Abstract
Background
Inflammatory markers may be associated with recurrent vascular events after stroke. We aimed to: (1) determine the association between interleukin-6, C-reactive protein, fibrinogen and white cell count and recurrent vascular events after stroke, and (2) compare the association between circulating inflammatory markers and the risk of death from vascular versus non-vascular causes.
Methods and Results
We prospectively recruited patients with acute stroke (n=817) and followed them for up to 4 years for the occurrence of: fatal or non-fatal recurrent stroke, myocardial infarction or fatal vascular events, and death from any cause (n=159). The delay to assessment was a median of 10 days. The adjusted incidence of the outcome cluster ‘recurrent stroke, myocardial infarction or vascular death’ after stroke was significantly higher with higher levels of interleukin 6 (75th to 25th centile: hazard ratio [HR] 1.56, 95 % CI 1.37 to 1.77), C-reactive protein (75th to 25th centile HR 1·08, 95% CI 1·04 to 1·11) and fibrinogen (75th to 25th centile HR 1.45, 95% CI 1.24 to 1.72). The associations between inflammatory markers and death were stronger than with recurrent vascular events. The associations of inflammatory markers with vascular and non-vascular deaths were similar.
Conclusions
Though inflammatory markers were associated with an increased risk of recurrent vascular events and vascular death after stroke, they were also associated with non-vascular causes of death, suggesting that inflammatory markers do not play a causal role specifically in the generation of recurrent vascular events after stroke. Future studies of the prediction of recurrent vascular events after stroke should concentrate on clinical variables, or different blood markers.
Keywords: Inflammation, stroke, prognosis
INTRODUCTION
In prospective studies of patients with prior stroke or transient ischemic attack (TIA), increased levels of markers of acute inflammation – C-reactive protein (CRP)1, interleukin-6 (IL-6)2, fibrinogen1, 3 and white cell count4 – were associated with increased incidence of recurrent stroke and myocardial infarction (MI) and the risk of death or disability at 6 months after stroke5. If high levels of acute-phase markers in the early stages of stroke contribute to recurrent vascular events, we hypothesised the association between inflammatory markers with fatal recurrent vascular events would be stronger than with other, non-vascular, deaths.
In a prospective cohort of patients with recent stroke we aimed to: (1) estimate the association between levels of circulating inflammatory markers and the incidence of ‘recurrent vascular events’ (recurrent stroke, MI and vascular death), and, (2) compare the strength of the association between inflammatory markers and the risk of death from vascular and non-vascular causes.
METHODS
The Edinburgh Stroke Study was a prospective, hospital-based cohort study of stroke patients followed up for recurrent stroke, MI and death. We have described the methods and process of data collection elsewhere6. In brief, we recruited consenting patients with ischemic stroke or intracerebral hemorrhage presenting to the Western General Hospital in Edinburgh between April 2002 and May 2005. We assigned ischemic stroke subtypes (i) according to the site and size of the causative infarct, modified if necessary by imaging findings with the Oxford community stroke project (OCSP) classification and (2) with a modified Trial of ORG10172 in Acute Stroke Treatment (TOAST) classification as described previously.6 We made a clinical assessment at baseline and contemporaneously drew blood for markers of inflammation (CRP, IL-6, fibrinogen and white cell count and glucose). We obtained follow up data on patients with multiple overlapping methods. We defined as ‘recurrent vascular events’ the outcome cluster ‘recurrent fatal or non-fatal stroke, subsequent fatal or non-fatal MI or other vascular death’. We defined ‘other vascular death’ as deaths due to athero-thrombo-embolic vascular diseases other than stroke or MI. For this study we classified deaths due to the qualifying – though not recurrent - stroke (which were most often due to pneumonia) or gastrointestinal hemorrhage as non-vascular. We did not routinely record the occurrence of infections or other complications between stroke onset and the measurement of vascular outcome or death. The Lothian Research Ethics Committee approved the project.
Measurement of blood markers
A clinical laboratory measured total white cell count (Beckman Coulter LH750 analyser) and blood glucose (Vitros Chemistry analyser). Blind to clinical details, we measured CRP and fibrinogen in plasma by immunonephelometry (Prospec, Dade Behring Milton Keynes, UK) using the manufacturer's reagents and standards. We assayed IL-6 by ELISA (R & D Systems, Oxford, UK). Intra- and inter-assay coefficients of variation were 4.7 and 8.3%, 2.6 and 5.3%, and 7.5 and 8.9%, respectively.
