Abstract
EBV nuclear antigen 3C (EBNA3C) is an essential transcription factor for EBV transformed lymphoblast cell line (LCL) growth. To identify EBNA3C-regulated genes in LCLs, microarrays were used to measure RNA abundances in each of three different LCLs that conditionally express EBNA3C fused to a 4-OH-Tamoxifen–dependent estrogen receptor hormone binding domain (EBNA3CHT). At least three RNAs were assayed for each EBNA3CHT LCL under nonpermissive conditions, permissive conditions, and nonpermissive conditions with wild-type EBNA3C transcomplementation. Using a two-way ANOVA model of EBNA3C levels, we identified 550 regulated genes that were at least 1.5-fold up- or down-regulated with false discovery rates < 0.01. EBNA3C-regulated genes overlapped significantly with genes regulated by EBNA2 and EBNA3A consistent with coordinated effects on cell gene transcription. Of the 550 EBNA3C-regulated genes, 106 could be placed in protein networks. A seeded Bayesian network analysis of the 80 most significant EBNA3C-regulated genes suggests that RAC1, LYN, and TNF are upstream of other EBNA3C-regulated genes. Gene set enrichment analysis found enrichment for MAP kinase signaling, cytokine–cytokine receptor interactions, JAK-STAT signaling, and cell adhesion molecules, implicating these pathways in EBNA3C effects on LCL growth or survival. EBNA3C significantly up-regulated the CXCL12 ligand and its CXCR4 receptor and increased LCL migration. CXCL12 up-regulation depended on EBNA3C's interaction with the cell transcription factor, RBPJ, which is essential for LCL growth. EBNA3C also up-regulated MYC 1.3-fold and down-regulated CDKN2A exons 2 and 3, shared by p16 and p14, 1.4-fold, with false discovery rates < 5 × 10−4.
Keywords: lymphoma, Notch
EBV is a γ-herpes virus that causes lymphoproliferative diseases, lymphomas, and Hodgkin's disease with increased incidence in immune-compromised people, as well as nasopharyngeal and some gastric carcinomas in people without evident immune deficiency (1). In vitro, and in primary human infection, EBV transforms B-lymphocytes into continuously proliferating lymphoblasts (LCLs; refs. 2 and 3). In LCLs, EBV expresses nuclear antigen proteins EBNA-LP, -1, -2, -3A, -3B, and -3C, Latent infection integral Membrane Proteins (LMP) LMP1, LMP2A, and LMP2B, nonpolyadenylated small RNAs EBER1 and -2, and more than 40 microRNAs (for review, see ref. 4). In primary B lymphocytes, EBNA2 and EBNALP are expressed first and turn on MYC, as well as EBNA3A, 3B, and 3C, LMP1, and LMP2. EBNA2, EBNALP, EBNA3A, EBNA3C, and LMP1 are essential for efficient B-cell transformation to LCLs, and EBNA1 is essential for efficient EBV episome persistence (for review, see ref. 4).
The experiments described here were undertaken to identify EBNA3C's effects on cell RNA abundances. EBNA3C, EBNA3B, and EBNA3A are sequentially encoded in the EBV genome, transcribed from the same promoter, spliced with similar introns and exons lengths, and are proteins of ∼1,000 aa. Furthermore, the EBNA3A, EBNA3B, and EBNA3C N termini are homologous domains, which associate with RBPJ/CSL (RBPJ), a DNA sequence-specific transcription factor that also mediates EBNA2 and Notch association with DNA. Therefore, EBNA3C association with RBPJ in LCLs may affect EBNA2-, EBNA3A-, EBNA3B-, or Notch-mediated transcription (5–8). For example, experimental two- to threefold EBNA3A overexpression decreases EBNA2 interaction with RBPJ, interrupts EBNA2 up-regulation of MYC, and stops LCL growth (9). Also, activated Notch expression can partially substitute for EBNA2 in maintaining LCL growth (10). Surprisingly, EBNA3A and EBNA3C are each uniquely important for initial and continued LCL growth, whereas EBNA3B is not required (11–16), although EBNA3B also alters cell gene transcription (17, 18). EBNA3C uniquely up-regulates LMP1 (19–24), CD21(CR2), TCL1A, and ITGA4 expression and represses JAG1, NCALD, p16, BIM, and FLNA expression (14–16, 18, 19, 25, 26). Furthermore, EBNA3C amino acids 365–545 or 724–826 repress or activate transcription when tethered to DNA by the Gal4 DNA binding domain (27, 28). Moreover, EBNA3C interacts with other transcription factors or regulators, including HDAC1, CtBP, SMN, DP103, p300, prothymosin alpha, Sin3A, NcoR, and NECDIN (29–35).
