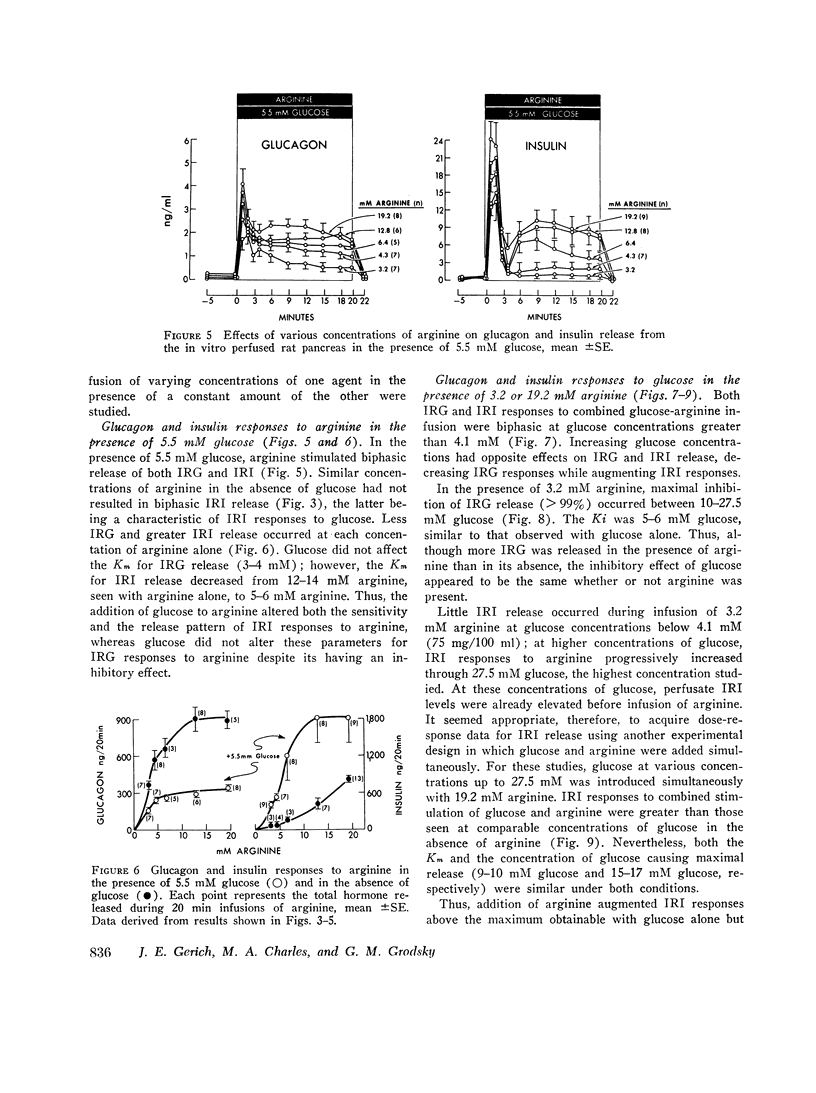
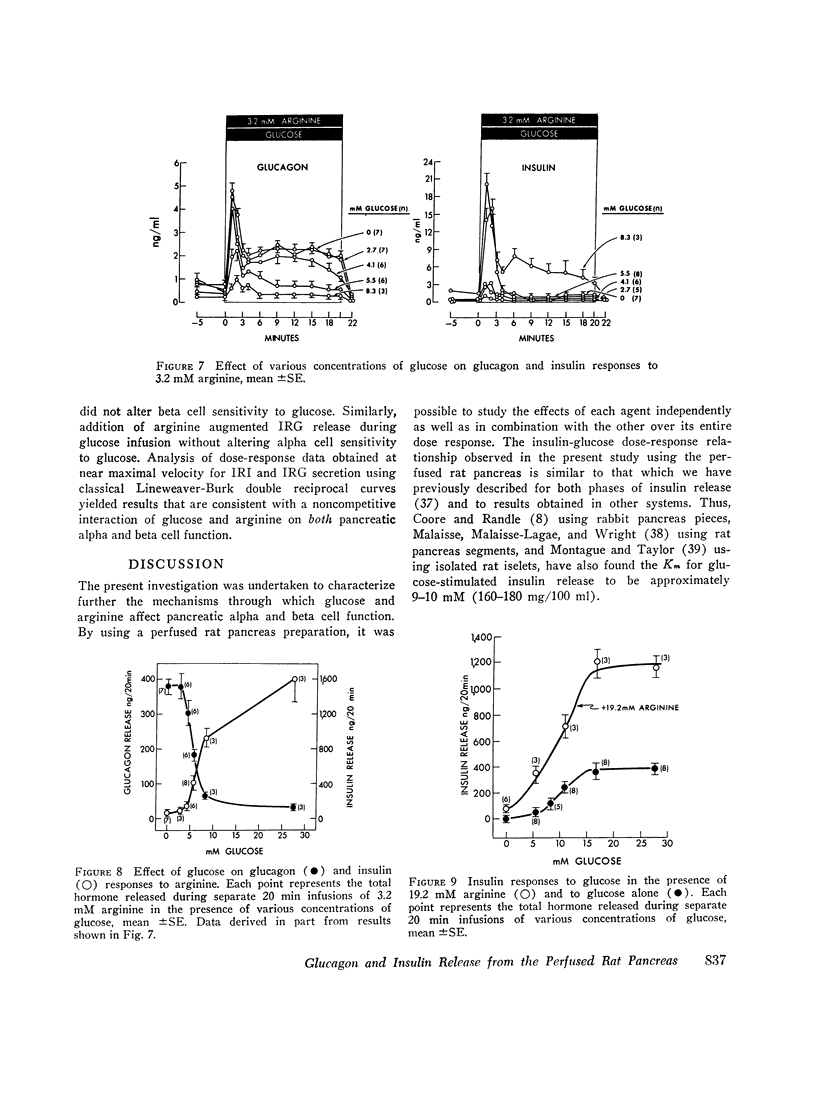
To characterize the mechanisms by which arginine and glucose affect pancreatic alpha and beta cell function, the effects of these agents over their full dose response, both alone and in various combinations, were studied using the perfused rat pancreas. Arginine (0-38 mM), in the absence of glucose, stimulated biphasic glucagon (IRG) secretion (Km≃3-4 mM) at concentrations less than 1 mM and caused nonphasic insulin (IRI) release (Km≃12-13 mM) but only at concentrations greater than 6 mM. Glucose (0-27.5 mM) alone stimulated biphasic IRI release (Km≃9-10 mM) at concentrations in excess of 5.5 mM and caused nonphasic inhibition of IRG secretion (Kt≃5-6 mM) at concentrations as low as 4.1 mM. These results demonstrate fundamental differences in pancreatic alpha and beta cell secretory patterns in response to glucose and arginine and suggest that glucagon secretion is more sensitive to the effect of both glucose and arginine. Various concentrations of arginine in the presence of 5.5 mM glucose stimulated biphasic IRG and IRI release: IRG responses were diminished and IRI responses were enhanced compared with those seen with arginine in the absence of glucose. Glucose (0-27.5 mM) in the presence of 3.2 or 19.2 mM arginine caused similar inhibition of IRG secretion (Km≃5-6 mM) and stimulation of IRI release (Km≃9-10 mM) as that seen with glucose alone, although greater IRG and IRI release occurred. This augmentation of IRI secretion was greater than that expected from mere additive effects of glucose and arginine. Classical Lineweaver-Burk analysis of these results indicates that glucose is a non-competitive inhibitor arginine-stimulated glucagon secretion and suggests that glucose and arginine affect pancreatic alpha and beta cell function via different mechanisms. In addition, comparison of simultaneous insulin and glucagon secretion patterns under various conditions suggests that endogenous insulin per se has little or no direct effect on IRG secretion and that endogenous glucagon does not appreciably affect pancreatic beta cell function.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basabe J. C., Lopez N. L., Viktora J. K., Wolff F. W. Insulin secretion studied in the perfused rat pancreas. I. Effect of tolbutamide, leucine and arginine; their interaction with diazoxide, and relation to glucose. Diabetes. 