Statistical analysis
We used Stata version 10 (Statacorp 2007) for analysis and prepared the paper with reference to the STrengthening the Reporting of OBservational studies in Epidemiology. (STROBE)7 guidelines. We measured survival to first recurrent vascular event, and separately to each of recurrent stroke, MI and vascular death censoring patients at the end of follow up or non-vascular death. We compared the baseline characteristics of patients who experienced a recurrent vascular event with those who did not with univariable Cox regression analysis. We examined the relationships between inflammatory markers with correlation coefficients, and used linear regression (after loge transformation of markers) to examine the relationship of markers with delay to blood draw. We used Kaplan Meier survival curves to compare event free survival between groups of patients defined by thirds of inflammatory biomarkers and compared curves with log rank trend tests. We used Cox regression analysis to calculate unadjusted hazard ratios (HR) and 95% confidence intervals (CI) per unit increase in marker levels. We built a multivariate Cox regression model to adjust for confounders, adding variables sequentially that were associated with recurrent vascular events in univariable analysis and had data completeness of over 95%, keeping those variables that significantly improved the fit of the model (likelihood ratio test P<0·05). When the model was complete we tested the proportional hazards assumption and its goodness of fit. We looked for first order interactions of inflammatory biomarker levels with other variables in the final model by adding multiplicative terms.
We made further assessment of the marker most strongly associated with recurrent vascular events (IL-6). We assessed the change in discrimination after the addition of IL-6 to a model containing only clinical variables by calculating Harrell's c-statistic for models with and without biomarkers. The c-statistic is analogous to the area under a receiver operator curve for Cox regression models; a value of 0.5 indicates no better discrimination than chance and a value of 1 perfect discrimination.
We replicated the analysis measuring time to death only, censoring at the end of the study. To adjust models examining the risk of death, we used previously validated covariates. 8 We also plotted, by thirds of marker levels, the competing risks of vascular deaths, death due to the initial stroke and death due to other causes using the ‘stcompet’ command, 9 which calculates the cumulative incidence of each outcome.
RESULTS
Baseline characteristics
877 of 1408 patients in the Edinburgh Stroke Study (62%) gave consent and were available for blood to be drawn for markers of inflammation. Of these, 817 (93%) had a definite ischemic stroke, 17 (2%) a probable ischemic stroke and 43 (5%) a hemorrhagic stroke. Of those patients who had blood drawn for blood markers, none was lost to follow up for the outcomes of death, recurrent stroke or myocardial infarction. We first assessed patients clinically at a median of 10 days (IQR 3 to 21 days) after stroke onset and drew blood at a median of 0 days (IQR 0 to 3 days) after assessment. The delay to assessment was longer for outpatients seen in a clinic (median 19 days) than in those admitted to the stroke unit (median 2 days). Patients who did not have blood drawn were similar to those with blood drawn for biomarker data in age, sex distribution and the proportions with hypertension, peripheral or cardiac vascular disease, diabetes or atrial fibrillation. On average, compared to those without blood sampling, patients with blood samples had milder strokes (proportion TACS 6.8 % vs 14.7% p=0.001), as patients admitted to hospital and those with more severe symptoms were less likely to be recruited because of practical barriers to obtaining and processing research blood samples and obtaining informed consent or assent.
During the 1866 person years of follow up (mean 2.12 years), 106 patients had a first recurrent stroke (92 ischemic, 5 hemorrhagic and 9 of uncertain type) and 34 had a myocardial infarction. There were 184 deaths: 113 from vascular causes (63 strokes, 35 from cardiac causes, and death from bowel ischemia, vascular dementia and presumed vascular renal failure) and 64 from other causes (33 cancers, 13 chest infections, 6 from COPD and the rest from pancreatitis, bowel perforation, hip fracture, and extra-pulmonary sepsis).
At the time of the clinical assessment of the index stroke, the median IL-6 was 4·0 (interquartile range [IQR] 2.4 to 7.2) pg/l, median CRP 3.5 (IQR 1.4 to 9.7) mg/l, median fibrinogen 4.5 (IQR 3.8 to 5.4) g/l, median white cell count 8 (IQR 6.6 to 9.7) ×109/l and median glucose 5.6 (IQR 5 to 6.8) mmol/l. The correlation coefficients were, between IL-6 and other variables were: CRP 0.59, fibrinogen 0.48, glucose 0.06 and white cell count 0.25. The correlation coefficients between loge marker levels and delay from symptom onset to blood draw were, for IL-6 −0.18, CRP −0.12, fibrinogen −0.11, white cell count −0.13 and glucose −0.06. The relationships of average marker levels with the delay from stroke to blood draw were not statistically significant after adjusting for the level of baseline neurological impairment and age.
Circulating inflammatory markers and recurrent stroke, MI and vascular death
There was a significant increase in recurrent vascular events for patients who: were older; or had a history of AF, heart failure or previous peripheral vascular disease, coronary heart disease or stroke (Table 1). The log hazard of stroke, MI or vascular death rose with each third of IL-6 and CRP, though not by thirds of glucose, fibrinogen or white cell count.
Table 1.
