Abstract
Hageman factor (factor XII) is activated by exposure to surfaces such as glass or by solutions of certain compounds, notably ellagic acid. Changes in the structure of Hageman factor accompanying activation have been examined in this study by circular dichroism spectroscopy. The spectrum of unactivated Hageman factor in aqueous solutions suggests that its conformation is mainly aperiodic. Various perturbants altered the conformation of Hageman factor in differing ways, demonstrating the sensitivity of Hageman factor to its environment.
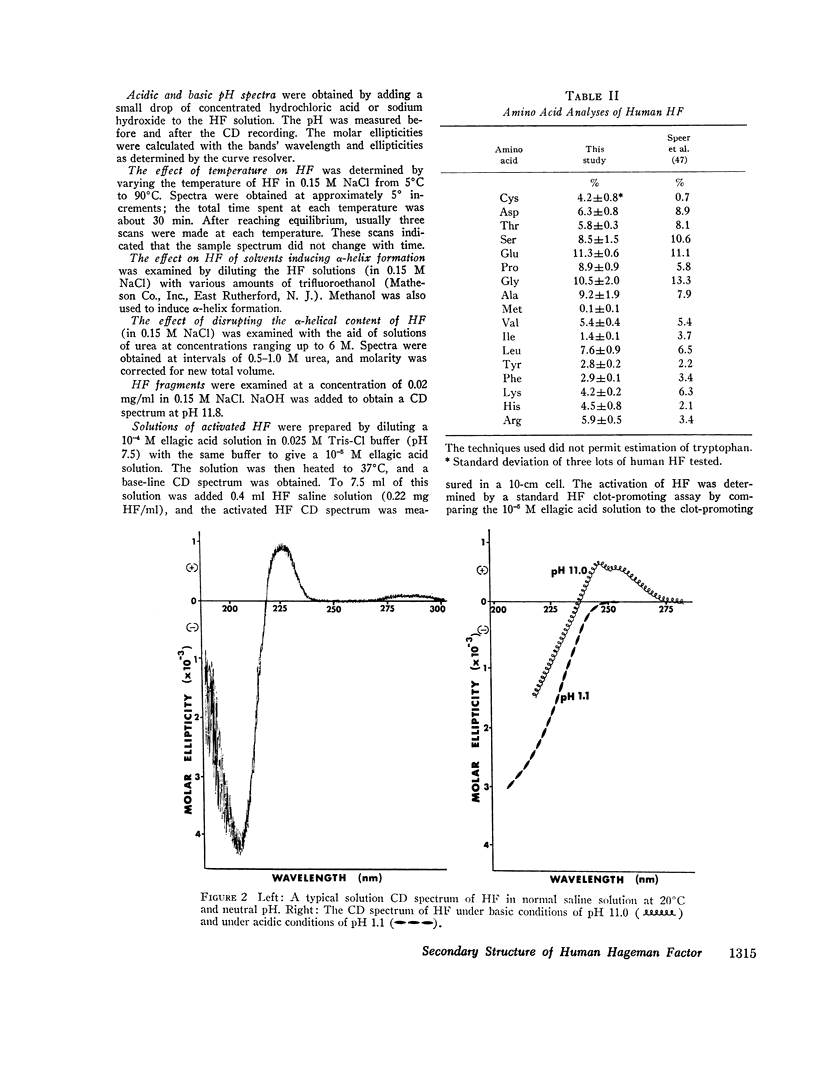
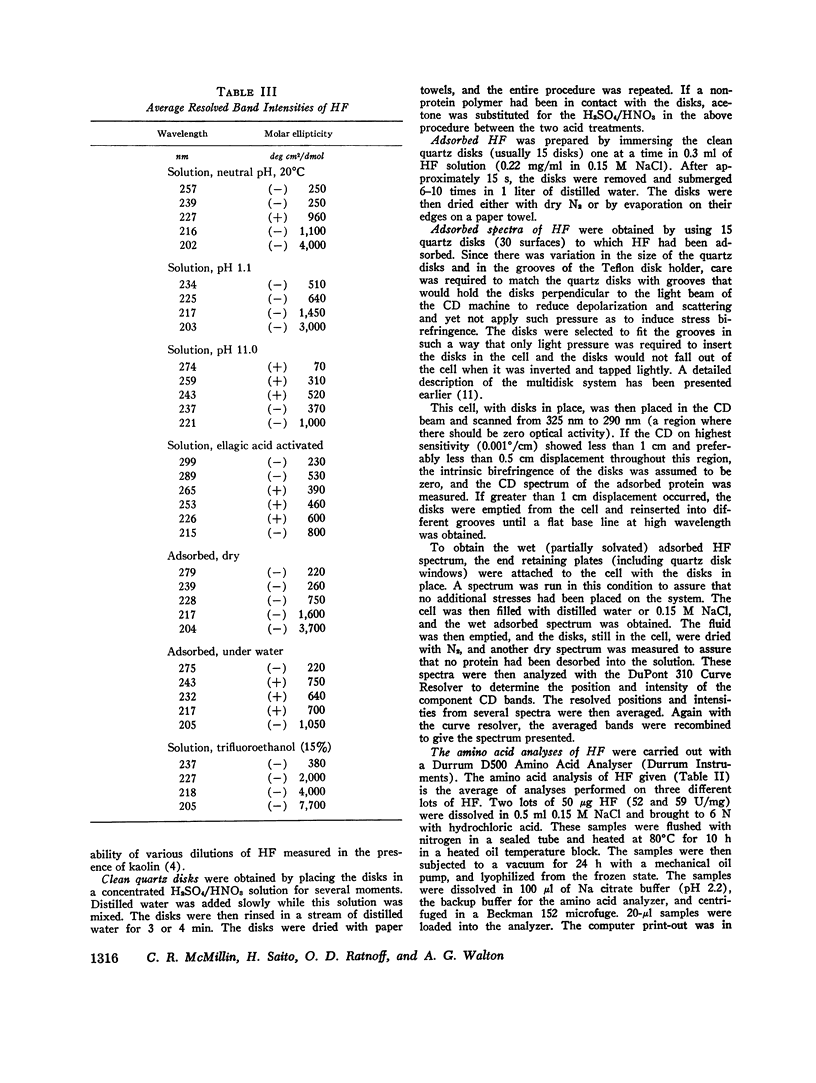
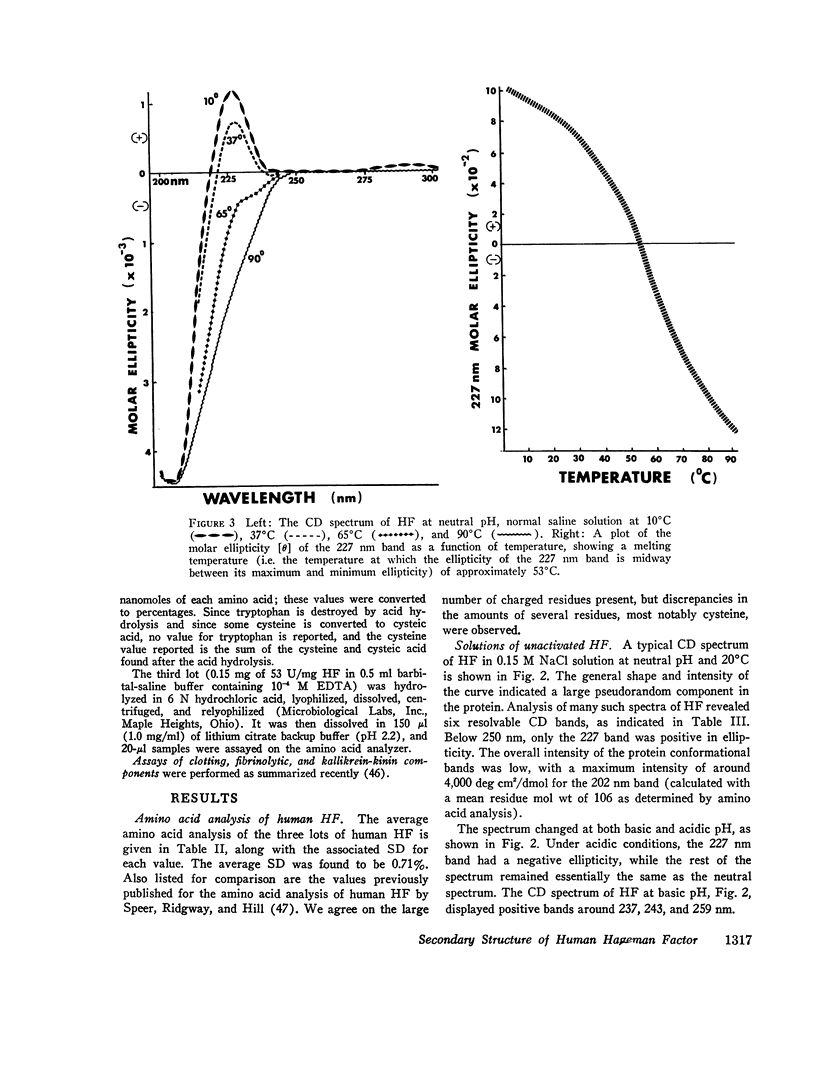
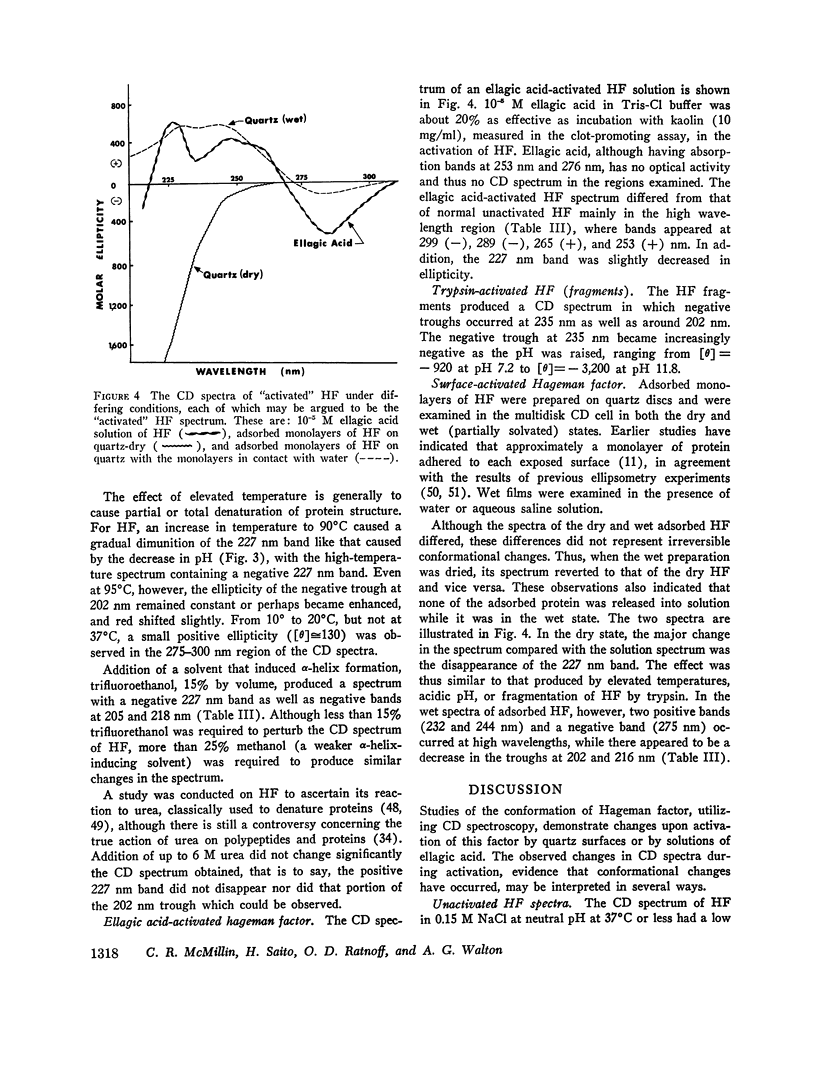
After activation of Hageman factor with solutions of ellagic acid, a negative trough appeared in the region of the circular dichroism spectrum commonly assigned to tyrosine residues, along with other minor changes in the peptide spectral region. Some of these changes are similar to changes that occurred upon partial neutralization of the basic residues at alkali pH. Activation of Hageman factor by adsorption to quartz surfaces (in an aqueous environment) also produced changes similar to those in the ellagic acid-activated Hageman factor, including the negative ellipticity in the tyrosine region.
