Abstract
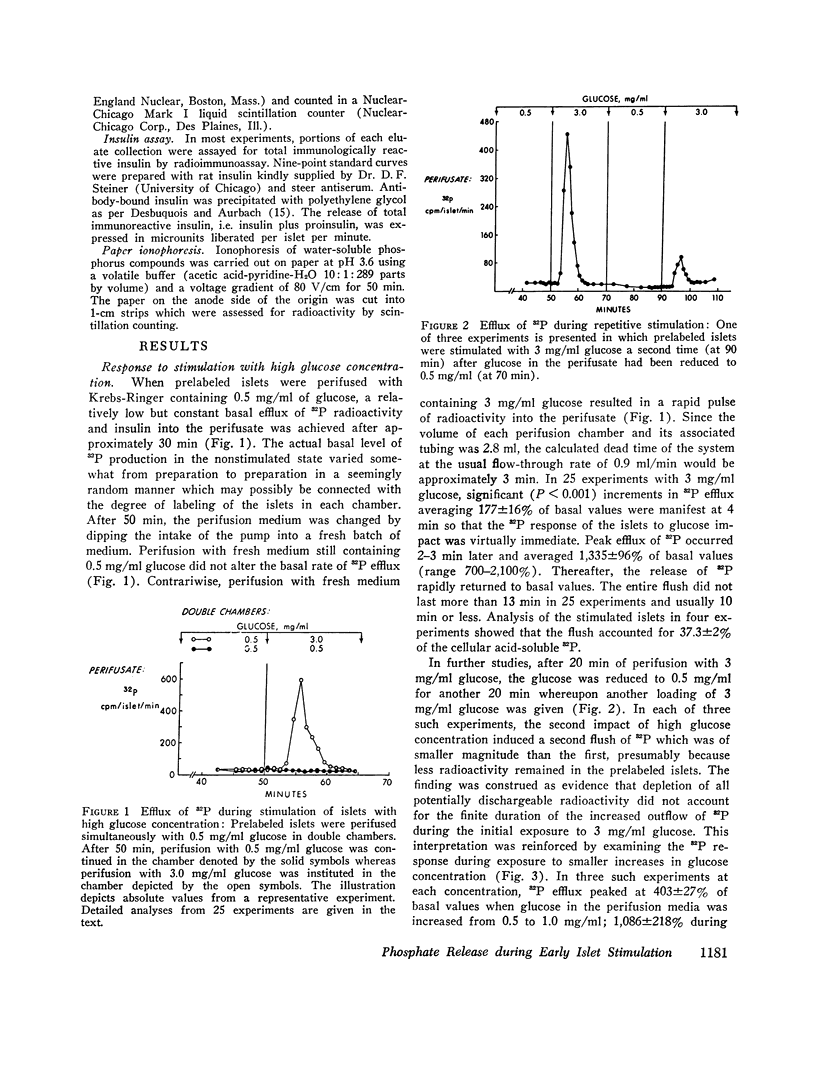
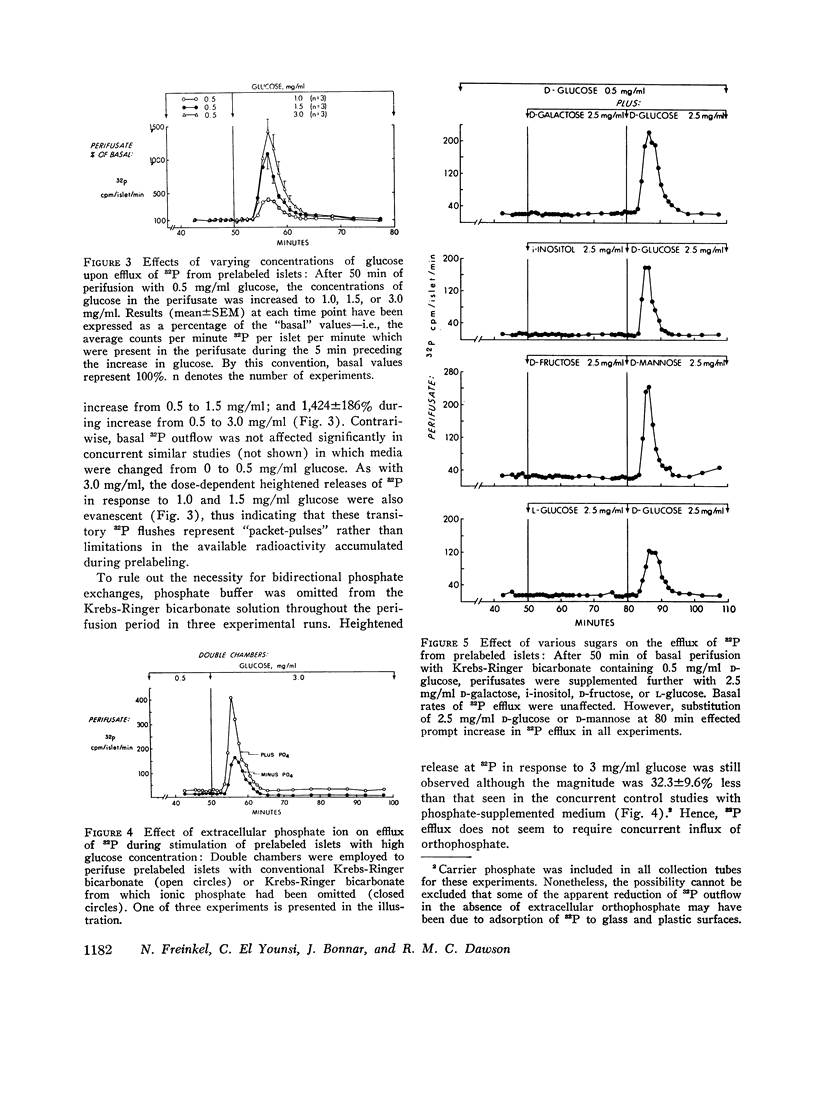
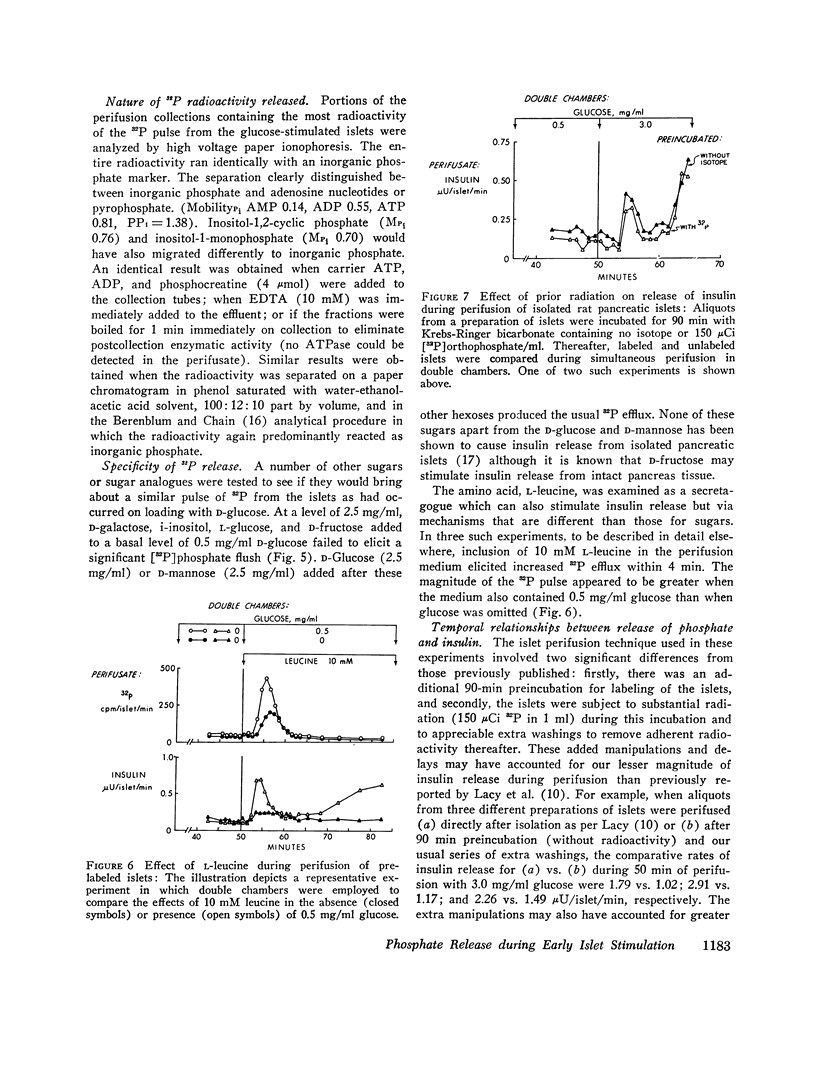
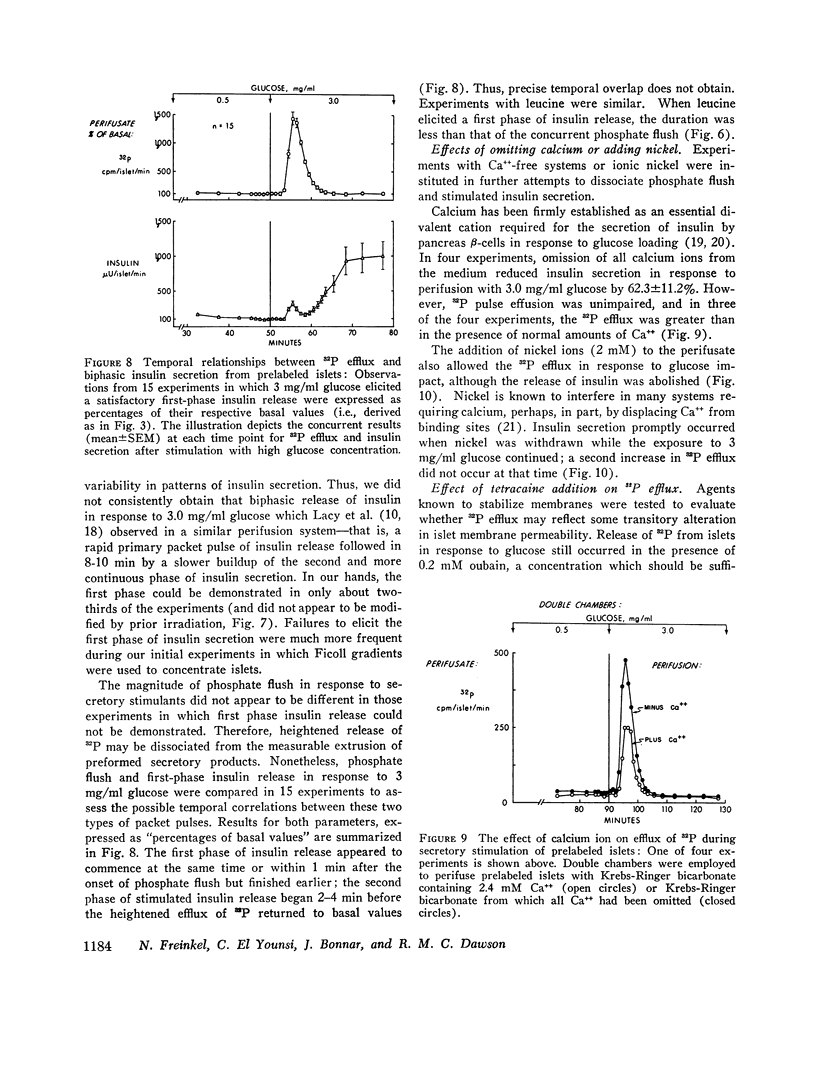
Anionic fluxes during the membrane realignments of stimulated insulin release have not been characterized previously although cations have been implicated in stimulus-secretion coupling. We have shown that a limited packet pulse of phosphate release (“phosphate flush”) begins at the same time that the first phase of insulin secretion may occur. To demonstrate this phenomenon, we have prelabeled islets, obtained from rat pancreas by collagenase digestions, by incubation with [32P]orthophosphate. When such prelabeled islets are perifused with Krebs-Ringer bicarbonate containing 0.5 mg/ml D-glucose, a basal rate of efflux of radioactivity is established; transfer to perifusates containing 3.0 mg/ml D-glucose elicits an increased 32P efflux within 1-2 min to peak values which are 7- to 21-fold greater than basal. The total duration of this “phosphate flush” approximates 10 min and exceeds the duration of the first phase of stimulated insulin secretion. With lesser concentrations of glucose, the flush exhibits dose-response relationships, and with 3 mg/ml glucose, a second flush can be elicited by restoring basal conditions and stimulating anew with 3 mg/ml glucose. The phenomenon is highly specific and can be reduplicated by other secretagogues (L-leucine) or sugars (D-mannose) which are also known to elicit insulin release but not by sugars which fail to affect insulin secretion (D-galactose, D-fructose, i-inositol, L-glucose). The efflux of radioactivity consists entirely of [32P]orthophosphate. Phosphate flush persists in phosphate-free media, Ca++-free media, and when insulin release