Baseline characteristics of stroke patients.
| Total | Recurrent Stroke, MI or vascular death |
No vascular event | Univariate HR (95% CI) |
|
|---|---|---|---|---|
| Number | 877 | 159 | 718 | |
| Demographic | ||||
| Age, years (mean, SD) | 71.4 (12.0) | 73.6 (10.7) | 70.9 (12.2) | 1.02 (1.00 – 1.04)† |
| Male sex, N (%) | 463 (52.8) | 80 (50.3) | 383 (53.3) | 0.9 (0.6 to 1.2) |
| Laboratory results | Median (IQR) | Median (IQR) | Median (IQR) | |
| Interleukin-6 (pg/ml) | 4.0 (2.4-7.2) | 4.8 (2.8-9.1) | 3.8 (2.3-6.6) | |
| CRP (mg/L) | 3.5 (1.4-9.7) | 5.9 (1.9-15.5) | 3.3 (1.2-8.8) | |
| Fibrinogen (g/L) | 4.5 (3.8-5.4) | 5.7 (3.9-5.7) | 4.4 (3.8-5.4) | |
| White cell count (×109/l) | 8 (6.6-9.7) | 8.4 (6.7-9.7) | 7.9 (6.6-9.7) | |
| Glucose (mmol) | 5.6 (5-6.8) | 5.7 (4.9-7.2) | 5.6 (5-6.7) | |
| Cholesterol (mmol/l) | 5.1 (4.4-6.0) | 4.9 (4.3-5) | 5.1 (4.4-6) | |
| Pathological type, index stroke | N (%) | N (%) | N (%) | |
| Definite ischemic | 817 (93.2) | 149 (93.7) | 668 (93.0) | 1.1 (0.6 to 2.1)‡ |
| Definite hemorrhagic | 43 (4.9) | 9 (5.7) | 34 (4.7) | 1.2 (0.6 to 2.4) ‡ |
| Pathological type unknown | 17 (1.9) | 1 (0.6) | 16 (2.2) | 0.3 (0.1 to 1.9) ‡ |
|
Clinical stroke syndrome of index stroke (OCSP*) |
||||
| TACI | 57 (6.8) | 10 (6.3) | 53 (7.4) | 1.2 (0.6 to 2.2) § |
| PACI | 376 (45.1) | 81 (50.9) | 308 (42.9) | 1.1 (0.8 to 1.6) § |
| LACI | 228 (27.3) | 38 (23.9) | 200 (27.9) | 0.8 (0.5 to 1.1) § |
| POCI | 131 (15.7) | 22 (13.8) | 121 (16.9) | 0.8 (0.5 to 1.3) § |
| Uncertain subtype | 42 (5.0) | 8 (5.0) | 36 (5.0) | 0.9 (0.5 to 1.9) § |
| Severity of index stroke | ||||
| Can't walk, can't lift both arms | 91 (10.4) | 12 (7.6) | 79 (11.0) | 0.9 (0.5 to 1.5) |
| Can't walk, can lift both arms | 119 (13.6) | 31 (19.5) | 88 (12.3) | 1.6 (1.1 to 2.4) |
| Can walk | 664 (76.0) | 115 (72.9) | 549 (76.7) | 0.7 (0.5 to 1.0) |
|
Aetiological classification of index stroke (TOAST) |
||||
| Cardioembolic | 117 (13.3) | 28 (17.6) | 89 (12.4) | 1.2 (0.8 to 2.0) ∥ |
| Large vessel disease | 72 (8.2) | 12 (7.6) | 60 (8.4) | 1.0 (0.7 to 1.6) ∥ |
| Mixed aetiology | 58 (6.6) | 16 (7.6) | 42 (5.9) | 1.8 (1.2 to 2.7) ∥ |
| Small vessel disease | 178 (20.3) | 24 (15.1) | 154 (21.5) | 0.8 (0.6 to 1.2) ∥ |
| Unclassified after complete investigation | 355 (40.5) | 65 (40.9) | 290 (40.4) | 0.9 (0.7 to 1.3) ∥ |
| Unclassified after incomplete investigation | 40 (15.8) | 14 (8.8) | 83 (11.6) | 0.8 (0.5 to 1.5) ∥ |
| Risk factors | ||||
| History of TIA | 143 (16.3) | 29 (18.2) | 114 (15.9) | 1.1 (0.8 to 1.7) |
| History of stroke | 166 (18.9) | 41 (25.8) | 125 (17.4) | 1.5 (1.1 to 2.2) |
| History of ischemic heart disease | 242 (27.6) | 62 (39.0) | 180 (25.1) | 1.9 (1.4 to 2.6) |