These observations suggest that the activation process may be related to a change in status of some of the basic amino acid residues, coupled with a specific change in the environment of some tyrosine residues. The importance of these changes during the activation process remains to be determined. The sensitivity of Hageman factor to its environment is consistent with the view that the initiation of clotting by exposure of plasma to appropriate agents is brought about by alterations in the conformation of Hageman factor that occur in the apparent absence of Fletcher factor or other recognized clotting factors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Greenfield N. J., Fasman G. D. Circular dichroism and optical rotatory dispersion of proteins and polypeptides. Methods Enzymol. 1973;27:675–735. doi: 10.1016/s0076-6879(73)27030-1. [DOI] [PubMed] [Google Scholar]
- Aebersold D., Pysh E. S. Optical properties of amorphous polypeptides. I. One state solutions. J Chem Phys. 1970 Sep 15;53(6):2156–2163. doi: 10.1063/1.1674309. [DOI] [PubMed] [Google Scholar]
- Brash J. L., Lyman D. J. Adsorption of plasma proteins in solution to uncharged, hydrophobic polymer surfaces. J Biomed Mater Res. 1969 Mar;3(1):175–189. doi: 10.1002/jbm.820030114. [DOI] [PubMed] [Google Scholar]
- Bush C. A., Gibbs D. E. Aromatic side-chain Cotton effects in cyclic hexapeptides. Biochemistry. 1972 Jun 20;11(13):2421–2427. doi: 10.1021/bi00763a006. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1285–1291. doi: 10.1016/s0006-291x(71)80225-5. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Revak S. D., Wuepper K. D. Activation of Hageman factor in solid and fluid phases. A critical role of kallikrein. J Exp Med. 1973 Dec 1;138(6):1564–1583. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Wuepper K. D. The first component of the kinin-forming system in human and rabbit plasma. Its relationship to clotting factor XII (Hageman Factor). J Exp Med. 1971 Oct 1;134(4):986–1004. doi: 10.1084/jem.134.4.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Donaldson V. H., Ratnoff O. D. Hageman factor: alterations in physical properties during activation. Science. 1965 Nov 5;150(3697):754–756. doi: 10.1126/science.150.3697.754. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Wheeler G. E., Moscarello M. A. Circular dichroism and proton magnetic resonance studies of random chain poly-L-lysine. Biopolymers. 1974;13(2):359–369. doi: 10.1002/bip.1974.360130211. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B., Cowburn D. A. Optical activity of biopolymers. Nature. 1969 May 3;222(5192):426–431. doi: 10.1038/222426a0. [DOI] [PubMed] [Google Scholar]
- Iizuka E., Yang J. T. Optical rotatory dispersion and circular dichroism of the beta-form of silk fibroin in solution. Proc Natl Acad Sci U S A. 1966 May;55(5):1175–1182. doi: 10.1073/pnas.55.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A., Lloyd K. O., Beychok S. Optical activity and conformation of carbohydrates. II. Opitical rotatory dispersion and circular dichroism studies on immunochemically reactive oligo- and polysaccharides containing amino sugars and their derivatives. Biochemistry. 1969 Mar;8(3):747–756. doi: 10.1021/bi00831a001. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med. 1971 Apr 1;133(4):696–712. doi: 10.1084/jem.133.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listowsky I., Englard S., Avigad G. An analysis of the circular dichroism spectra of uronic acids. Biochemistry. 1969 May;8(5):1781–1785. doi: 10.1021/bi00833a001. [DOI] [PubMed] [Google Scholar]
- MARGOLIS J. Activation of plasma by contact with glass: evidence for a common reaction which releases plasma kinin and initiates coagulation. J Physiol. 1958 Nov 10;144(1):1–22. doi: 10.1113/jphysiol.1958.sp006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIS J. The interrelationship of coagulation of plasma and release of peptides. Ann N Y Acad Sci. 1963 Feb 4;104:133–145. doi: 10.1111/j.1749-6632.1963.tb17659.x. [DOI] [PubMed] [Google Scholar]
- Madison V., Schellman J. Optical activity of polypeptides and proteins. Biopolymers. 1972;11(5):1041–1076. doi: 10.1002/bip.1972.360110509. [DOI] [PubMed] [Google Scholar]
- Martin A. R. Spectroscopic methods in biology, with application to neurophysiology. Adv Biol Med Phys. 1973;14:225–284. doi: 10.1016/b978-0-12-005214-1.50009-1. [DOI] [PubMed] [Google Scholar]