1971 Jul;20(7):449–456. doi: 10.2337/diab.20.7.449. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., Mawhinney W. A. Glucagon release from isolated pancreas in streptozotocin-treated rats. Diabetes. 1973 Nov;22(11):797–800. doi: 10.2337/diab.22.11.797. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R. Diabetes mellitus--a disorder of cellular information transmission? Horm Metab Res. 1970 Jul;2(4):246–249. doi: 10.1055/s-0028-1095082. [DOI] [PubMed] [Google Scholar]
- Chesney T. M., Schofield J. G. Studies on the secretion of pancreatic glucagon. Diabetes. 1969 Sep;18(9):627–632. doi: 10.2337/diab.18.9.627. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Edgar P., Rabinowitz D., Merimee T. J. Effects of amino acids on insulin release from excised rabbit pancreas. Endocrinology. 1969 Apr;84(4):835–843. doi: 10.1210/endo-84-4-835. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Hellerström C., Petersson B., Taylor K. W. Oxidation of glucose and fatty acids in normal and in A 2 -cell rich pancreatic islets isolated from guinea-pigs. Diabetologia. 1972 Apr;8(2):93–98. doi: 10.1007/BF01235632. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Taylor K. W. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Biochim Biophys Acta. 1970 Aug 14;215(2):310–315. doi: 10.1016/0304-4165(70)90029-2. [DOI] [PubMed] [Google Scholar]
- Efendic S., Cerasi E., Luft R. Role of glucose in arginine-induced insulin release in man. Metabolism. 1971 Jun;20(6):568–579. doi: 10.1016/0026-0495(71)90005-9. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Pek S., Weissman P., Conn J. W. Amino acids and insulin release in vivo. Isr J Med Sci. 1972 Mar;8(3):233–243. [PubMed] [Google Scholar]
- Frankel B. J., Gerich J. E., Hagura R., Fanska R. E., Gerritsen G. C., Grodsky G. M. Abnormal secretion of insulin and glucagon by the in vitro perfused pancreas of the genetically diabetic Chinese hamster. J Clin Invest. 1974 Jun;53(6):1637–1646. doi: 10.1172/JCI107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRODSKY G. M., BATTS A. A., BENNETT L. L., VCELLA C., MCWILLIAMS N. B., SMITH D. F. EFFECTS OF CARBOHYDRATES ON SECRETION OF INSULIN FROM ISOLATED RAT PANCREAS. Am J Physiol. 1963 Oct;205:638–644. doi: 10.1152/ajplegacy.1963.205.4.638. [DOI] [PubMed] [Google Scholar]