| History of PVD | 69 (7.9) | 22 (13.9) | 47 (6.6) | 2.0 (1.3 to 3.2) |
| Ipsilat. carotid stenosis >70 | 97 (12.5) | 21 (14.9) | 76 (12.0) | 1.2 (0.8 to 2.0) |
| Ever AF | 168 (20.3) | 42 (26.4) | 126 (17.6) | 1.7 (1.3 to 2.5) |
| Prior treated hypertension | 467 (53.3) | 96 (60.4) | 371 (51.7) | 1.4 (1.0 to 1.9) |
| Diabetes | 110 (12.5) | 26 (16.4) | 84 (11.7) | 1.4 (0.9 to 2.2) |
| Ever smoker | 602 (69.8) | 113 (71.1) | 489 (69.5) | 1.1 (0.8 to 1.5) |
| Heart failure | 40 (4.58) | 14 (8.7) | 26 (3.6) | 2.8 (1.6 to 4.8) |
| Any antiplatelet at baseline | 369 (46.1) | 11 (6.9) | 33 (4.6) | 1.7 (0.9 to 3.1) |
| Warfarin at baseline | 43 (4.9) | 32 (4.5) | 11 (6.9) | 1.5 (0.8 to 2.7) |
| Systolic BP (Mean, No.observations) | 147.2 (874) | 147.3 (159) | 147.2 (715) | 1.00 (0.99 to 1.01) † |
| Diastolic BP (Mean, No. observations) | 80.0 (874) | 80.1 (159) | 80.0 (715) | 1.00 (0.99 to 1.01) † |
OCSP=Oxfordshire Community Stroke Project Classification (ischemic and probable), TACS=total anterior circulation stroke, PACS=partial anterior circulation stroke, LACS=lacunar stroke, POCS=posterior circulation stroke.
per unit increase
vs other pathological types
vs all others in OCSP classification
vs all others in TOAST classification.
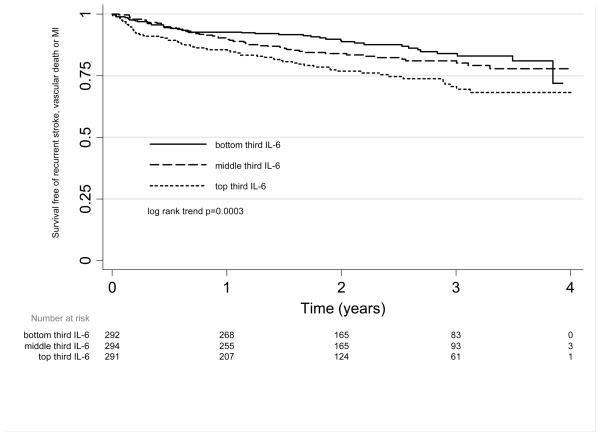
In unadjusted Kaplan-Meier survival analyses, patients survived free of recurrent vascular events for a shorter time in the highest third of IL-6 (log rank trend χ2=13.2, p=0.0003) (figure 1) and CRP (log rank trend χ2=13.9, p=0.0002). This relationship did not reach statistical significance for fibrinogen (log rank trend χ2=2.8, p=0.09), glucose (log rank trend χ2=1.1, p=0.3) or white cell count (log rank trend χ2=3.1, p=0.08). However, a linear model fitted the data well for each marker.
Figure 1.
Unadjusted Kaplan Meier survival curve and life table, for survival free from recurrent stroke, myocardial infarction or vascular death by third of interleukin 6.
Table 2 shows the association between circulating inflammatory markers and recurrent vascular events. In univariate analyses, all markers except glucose were significantly associated with recurrent vascular events. The relative hazard for an increase of 1 pg/ml of IL-6 was 1.07 (95% CI: 1.04 to 1.10) per pg/ml. The unadjusted associations between IL-6 and recurrent fatal or non-fatal stroke alone (HR 1.04 95% CI 1.00 to 1.08 per pg/ml) were weaker while the HRs for a mg/l increase of CRP, a g/l increase of fibrinogen, a 1×109 increase in white cell count or a mmol/l increase of glucose were not significantly different from 1. The unadjusted association between IL-6 and fatal or non-fatal MI alone (HR 1.09, 95% CI 1.03 to 1.15) was stronger than for recurrent stroke alone.
Table 2.