- NIEWIAROWSKI S., PROU-WARTELLE O. [Role of the contact factor (Hageman factor) in fibrinolysis]. Thromb Diath Haemorrh. 1959 Sep 1;3:593–603. [PubMed] [Google Scholar]
- Pflumm M. N., Beychok S. Optical activity of cystine-containing proteins. II. Circular dichroism spectra of pancreatic ribonuclease A, ribonuclease S, and ribonuclease S-protein. J Biol Chem. 1969 Jul 25;244(14):3973–3981. [PubMed] [Google Scholar]
- Quadrifoglio F., Urry D. W. Ultraviolet rotatory properties of polypeptides in solution. II. Poly-L-serine. J Am Chem Soc. 1968 May 22;90(11):2760–2765. doi: 10.1021/ja01013a005. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., CRUM J. D. ACTIVATION OF HAGEMAN FACTOR BY SOLUTIONS OF ELLAGIC ACID. J Lab Clin Med. 1964 Mar;63:359–377. [PubMed] [Google Scholar]
- RATNOFF O. D., DAVIE E. W. The purification of activated Hageman factor (activated factor XII). Biochemistry. 1962 Nov;1:967–975. doi: 10.1021/bi00912a005. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., ROSENBLUM J. M. Role of Hageman factor in the initiation of clotting by glass; evidence that glass frees Hageman factor from inhibition. Am J Med. 1958 Aug;25(2):160–168. doi: 10.1016/0002-9343(58)90023-8. [DOI] [PubMed] [Google Scholar]
- Rippon W. B., Walton A. G. Optical properties of the polyglycine II helix. Biopolymers. 1971;10(7):1207–1212. doi: 10.1002/bip.360100710. [DOI] [PubMed] [Google Scholar]
- Ronish E. W., Krimm S. Theoretical calculation of the circular dichroism of unordered polypeptide chains. Biopolymers. 1972;11(9):1919–1928. doi: 10.1002/bip.1972.360110912. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. The optical rotatory dispersion of aromatic amino acids and the side chain-dependent Cotton effects in proteins. J Biol Chem. 1966 Nov 10;241(21):5119–5125. [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Donaldson V. H. Defective activation of clotting, fibrinolytic, and permeability-enhancing systems in human Fletcher trait plasma. Circ Res. 1974 May;34(5):641–651. doi: 10.1161/01.res.34.5.641. [DOI] [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Marshall J. S., Pensky J. Partial purification of plasma thromboplastin antecedent (factor XI) and its activation by trypsin. J Clin Invest. 1973 Apr;52(4):850–861. doi: 10.1172/JCI107249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. A new basis for interpreting the circular dichroic spectra of proteins. Proc Natl Acad Sci U S A. 1971 May;68(5):969–972. doi: 10.1073/pnas.68.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers J. G., Matze R., Haanen C., Zilliken F. Hageman factor, a novel sialoglycoprotein with esterase activity. Biochim Biophys Acta. 1965 Jul 1;101(2):166–176. doi: 10.1016/0926-6534(65)90047-3. [DOI] [PubMed] [Google Scholar]
- Simmons N. S., Glazer A. N. An analysis of the tyrosine circular dichroism bands in ribonuclease. J Am Chem Soc. 1967 Sep 13;89(19):5040–5042. doi: 10.1021/ja00995a037. [DOI] [PubMed] [Google Scholar]
- Speer R. J., Ridgway H., Hill J. M. Activated human Hageman factor (XII). Thromb Diath Haemorrh. 1965 Sep 1;14(1-2):1–11. [PubMed] [Google Scholar]
- Stevens L., Townend R., Timasheff S. N., Fasman G. D., Potter J. The circular dichroism of polypeptide films. Biochemistry. 1968 Oct;7(10):3717–3720. doi: 10.1021/bi00850a051. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Tiffany M. L., Krimm S. New chain conformations of poly(glutamic acid) and polylysine. Biopolymers. 1968;6(9):1379–1382. doi: 10.1002/bip.1968.360060911. [DOI] [PubMed] [Google Scholar]
- VROMAN L. A RESEMBLANCE BETWEEN THE CLOTTING OF BLOOD PLASMA AND THE BREAKDOWN OF CYTOPLASM. Nature. 1965 Jan 30;205:496–497. doi: 10.1038/205496a0. [DOI] [PubMed] [Google Scholar]
- Vroman L., Adams A. L., Klings M. Interactions among human blood proteins at interfaces. Fed Proc. 1971 Sep-Oct;30(5):1494–1502. [PubMed] [Google Scholar]
- Wong K. P., Tanford C. Denaturation of bovine carbonic anhydrase B by guanidine hydrochloride. A process involving separable sequential conformational transitions. J Biol Chem. 1973 Dec 25;248(24):8518–8523. [PubMed] [Google Scholar]
- Ziegler S. M., Bush C. A. Circular dichroism of cyclic hexapeptides with one and two side chains. Biochemistry. 1971 Apr 13;10(8):1330–1335. doi: 10.1021/bi00784a009. [DOI] [PubMed] [Google Scholar]