Results
EBNA3C Regulates LCL RNA Levels.
To define EBNA3C's effects on LCL RNA abundances, RNAs were prepared from three different LCLs transformed by recombinant EBVs that express a conditional EBNA3C (EBNA3CHT), which has a 4-Hydroxy Tamoxifen (4HT)-dependent mutant estrogen receptor hormone binding domain fused to the C terminus of EBNA3C (36). In medium with 4HT, EBNA3CHT-transformed LCLs grow similarly to wild-type LCLs. In medium without 4HT, EBNA3CHT levels fall substantially by day 7; LCL growth slows after day 7 and stops at day 10–14, when p16 RNA and protein have substantially increased, pRb is hypo-phosphorylated, and cells have accumulated in G0/G1 (14, 15). Because EBNA3CHT protein levels are substantially decreased at 7 d in medium without 4HT and LCL growth decreases 1–3 d later, RNA levels were evaluated at day 7, to potentially identify RNA changes that may cause decreased LCL growth and avoid RNA changes that are secondary to slow growth.
Quadruplicate RNA levels were determined from each of the three LCLs grown in media with 4HT or without 4HT, using 24 Affymetrix U133 Plus 2.0 oligonucleotide arrays. These arrays have 54,675 probe sets that assess 47,400 transcripts from 19,621 unique genes (based on mappings in the Bioconductor annotation package, hgu133Plus 2.db version 2.3.5). The expression data passed quality control, as assessed by NUSE and RLE scores, and were normalized by RMA (37, 38). After correction for multiple testing hypotheses, comparison of RNA abundances in the EBNA3CHT LCLs grown, under permissive and nonpermissive conditions, revealed only one gene to be differentially expressed, with a false discovery rate (FDR) < 0.01 (Dataset S1). Overall, the low number of differentially expressed genes is consistent with the possibility that, under permissive conditions, EBNA3CHT may be hypomorphic in effect relative to wild-type EBNA3C.
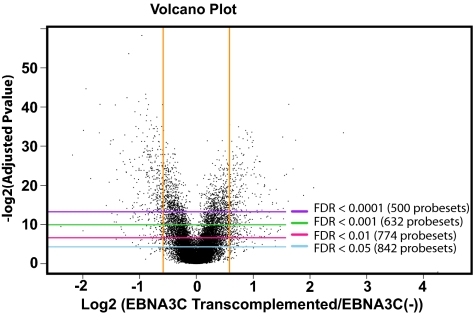
EBNA3CHT LCLs transcomplemented by transfection with an OriP episome that expresses wild-type EBNA3C and grown in medium without 4HT lose EBNA3CHT expression and self-select for EBNA3C expression at wild-type levels (15, 16). Triplicate RNAs levels in each of the three EBNA3CHT LCLs after EBNA3C transcomplementation were compared with quadruplicate RNA levels from the same cell lines growing in media with 4HT and with quadruplicate RNA levels from the same cell lines growing for 7 d in media without 4HT. A two-way ANOVA model, allowing for up or down effects, was fitted to the expression of each gene in the data set with Benjamini–Hochberg correction for multiple testing hypotheses (39). Where multiple probe sets mapped to a single gene, the probe set associated with the minimum adjusted P value was retained. With these approaches, 774 probe sets corresponding to 550 unique genes were statistically significantly EBNA3C effected, with a FDR < 0.01 and >1.5-fold change between transcomplemented and nonpermissive states (Fig. 1 and Dataset S2). In contrast to EBNA2 effects, which are almost all up-regulatory in LCLs, EBNA3C effected 550 genes, of which 266 were up-regulated and 284 were down-regulated (Dataset S2).