The association between marker level and recurrent stroke, MI or vascular death, assuming a linear association between marker level and log hazards
| Hazard ratio per unit increase* in marker (95% CI) | Hazard ratio comparing 75th to 25st centile† | |||
|---|---|---|---|---|
| Unadjusted | Adjusted‡ | Adjusted for all markers§ |
Adjusted‡ | |
| Interleukin-6 (pg/ml) | 1.07 (1.04 to 1.10) | 1.06 (1.03 to 1.09) | 1.05 (1.01 to 1.09) | 1.33 (1.15 to 1.53) |
| C-reactive protein (mg/l) | 1.01 (1.00 to 1.01) | 1.01 (1.00 to 1.01) | 1.00 (1.00 to 1.03) | 1.06 (1.02 to 1.09) |
| Fibrinogen (g/l) | 1.16 (1.05 to 1.28) | 1.12 (1.01 to 1.25) | 1.02 (0.97 to 1.17) | 1.20 (1.01 to 1.43) |
| White cell count (×109/l) | 1.06 (1.02 to 1.10) | 1.05 (1.00 to 1.11) | 1.03 (0.97 to 1.09) | 1.17 (0.98 to 1.38) |
| Glucose (mmol/l) | 1.04 (0.99 to 1.09) | 1.03 (0.98 to 1.08) | 1.02 (0.97 to 1.08) | 1.06 (0.97 to 1.15) |
for IL-6 pg/ml; CRP mg/l; fibrinogen g/l; white cell count ×109/l; glucose mmol/l
25th and 75th percentile respectively for: IL6 2.39 and 7.22 pg/ml; CRP 1.39 and 9.65 mg/l; fibrinogen 3.81 and 5.41 g/l; white cell count 6.6 and 9.7 ×109/l; glucose 5.0 and 6.8 mmol/l
Adjusted for confounders: age, cardiac failure, atrial fibrillation (current or past), or prior stroke, TIA, peripheral vascular disease or MI.
adjusted for all confounder in previous column, and other markers.
We adjusted for the following confounders in the final model: age, prior stroke or TIA or ischemic heart disease, current or prior AF and cardiac failure. Adding markers of stroke severity (i.e. ability to walk or lift arms off bed), blood pressure at assessment, stroke pathological type, diabetes, carotid stenosis, delay to blood taking or smoking did not significantly improve models containing a single inflammatory marker. After adjustment, there was still a significant association between recurrent vascular events and increasing levels of IL-6, CRP and fibrinogen (Table 2). In this cohort, those patients with highest blood levels (75th centile) of interleukin 6 had a 1.33 fold increase in the incidence of recurrent vascular events compared with those with the lowest levels (25th centile). A similar relative increase in incidence was seen for fibrinogen (HR 1.20), and less for C-reactive protein (HR 1.06).
We added markers sequentially as continuous variables, in order of the strength of their association with recurrent vascular events, to a model containing only clinical variables (age, prior stroke or TIA or heart disease, current or prior AF or cardiac failure). Addition of IL-6 significantly improved the model (likelihood ratio (LR) test χ2=14.0, p<0.001), though further addition of CRP (LR test χ2=0.3, p=0.56), fibrinogen (LR test χ2=0.2, p=0.68), white cell count (LR test χ2=0.7, p=0.40), or glucose (LR test χ2=1.3, p=0.25) did not make further improvement, probably as the markers were correlated. After adjustment for all markers, only the association between IL-6 and recurrent vascular events remained statistically significant. The final model including IL-6 fulfilled the proportional hazards assumption and fitted the data well.
A model with only clinical variables (age, prior TIA, MI or stroke and AF) had a Harrell's c statistic of 0.62; when we added interleukin-6 to this model, the Harrell's c statistic increased by a small amount, to 0.64.
Modification of associations by baseline variables
Multiplicative interaction terms between delay to blood taking after stroke, large vessel stroke versus all others (with a modified TOAST algorithm), age, ability to walk and level of IL-6 did not make important changes to the association between IL-6 and recurrent vascular events (and none significantly improved the fit of the final Cox proportional hazards model). This means that, for example for the variable ‘delay to blood taking’, we were unable to demonstrate a difference in the association between IL-6 and recurrent vascular events in those where blood was taken early (<5 days) or later (>5 days) after stroke. There were no significant two way interactions between other blood markers and IL-6.
Circulating inflammatory markers and death
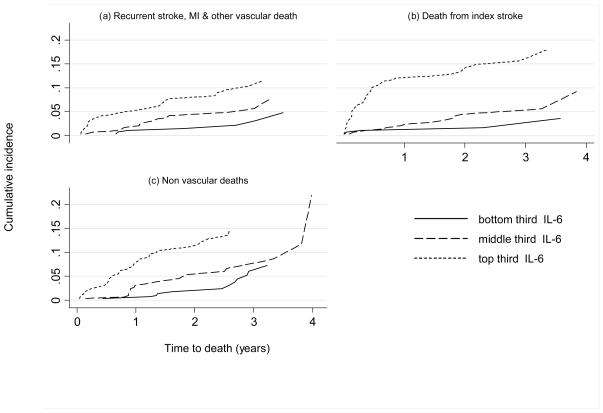
All markers were significantly associated with an increased risk of death (Table 3). After adjustment for factors which are known reliably to influence survival after the index stroke (age, being able to walk or talk, independence of daily activities prior to stroke, being able to lift arms from the bed), these associations remained statistically significant though attenuated. IL-6, CRP, fibrinogen and glucose were more strongly associated with death than with recurrent vascular events, though white cell count was less strongly associated. After additional adjustment for all other markers, only the associations of IL-6 and fibrinogen with death remained statistically significant. The association between higher levels of IL-6, CRP and fibrinogen and an increased incidence of death was consistent for each of the separate causes of death (vascular deaths, deaths due to the initial stroke and deaths due to other causes) (figure 2, data for CRP and fibrinogen not shown). Where the cause of death was the qualifying stroke, patients in the top third of the IL-6 distribution had the shortest survival time.