is obtunded by adding Ni++ (2 mM) to the perifusates. Thus, efflux of [32P]orthophosphate can be dissociated from insulin extrusion, and from net influx of ionic phosphate or calcium. Membrane stabilization with D2O or 1.0 mM tetracaine reversibly inhibits phosphate flush. Although the mechanism by which this effect occurs has not yet been established, the phosphate flush appears to constitute one of the earliest and hitherto unknown indices of the excitatory state in pancreatic islets.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABOOD L. G., KOKETSU K., KOYAMA I. Outflux of inorganic and organic phosphate during membrane depolarization of excitable tissues. Nature. 1961 Jul 22;191:395–396. doi: 10.1038/191395a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger W. F., Lacy P. E. Transplantation of intact pancreatic islets in rats. Surgery. 1972 Aug;72(2):175–186. [PubMed] [Google Scholar]
- Beigelman P. M., Shu M. J., Thomas L. J., Jr Insulin from individual isolated mouse islets of Langerhans. Biochem Med. 1973 Feb;7(1):91–96. doi: 10.1016/0006-2944(73)90103-8. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson G. R., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. XI. Effects of theophylline and epinephrine on Ca efflux from perifused islets. Metabolism. 1973 Mar;22(3):455–465. doi: 10.1016/0026-0495(73)90037-1. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R. The plasma insulin response to glucose infusion in healthy subjects and in diabetes mellitus. Acta Endocrinol (Copenh) 1967 Jun;55(2):278–304. doi: 10.1530/acta.0.0550278. [DOI] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968 Sep;83(3):572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Freinkel N., Jungalwala F. B., Clarke N. The enzymic formation of myoinositol 1:2-cyclic phosphate from phosphatidylinositol. Biochem J. 1971 May;122(4):605–607. doi: 10.1042/bj1220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells. Nature. 