Fig. 1.
Volcano plot of EBNA3C effects on cell RNA levels. Quadruplicate RNAs prepared from each of three EBNA3CHT LCLs grown under nonpermissive or permissive conditions (12 samples, each) for 7 d were compared with triplicate total RNAs from each of three EBNA3CHT LCLs grown under nonpermissive conditions and transcompletmented with EBNA3C for 12 wk (9 samples). RNAs were profiled using U133 Plus 2.0 arrays. Each probe set was represented by a dot placed in 2D space determined on the y axis by log2 P value after adjustment for multiple testing hypotheses and on the x axis by log2 (fold change in RNA levels comparing nonpermissive EBNA3CHT LCL RNA with RNA from the same LCLs transcomplemented with wild-type EBNA3C). P values were adjusted using Benjamini–Hochberg correction. Horizontal lines indicate FDRs of 0.05, 0.01, 0.001, 0.0001, from bottom to top. The vertical lines indicate 1.5-fold change in EBNA3C up- or down-effect.
Before focusing exclusively on this 550 unique gene data set, we note: (i) EBNA3C up-regulated 50 genes and down-regulated 49 genes greater than twofold with FDR < 0.01 (Fig. 2). (ii) Several potentially important genes for LCL cell growth or survival changed with FDR < 0.05 significance or with <1.5-fold effect. For example, EBNA3C up-regulated MYC 1.3-fold, with FDR <9 × 10−5. Although EBNA2 has a much larger initial effect on MYC up-regulation in LCLs, EBNA3C 1.3-fold up-regulation could be important at a specific point in cell cycle or under specific growth conditions, as has been described for the EBNA3C effect on EBV LMP1 expression in Latency III infected Raji BL cells (9, 40, 41). Also, TCL1A was EBNA3C up-regulated 1.7-fold, with FDR < 0.05. TCL1A increases AKT activity, an important activity for MYC over-expression related survival. Increased TCL1A expression and nuclear translocation are associated with high MYC levels in lymphomas (42–47). EBNA3C also 1.4-fold down-regulated p16 and p14 RNAs encoded from the shared CDKN2A exon 2 and 3 probe sets, with FDR <5 × 10−4. EBNA3C repression of p16 and p14 is likely central to EBNA3C and EBNA3A combined effects in suppressing these inhibitors of LCL growth (13–15).
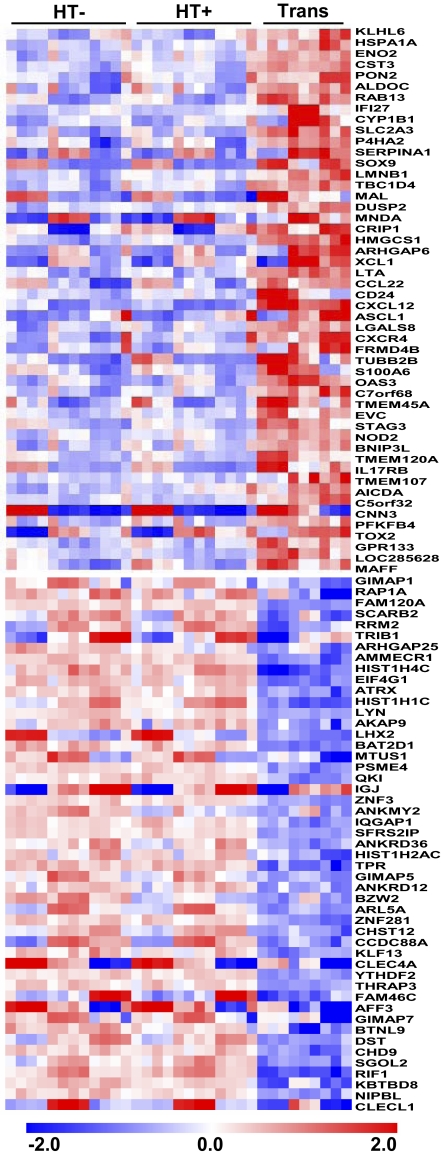
Fig. 2.