Table 3.
The association between marker level and death from any cause, assuming a linear association between marker level and log hazards
| Hazard ratio per unit increase* in marker (95% CI) | Hazard ratio comparing 75th to 25th centile† | |||
|---|---|---|---|---|
| Unadjusted | Adjusted‡ | Adjusted for all markers§ |
Adjusted‡ | |
| Interleukin-6 (pg/ml) | 1.13 (1.10 to 1.15) | 1.10 (1.07 to 1.12) | 1.07 (1.04 to 1.12) | 1.56 (1.37 to 1.77) |
| C-reactive protein (mg/l) | 1.01 (1.00 to 1.01) | 1.01 (1.00 to 1.01) | 1.00 (1.00 to 1.81) | 1.08 (1.04 to 1.11) |
| Fibrinogen (g/l) | 1.37 (1.26 to 1.49) | 1.26 (1.14 to 1.40) | 1.14 (1.01 to 1.28) | 1.45 (1.24 to 1.72) |
| White cell count (×109/l) | 1.07 (1.02 to 1.12) | 1.05 (1.00 to 1.11) | 1.03 (0.97 to 1.09) | 1.17 (1.00 to 1.37) |
| Glucose (mmol/l) | 1.95 (1.02 to 1.10) | 1.06 (1.02 to 1.11) | 1.04 (0.99 to 1.09) | 1.12 (1.03 to 1.21) |
for IL-6 pg/ml; CRP mg/l; fibrinogen g/l; white cell count ×109/l; glucose mmol/l
25th and 75th percentile respectively for: IL6 2.39 and 7.22 pg/ml; CRP 1.39 and 9.65 mg/l; fibrinogen 3.81 and 5.41 g/l; white cell count 6.6 and 9.7 ×109/l; glucose 5.0 and 6.8 mmol/l.
Adjusted for confounders: age, ability to walk, living alone, independent prior to stroke, orientated to place time and person, able to lift arms from bed.
adjusted for all confounder in previous column, and other markers
Figure 2.
Unadjusted cumulative incidence curves of (a) death from recurrent stroke, MI or other vascular causes, (b) death from the initial stroke with no evidence of recurrent stroke or (c) other non-vascular deaths, by thirds of interleukin 6, estimated from a competing risk analysis.
DISCUSSION
In this cohort consisting of stroke patients on the stroke unit assessed soon after onset and of outpatients with milder strokes seen after a short interval, higher levels of IL-6, CRP and fibrinogen were associated with a higher incidence of recurrent stroke, MI or vascular death, independent of atrial fibrillation, prior vascular events and age. In addition, higher levels of each inflammatory marker were associated with a higher incidence of death from all causes, an association that was stronger than for all vascular events. The association with recurrent stroke alone was weak for IL6, and did not reach statistical significance in this cohort for the other markers.
We found no consistent evidence of different strengths of association between higher levels of IL-6 with large vessel type strokes versus other stroke subtypes, strokes of different severity or different times from stroke onset to blood draw. Stroke patients had qualitatively similar associations between IL-6, CRP and fibrinogen and deaths from vascular and from non vascular causes. However, there was a suggestion that early deaths from the index stroke might be more strongly associated with higher levels of inflammation.
Strengths and limitations
This study had a number of methodological strengths: the cohort included both mild and severe strokes; we used several overlapping methods to ensure we detected all recurrent vascular events; we determined vital status at the end of the follow up period for all of the cohort; data on all suspected outcome events were checked by the study clinicians, either directly or by review of the medical and imaging records. The majority of patients with recurrent strokes underwent repeat brain imaging (93%).
We were unable to draw blood for inflammatory markers from all patients. The most common reasons for not drawing blood were: either the patient did not consent or the practical constraints in inpatients (chiefly the working hours of research laboratories handling the samples). Patients without blood samples tended to have more severe strokes though were otherwise similar (there was no evidence of an interaction between stroke severity and the association between inflammatory markers and either recurrent vascular events or death). These selection biases may have influenced the results.