1968 Jul 27;219(5152):389–390. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- FREINKEL N. Action of pituitary thyrotropin on the inorganic phosphorous of thyroid tissue in vitro. Nature. 1963 Jun 1;198:889–891. doi: 10.1038/198889a0. [DOI] [PubMed] [Google Scholar]
- FREINKEL N. Pathways of thyroidal phosphorus metabolism: the effect of pituitary thyrotropin upon the phospholipids of the sheep thyroid gland. Endocrinology. 1957 Oct;61(4):448–460. doi: 10.1210/endo-61-4-448. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Quibrera R., Pek S., Floyd J. C., Jr, Christensen H. N., Conn J. W. Stimulation of insulin release in the dog by a nonmetabollizable amino acid. Comparison with leucine and arginine. J Clin Endocrinol Metab. 1971 Jul;33(1):35–41. doi: 10.1210/jcem-33-1-35. [DOI] [PubMed] [Google Scholar]
- Friedmann N. Effects of glucagon and cyclic AMP on ion fluxes in the perfused liver. Biochim Biophys Acta. 1972 Jul 3;274(1):214–225. doi: 10.1016/0005-2736(72)90295-7. [DOI] [PubMed] [Google Scholar]
- Getz G. S., Jakovcic S., Heywood J., Frank J., Rabinowitz M. A two-dimensional thin-layer chromatographic system for phospholipid separation. The analysis of yeast phospholipids. Biochim Biophys Acta. 1970 Dec 15;218(3):441–452. doi: 10.1016/0005-2760(70)90007-x. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Bennett L. L. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966 Dec;15(12):910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The displacement of calcium ions from phospholipid monolayers by pharmacologically active and other organic bases. Biochem J. 1968 Oct;109(5):909–916. doi: 10.1042/bj1090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerström C. Effects of carbohydrates on the oxygen consumption of isolated pancreatic islets of mice. Endocrinology. 