EBNA3C-regulated genes. Expression level for each sample was normalized by RMA and log base 2 transformed. Each gene was mean centered. The figure shows 99 EBNA3C greater than twofold up- or down-regulated genes, with FDR < 0.01 after multiple testing correction.
Bayesian Network Analysis.
A seeded Bayesian network analysis (48) was used to construct a predictive model of the directionality of EBNA3C effects on the 80 genes with the most significant FDRs and at least a 1.5-fold change. The network was constructed using literature derived relationships. The initial network was then allowed to evolve given the data and conditional probability tables calculated for all relationships. The results (Fig. 3) position EBNA3C down-regulation of IQGAP1 as up-stream of ACTR2 and RAC1, which were also down-regulated. IQGAP1 is increasingly implicated in SHC, Ras, and RAC1 cytoskeletal and mTOR signaling effects. IQGAP effects on RAC1, which was EBNA3C down-regulated are also up-stream of TNF, JUNB, CD97, PEA15, STAT3, SOCS1, AP2B1, RAB13, IL23A, CSF2RB, and UPB1, which were EBNA3C up-regulated, and of LYN, RASA2, GIT2, WNK1, E2F3, BCLAF1, TPR, SFPQ, RIF1, CD47, RBM4, EI24, LRRFIP1, AAK1, SRPK2, CAMK2G, CCAR1, MS4A1, SFRS2IP, YTHDF2, KIAA0100, and PUM1, which were EBNAC down-regulated (Fig. 3). Thus, EBNA3C regulation of IQGAP1, RAC1, LYN, and TNF may be upstream of many other EBNA3C transcriptional effects.
Fig. 3.
Seeded Bayesian network for the 80 most significantly EBNA3C up-regulated (red) or down-regulated (blue) regulated genes, showing literature curated interactions (edges). Directionality was determined by an effect of the up-stream gene on the down-stream gene. The BN used 100 bootstrap iterations and a confidence threshold of 0.7.
EBNA3C-Regulated Cell Genes Are Enriched for Specific Cell Pathways.
We searched the EBNA3C-regulated gene set for enrichment of biological pathways, defined in the Kyoto Encyclopedia of Genes and Genomes (KEGG), using gene set enrichment analysis (GSEA) (49). We found 55 significant pathways with a permuted P value <0.001. The top 10 pathways are shown in Table 1. Most of these 10 pathways involve cytokine, chemokine, IFN, or other cell ligands that activate transcription factors involved in cell proliferation, differentiation, migration, or survival.
Table 1.
Top 10 pathways affected by EBNA3C
| EBNA3C | |||||
| KEGGID | Pathway name | P value | Size | Up-regulated | Down-regulated |
| 4010 | MAPK signaling pathway | <0.001 | 257 | 11 | 6 |
| 4060 | Cytokine-cytokine receptor interaction | <0.001 | 249 | 15 | 5 |
| 4062 | Chemokine signaling pathway | <0.001 | 176 | 7 | 5 |
| 4630 | Jak-STAT signaling pathway | <0.001 | 150 | 5 | 4 |
| 4530 | Tight junction | <0.001 | 128 | 2 | 0 |
| 4514 | Cell adhesion molecules (CAMs) | <0.001 | 125 | 8 | 3 |
| 4620 | Toll-like receptor signaling pathway | <0.001 | 98 | 4 | 1 |
| 4912 | GnRH signaling pathway | <0.001 | 95 | 1 | 1 |
| 1070 | Biosynthesis of plant hormones | <0.001 | 85 | 5 | 0 |
| 4640 | Hematopoietic cell lineage | <0.001 | 81 | 4 | 2 |
Functional enrichment of the 550 EBNA3C-regulated genes for Gene Ontology categories was also assessed using Fisher's exact test (Dataset S3). EBNA3C-regulated genes were enriched for Biological Process such as “B cell activation,” “immune response,” or “programmed cell death,” which are highly relevant to EBV transformation of primary B cells to LCLs. Enrichment for KEGG pathways (Dataset S4) included “cytokine–cytokine receptor interaction” and “B cell receptor signaling pathway”.