We drew blood as soon as possible after assessment (median 2 days among patient admitted to hospital); hence levels of IL-6 and CRP were higher than in previous studies (delay to blood draw in these studies was between 12 hours and 30 days), possibly due in some cases to stroke complications such a pneumonia or deep vein thrombosis. As we were unable to adjust for these in our analysis, the observed association may have been due to confounding by these complications. However, in the 60% of patients seen as outpatients, who had milder strokes, and probably fewer infections or other complications, the median time to blood draw was 19 days. Despite this, we were unable to demonstrate effect modification by the time to blood draw after stroke on the association between either IL-6 or CRP and recurrent vascular events, though statistical tests for effect modification are of low power. It is also possible that among patients in whom the initial assessment was delayed, we may have overlooked some early recurrent strokes.
We did not find that large vessel stroke subtypes had a stronger association between marker levels and recurrent events. However, a relatively large number of strokes were unclassified by the TOAST classification, so we cannot exclude the possibility that an association exists.
We used a competing risks survival analysis to examine the association of IL-6, fibrinogen and CRP with of the three main causes of death; vascular, non-vascular and deaths due to the initial stroke. It is possible that there was some misclassification of the cause of death, particularly for deaths occurring soon after stroke when accurate attribution of the cause of death is difficult, even if autopsy is performed.
The epidemiological association between inflammatory markers and recurrent vascular events appears consistent and strong, and similar for IL-6 and CRP, though a somewhat weaker for fibrinogen. However, the degree of extra clinical utility obtained by adding an inflammatory marker to a clinical predictive model is not merely determined by the fact that it is a statistically independent predictor in multivariate models. The small increase in the c-statistic achieved by adding IL-6 to a model based upon clinical variables makes it unlikely that it will add clinically useful prediction to models based on variables that do not require blood draw. We did not calculate a threshold of IL-6 that best predicted recurrent vascular events, as such calculations usually lead to a biased assessment of the strength of predictive ability of markers and are unlikely to be replicated in validation cohorts.10
Interpretation
These data suggest that CRP or IL-6 may not have a major causal role in recurrent vascular events after stroke. It is more likely that the observed association reflects an inflammatory response either to atherosclerosis or to its risk factors, or to an as yet unidentified trigger. In support of this, studies that have examined functional CRP and IL-6 polymorphisms (which produce differences in baseline CRP or IL-6 levels) found different polymorphisms were not associated with an increased risk of stroke11 or other occlusive vascular events12. However, to determine reliably any causal relationship between the physiological inflammatory response and recurrent vascular events in humans would require a randomised trial of an agent which was an effective anti-inflammatory but had no direct effect on other vascular risk factors such as cholesterol or blood pressure.
Generalisability
Our finding that IL-6 showed the strongest association with recurrent vascular events and with death in this cohort of stroke patients, is consistent with recent reports from population-based prospective studies. 13 14 Our estimates of the association between: (i) CRP, IL-6, fibrinogen and recurrent stroke, 1, 15 16 and (ii) CRP, fibrinogen and death16, 17 are consistent with previous studies (supplementary table 1).
Conclusions
We have demonstrated an association between higher levels of IL-6, CRP and fibrinogen and an increased incidence of occlusive vascular events in patients after stroke. The association between IL-6, CRP and fibrinogen and fatal vascular and non-vascular events after stroke seems similar. Future studies of prediction of recurrent vascular events after stroke might better study easily measured clinical variables, or examine the effect of different blood markers.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to Aidan Hutchison for his help with querying the ESS database and to Mike McDowall and the Edinburgh staff of the Scottish Stroke Care Audit for storing and managing data for the ESS., the Wellcome Trust Clinical Research Facility in Edinburgh, the many stroke and research fellows who helped to recruit patients, and in particular the patients themselves.