- GRODSKY G. M., FORSHAM P. H. An immunochemical assay of total extractable insulin in man. J Clin Invest. 1960 Jul;39:1070–1079. doi: 10.1172/JCI104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardino J. J., Martin J. M. Studies on the mechanism of insulin release. Metabolism. 1966 Dec;15(12):1068–1075. doi: 10.1016/0026-0495(66)90095-3. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Schneider V., Dippe S. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Characterization of the glucagon response to hypoglycemia in man. J Clin Endocrinol Metab. 1974 Jan;38(1):77–82. doi: 10.1210/jcem-38-1-77. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest. 1972 Aug;51(8):2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L., Smith D. F., Schmid F. G. Effect of pulse administration of glucose or glucagon on insulin secretion in vitro. Metabolism. 1967 Mar;16(3):222–233. doi: 10.1016/0026-0495(67)90171-0. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Evidence for mediated transport of glucose in mammalian pancreatic -cells. Biochim Biophys Acta. 1971 Jul 6;241(1):147–154. doi: 10.1016/0005-2736(71)90312-9. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. Uptake of alanine, arginine and leucine by mammalian pancreatic beta-cells. Endocrinology. 1971 Dec;89(6):1432–1439. doi: 10.1210/endo-89-6-1432. [DOI] [PubMed] [Google Scholar]
- Hertelendy F., Machlin L. J., Takahashi Y., Kipnis D. M. Insulin release from sheep pancreas in vitro. J Endocrinol. 1968 Aug;41(4):605–606. doi: 10.1677/joe.0.0410605. [DOI] [PubMed] [Google Scholar]
- Iversen J. Secretion of glucagon from the isolated, perfused canine pancreas. J Clin Invest. 1971 Oct;50(10):2123–2136. doi: 10.1172/JCI106706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube H., Fussgänger R. D., Maier V., Pfeiffer E. F. Hyperglucagonemia of the isolated perfused pancreas of diabetic mice (db-db). Diabetologia. 1973 Oct;9(5):400–402. doi: 10.1007/BF01239436. [DOI] [PubMed] [Google Scholar]
- Laube H., Fussgänger R., Goberna R., Schröder K., Straub K., Sussman K., Pfeiffer E. F. Effects of tolbutamide on insulin and glucagon secretion of the isolated perfused rat pancreas. Horm Metab Res. 1971 Jul;3(4):238–242. doi: 10.1055/s-0028-1094161. [DOI] [PubMed] [Google Scholar]
- Leclercq-Meyer V., Brisson G. R., Malaisse W. J. Effect of adrenaline and glucose on release of glucagon and insulin in vitro. Nat New Biol. 1971 Jun 23;231(25):248–249. doi: 10.1038/newbio231248a0. [DOI] [PubMed] [Google Scholar]
- Lernmark A. Effects of neutral and dibasic amino acids on the in vitro release of insulin. Hormones. 1972;3(1):22–30. [PubMed] [Google Scholar]