EBNA3C-Regulated Genes Are Enriched in Distinct Protein Networks.
To search for other functional interactions among EBNA3C-regulated proteins, we used Pathway Pallet (http://blaispathways.dfci.harvard.edu/Palette.html) to assemble protein–protein interactions identified by in vivo, in vitro, mass spectroscopy, or yeast two-hybrid assays in HPRD (www.hprd.org) and BioGRID (http://thebiogrid.org). The largest protein network has 90 of the 550 EBNA3C-regulated genes linked by 121 interactions or edges. Networks of 10 genes with 9 edges and 6 genes with 5 edges were also detected (Fig. S1). Extensive overlap between the EBNA3C-regulated protein interaction KEGG pathways and the pathways identified by GSEA was also observed, such as MAPK signaling, cytokine–cytokine receptor, JAK-STAT signaling, and cell adhesion.
EBNA3C Up-Regulates CXCL12 Chemokine and CXCR4 Receptor Expression and Increases LCL Migration.
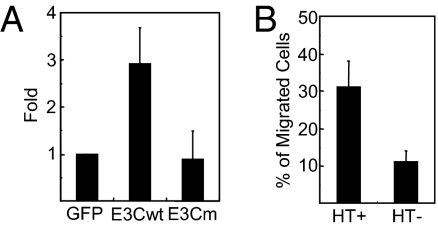
EBNA3C up-regulated the CXCL12 chemokine RNA 6-fold with FDR <9 × 10−11 and its CXCR4 receptor 2.8-fold with FDR < 0.01. Because CXCL12 activation of CXCR4 effects LCL malignant growth in SCID mice (50), EBNA3C effects on CXCL12 expression and CXCR4 activation were further investigated. EBNA3C up-regulated CXCL12 RNA levels 2.9-fold by qRT-PCR, whereas EBNA3C with a null point mutation in interaction with RBPJ had no effect on CXCL12 RNA levels (Fig. 4A). These results position EBNA3C effects through RBPJ in CXCL12 up-regulation.
Fig. 4.
EBNA3C up-regulates the CXCL12 ligand and its receptor CXCR4. (A) EBNA3CHT LCLs were transfected with an OriP vector expressing GFP, EBNA3C, or an EBNA3C null point mutant in RBPJ binding, and grown under nonpermissive conditions for 14 d. RNA was prepared and qRT-PCR was used to measure CXCL12 normalized to GAPDH RNA. (B) EBNA3CHT LCLs were grown under permissive or nonpermissive condition for 7 d, and 3 × 105 cells were seeded in the upper chamber of a transwell. The number of cells migrating at 37 °C through the transwell membrane to CXCL12 in the lower chamber was counted 4 h later. The cell fraction migrating to CXCL12 was corrected for cell migration in the absence of CXCL12.
A CXCL12- and CXCR4-dependent transwell migration assay was used to assess EBNA3C effects through CXCR4. EBNA3CHT LCLs grown under EBNA3CHT permissive versus nonpermissive conditions for 7 d were tested for migration to recombinant CXCL12 through a transwell filter. In repeat assays, 31% of EBNA3CHT LCLs grown under permissive conditions migrated through the transwell to CXCL12, versus only 11% of LCLs grown under nonpermissive condition (Fig. 4B). These data indicate that EBNA3C up-regulates LCL CXCL12- and CXCR4-mediated migration responses.
EBNA3C-Regulated Genes Overlap with EBNA2- and EBNA3A-Regulated Genes.
Because EBNA2 and EBNA3A regulation of transcription through RBPJ is essential for LCL growth, we investigated the overlap of EBNA3C and EBNA2 effects on transcript abundance in LCLs (41, 51) and in BL cells (52). Notably, all significant EBNA2 effects on LCL RNAs are up-regulatory (41, 51). Of the 461 EBNA3C-regulated genes common to HU133A and Agilent arrays used in EBNA2 regulated LCL and BL RNA analyses, 55 RNAs were effected by EBNA3C and EBNA2, P value of <1.7 × 10−14 (Fig. S2 and Dataset S5). Of the 55 EBNA3C and EBNA2 regulated genes, 21 were EBNA3C and EBNA2 up-regulated. Among these, 17 were up-regulated in LCLs and potentially relevant to LCL growth or survival, including OAS3, IRF4, MAFF, ZNF638, ASCL1, CRIP1, ZBTB32, CD21, CCL5, RGS1, CDH1, SLC1A4, P2RX5, KTN1, DNMBP, FNBP1, and ATRX.