FUNDING SOURCES
Dr Whiteley is supported by a Chief Scientist's Office Clinical Academic Training Fellowship from the Scottish Government. Dr Sudlow was supported by a Clinician Scientist Award from the Wellcome Trust (063668/Z/01/A) and is now funded by the Scottish Funding Council. Dr Jackson was supported by the Wellcome Trust (063668/Z/01/A) and a Binks Trust Research Fellowship. Prof Wardlaw was supported by the Scottish Funding Council through the Scottish Imaging Network, a Platform for Scientific Excellence Collaboration (www.sinapse.ac.uk). The imaging was conducted in the SFC Brain Imaging Research Centre at the University of Edinburgh (www.sbirc.ed.ac.uk), a SINAPSE centre
Footnotes
DISCLOSUURES
None
Reference List
- 1.Woodward M, Lowe GDO, Campbell DJ, Colman S, Rumley A, Chalmers J, Neal BC, Patel A, Jenkins AJ, Kemp BE, MacMahon SW. Associations of Inflammatory and Hemostatic Variables With the Risk of Recurrent Stroke. Stroke. 2005 Oct 1;36(10):2143–7. doi: 10.1161/01.STR.0000181754.38408.4c. [DOI] [PubMed] [Google Scholar]
- 2.Welsh P, Lowe GDO, Chalmers J, Campbell DJ, Rumley A, Neal BC, MacMahon SW, Woodward M. Associations of Proinflammatory Cytokines With the Risk of Recurrent Stroke. Stroke. 2008 Aug 1;39(8):2226–30. doi: 10.1161/STROKEAHA.107.504498. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell PM, Howard SC, Power DA, Gutnikov SA, Algra A, van GJ, Clark TG, Murphy MF, Warlow CP. Fibrinogen concentration and risk of ischemic stroke and acute coronary events in 5113 patients with transient ischemic attack and minor ischemic stroke. Stroke. 2004 Oct;35(10):2300–5. doi: 10.1161/01.STR.0000141701.36371.d1. [DOI] [PubMed] [Google Scholar]
- 4.Grau AJ, Boddy AW, Dukovic DA, Buggle F, Lichy C, Brandt T, Hacke W, for the CAPRIE Investigators Leukocyte Count as an Independent Predictor of Recurrent Ischemic Events. Stroke. 2004 May 1;35(5):1147–52. doi: 10.1161/01.STR.0000124122.71702.64. [DOI] [PubMed] [Google Scholar]
- 5.Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, Wardlaw J, Dennis M, Sudlow C. Inflammatory Markers and Poor Outcome after Stroke: A Prospective Cohort Study and Systematic Review of Interleukin-6. PLoS Med. 2009 Sep 8;6(9):e1000145. doi: 10.1371/journal.pmed.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lewis SC, Sudlow CLM. Differences Between Ischemic Stroke Subtypes in Vascular Outcomes Support a Distinct Lacunar Ischemic Stroke Arteriopathy: A Prospective, Hospital-Based Study. Stroke. 2009 Dec 1;40(12):3679–84. doi: 10.1161/STROKEAHA.109.558221. [DOI] [PubMed] [Google Scholar]
- 7.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007 Oct;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 8.Counsell C, Dennis M, McDowall M. Predicting functional outcome in acute stroke: comparison of a simple six variable model with other predictive systems and informal clinical prediction. J Neurol Neurosurg Psychiatry. 2004 Mar 1;75(3):401–5. doi: 10.1136/jnnp.2003.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coviello V. Cumulative incidence estimation in the presence of competing risks. Stata Journal. 2004;4(2):103–12. [Google Scholar]
- 10.Royston P, Moons KGM, Altman DG, Vergouwe Y. Prognosis and prognostic research: Developing a prognostic model. BMJ. 2009 Mar 31;338(mar31_1):b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 11.Ladenvall C, Jood K, Blomstrand C, Nilsson S, Jern C, Ladenvall P. Serum C-Reactive Protein Concentration and Genotype in Relation to Ischemic Stroke Subtype. Stroke. 2006 Aug 1;37(8):2018–23. doi: 10.1161/01.STR.0000231872.86071.68. [DOI] [PubMed] [Google Scholar]
- 12.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically Elevated C-Reactive Protein and Ischemic Vascular Disease. N Engl J Med. 2008 Oct 30;359(18):1897–908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 13.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JPT, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GDO, Gudnason V. Long-Term Interleukin-6 Levels and Subsequent Risk of Coronary Heart Disease: Two New Prospective Studies and a Systematic Review. PLoS Medicine. 2008 Apr 1;5(4):e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson CC, Smith AE, Yarnell JWG, Rumley A, Ben-Shlomo Y, Lowe GDO. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: The Caerphilly Study. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2009.09.030. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 15.Campbell DJ, Woodward M, Chalmers JP, Colman SA, Jenkins AJ, Kemp BE, Neal BC, Patel A, MacMahon SW. Soluble vascular cell adhesion molecule 1 and N-terminal pro-B-type natriuretic peptide in predicting ischemic stroke in patients with cerebrovascular disease. Arch Neurol. 2006 Jan;63(1):60–5. doi: 10.1001/archneur.63.1.noc50221. [DOI] [PubMed] [Google Scholar]
- 16.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. 2006 Oct 23;166(19):2073–80. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 17.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001 Apr;32(4):917–24. doi: 10.1161/01.str.32.4.917. [DOI] [PubMed] [Google Scholar]
- 18.Di Napoli M, Papa F, Bocola V. Prognostic Influence of Increased C-Reactive Protein and Fibrinogen Levels in Ischemic Stroke. Stroke. 2001 Jan 1;32(1):133–8. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- 19.Rallidis LS, Vikelis M, Panagiotakos DB, Liakos GK, Krania E, Kremastinos DT. Usefulness of inflammatory and haemostatic markers to predict short-term risk for death in middle-aged ischaemic stroke patients. Acta Neurologica Scandinavica. 2008;117:415–20. doi: 10.1111/j.1600-0404.2007.00971.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.