- Levin S. R., Grodsky G. M., Hagura R., Smith D. F., Forsham P. H. Relationships between arginine and glucose in the induction of insulin secretion from the isolated, perfused rat pancreas. Endocrinology. 1972 Mar;90(3):624–631. doi: 10.1210/endo-90-3-624. [DOI] [PubMed] [Google Scholar]
- Levin S. R., Karam J. H., Hane S., Grodsky G. M., Forsham P. H. Enhancement of arginine-induced insulin secretion in man by prior administration of glucose. Diabetes. 1971 Mar;20(3):171–176. doi: 10.2337/diab.20.3.171. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Insulin secretion: multifactorial regulation for a single process of release. The Minkowski award lecture delivered on September 7, 1972 before the European Association for the study of Diabetes at Madrid, Spain. Diabetologia. 1973 Jun;9(3):167–173. doi: 10.1007/BF01219778. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. Stimulation of insulin secretion by noncarbohydrate metabolites. J Lab Clin Med. 1968 Sep;72(3):438–448. [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. E., Krzanowski J., Kotler-Brajtburg J., Landgraf R., Fertel R. The dual function of glucose in islets of Langerhans. J Biol Chem. 1971 Feb 25;246(4):1007–1011. [PubMed] [Google Scholar]
- Milner R. D. The stimulation of insulin release by essential amino acids from rabbit pancreas in vitro. J Endocrinol. 1970 Jul;47(3):347–356. doi: 10.1677/joe.0.0470347. [DOI] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Islet-cell metabolism during insulin release. Effects of glucose, citrate, octanoate, tolbutamide, glucagon and theophylline. Biochem J. 1969 Nov;115(2):257–262. doi: 10.1042/bj1150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971 Sep;50(9):1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda A., Aguilar-Parada E., Eisentraut A. M., Unger R. H. Control of pancreatic glucagon secretion by glucose. Diabetes. 1969 Jan;18(1):1–10. doi: 10.2337/diab.18.1.1. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr The glucose receptor. A defective mechanism in diabetes mellitus distinct from the beta adrenergic receptor. J Clin Invest. 1973 Apr;52(4):870–876. doi: 10.1172/JCI107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha D. M., Faloona G. R., Unger R. H. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972 Sep;51(9):2346–2351. doi: 10.1172/JCI107046. [DOI] [PMC free article] [PubMed] [Google Scholar]