To consider the potential overlap of EBNA3C and EBNA3A transcriptional effects, we compared EBNA3C effects on RNA levels with putative EBNA3A effects, which have been assessed by comparison of RNA levels in LCLs with RNA levels in B cells grown on feeder cells after infection with an EBNA3A-negative EBV (53). Overall, 52 genes were significantly regulated by both EBNA3C and EBNA3A, with a P value of <3 × 10−23 (Fig. S2 and Dataset S6). Genes regulated by both EBNA3C and EBNA3A encoded components of MAPK, Cytokine-Receptor, Chemokine, or JAK-STAT pathways, such as OAS1, OAS3, IFI6, IFI27, IFIT1, GAS7, LTA, TNF, TNFAIP2, CD24, CCL5, TMEM45A, IL17RB, RAB13, LGALS8, CST3, STAG3, S100A6, MNDA, DDX60, DMD, XAF1, S100A4, GNPTAB, KCNN3, which were EBNA3C and EBNA3A up-regulated, and JAK1, IL18R1, GIMAP6, FOXO1, SLC1A4, CITED2, MED13L, TXNDC5, TRIB1, ANKMY2, GIMAP5, ARHGAP25, MTUS1, IGJ, BZW2, and SCARB2, which were EBNA3C and EBNA3A down-regulated. Also, CDH1, KIAA1659, CXCR4, PTPN13, RAI14, and NMT2 were EBNA3C up-regulated and EBNA3A down-regulated, whereas RB1, ZNF83, SELL, SYNE2, and CHST12 were EBNA3C down-regulated and EBNA3A up-regulated. The extensive and highly significant overlap in EBNA3C and EBNA3A effects indicates that EBNA3C and EBNA3A are more similar to each other in RNA regulation than was evident from previous studies (5, 6, 8, 28, 29, 31, 54–56).
Discussion
EBNA3C reverse genetic analyses identified interactions through RBPJ, as well as through less well-defined targets of amino acids 51–150 and 851–900, to be essential for LCL growth, whereas effects through CtBP and a SUMO homology domain were less important for LCL growth (15, 16). Thus, EBNA3C effects on cell RNAs important for LCL growth or survival were anticipated, although the broad spectrum of EBNA3C >1.5-fold effects on 550 cell RNAs, with FDR < 0.01, was surprising. EBNA3C-regulated RNAs were enriched in MAP kinase, Cytokine and Cytokine Receptor interactions, Chemokine Signaling, JAK/STAT signaling, Cell Adhesion Molecules, Pancreatic Cancer, Toll Receptor, and Hematopoietic Lineage signaling pathways. Many of the 80 most highly significant EBNA3C-regulated RNAs encode proteins that are implicated in IQGAP and RAC1 signaling. Interestingly, RAC1 signaling is also implicated in Kaposi's sarcoma herpes virus and human papilloma virus (HPV) tumor pathogenesis (57–59). The principal EBNA3C up-regulated pathway components are RNAs downstream of LYN or TNF. The seeded Bayesian network analysis suggests that EBNA3C down-regulation of LYN and up-regulation of TNF have significant effects on cell signaling or gene expression. TNF, together with other EBNA3C up-regulated genes, including CD40, LTA, IL17RB, TNFSF13B (BAFF), and IL1R1 (which are not among the 80 most significantly regulated genes shown in the Bayesian network) are involved in LCL NF-κB activation, proliferation, and survival and IL17 and BAFF enhance primary B lymphocyte survival through NF-κB activation (60, 61). EBNA3C also up-regulates EBV LMP1 expression, which constitutively activates NF-κB and augments CD40-, LTA-, and IL17RB-mediated NF-κB activation and cell survival (19, 21).