1967 Jul;81(1):105–112. doi: 10.1210/endo-81-1-105. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B., Freinkel N., Dawson R. M. The metabolism of phosphatidylinositol in the thyroid gland of the pig. Biochem J. 1971 Jun;123(1):19–33. doi: 10.1042/bj1230019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBella F., Dular R., Vivian S., Queen G. Pituitary hormone releasing or inhibiting activity of metal ions present in hypothalamic extracts. Biochem Biophys Res Commun. 1973 Jun 8;52(3):786–791. doi: 10.1016/0006-291x(73)91006-1. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Klein N. J., Fink C. J. Effect of cytochalasin B on the biphasic release of insulin in perifused rat islets. Endocrinology. 1973 May;92(5):1458–1468. doi: 10.1210/endo-92-5-1458. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Walker M. M., Fink C. J. Perifusion of isolated rat islets in vitro. Participation of the microtubular system in the biphasic release of insulin. Diabetes. 1972 Oct;21(10):987–998. doi: 10.2337/diab.21.10.987. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. Phosphatidylinositol metabolism in cells receiving extracellular stimulation. FEBS Lett. 1973 Apr 1;31(1):1–10. doi: 10.1016/0014-5793(73)80061-4. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G. R., Baird L. E. Stimulus-secretion coupling of glucose-induced insulin release. X. Effect of glucose on 45 Ca efflux from perifused islets. Am J Physiol. 1973 Feb;224(2):389–394. doi: 10.1152/ajplegacy.1973.224.2.389. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Walker M. O., Lacy P. E. The stimulus-secretion coupling of glucose-induced insulin release. V. The participation of a microtubular-microfilamentous system. Diabetes. 1971 May;20(5):257–265. doi: 10.2337/diab.20.5.257. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J. Role of calcium in insulin secretion. Isr J Med Sci. 1972 Mar;8(3):244–251. [PubMed] [Google Scholar]
- Matschinsky F. M., Ellerman J. Dissociation of the insulin releasing and the metabolic functions of hexoses in islets of Langerhans. Biochem Biophys Res Commun. 1973 Jan 23;50(2):193–199. doi: 10.1016/0006-291x(73)90826-7. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Klingenberg M. Correlation between H+ and anion movement in mitochondria and the key role of the phosphate carrier. Eur J Biochem. 1971 Jun 11;20(3):392–399. doi: 10.1111/j.1432-1033.1971.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. 1967 Mar;3(1):47–49. doi: 10.1007/BF01269910. [DOI] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Pentitols and insulin release by isolated rat islets of Langerhans. Biochem J. 1968 Sep;109(3):333–339. doi: 10.1042/bj1090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace C. S., Price S. Electrical responses of pancreatic islet cells to secretory stimuli. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1557–1563. doi: 10.1016/0006-291x(72)90785-1. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Pupo A. A. Insulin responses to glucose: evidence for a two pool system in man. J Clin Invest. 1969 Dec;48(12):2309–2319. doi: 10.1172/JCI106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B., Tenenhouse A. The role of cyclic AMP and calcium in cell activation. CRC Crit Rev Biochem. 1972 Feb;1(1):95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]