EBNA3C up-regulated RNAs encode the CXCL12 ligand, its CXCR4 receptor, and STAT3, their downstream inducer of gene expression, a pathway essential for LCL proliferation and survival in a mouse model (50). Immune-deficient mice injected with LCLs survive longer with neutralizing CXCL12 antibody or with inhibition of CXCR4 signaling. Ex vivo lymphoma cell treatment with CXCL12 neutralizing antibody or CXCR4 antagonists decreases cell proliferation and increases apoptosis (50), whereas CXCL12 induced CXCR4-mediated JAK2 activation and STAT3 phosphorylation (62). Phosphorylated STAT3 binding to DNA activates genes important for proliferation, survival, and angiogenesis and suppresses tumor immune responses (63). JAK/STAT activation also induces SOCS1 and SOCS3, which modulate JAK/STAT activity (64). EBNA3C directly or indirectly up-regulated SOCS1 and SOCS3 and down-regulated PIAS1, which is a negative STAT3 regulator. Thus, EBNA3C has multiple effects on JAK/STAT signaling.
Although EBNA3C down-regulated pRb RNA 1.5-fold, with FDR < 0.001, under nonpermissive conditions for EBNA3CHT expression, EBNA3CHT levels decreased, but pRb protein levels did not increase, suggesting that pRb turnover is increased in the absence of EBNA3C or that pRb is less well detected (14). EBNA3C also down-regulated p16 and p14 RNAs 1.4-fold with FDR <5 × 10−4 and p16 expression likely caused pRb hypo-phosphorylation. Hypo-phosphorylated pRb may not be accurately assessed by Western blots. Although EBNA3C lacks a LXCXE motif similar to HPV E7, EBNA3C has been implicated in decreasing pRb levels (65–67). EBNA3C also binds CtBP, which can recruit CtBPIP. CtBPIP has an LXCXE motif, similar to E7, and may affect pRb stability.
Materials and Methods
Cell Culture, Transfection, and Migration Assay.
EBNA3CHT LCLs were cultured and transfected with Ori-P based EBNA3C expression vectors (14, 16). Migration assays were performed as described (18).
RNA Preparation, Transcription Profiling, and Real-Time RT-PCR.
Total RNA samples from EBNA3CHT LCLs transfected with an Ori-P based EBNA3C expression plasmid and grown in the absence of 4HT for 12–16 wk or from EBNA3CHT LCLs grown in media in the presence or absence of 4HT for 7 d at concentrations of 2–4 × 105 per mL were prepared with RNA-bee (Tel-Test, Friendswood, TX). Gene expression profiles were assayed using Affymetrix U133 Plus 2.0 Gene Chips (Santa Clara, CA). Real-time RT-PCR used Power SYBR Green RNA-to-CT 1-Step Kit (Applied Biosystems, Foster City, CA). Fold changes were determined using delta delta Ct normalized with GAPDH.
Microarray Preprocessing Analysis.
Expression data were normalized using RMA and array quality assessed using NUSE and RLE scores (37). The data were annotated using MIAME standards (68) and are available for download from the Gene Expression Omnibus (accession number GSE24362).
Determining Genes Significantly Regulated by EBNA3C.
Gene-specific RNA levels significantly altered by EBNA3C were determined by using a two-way ANOVA model with covariates representing clonal and EBNA3C effects. The clonal covariate adjusted for potential clone-specific effects resulting from C15, C51, and C19 LCLs lines used in the experiment. The EBNA3C activity covariate accounted for the consecutive degrees of EBNA3C activity from the C15, C51, and C19 LCLs in HT− (least), versus HT+ (intermediate), versus HT− after EBNA3C transcomplementation (highest) as specified in this ANOVA model.
Hierarchical Clustering.
Heatmaps were used to visualize the expression changes for the 550 EBNA3C-regulated genes, with an average-linkage agglomerative hierarchical clustering, where distance was based on the Pearson correlation coefficient (69). The cluster analysis and heatmap visualization was performed using MeV (70).
Bayesian Network Analysis.
Seeded Bayesian network analysis used an implementation in the freely available, open-source software MeV version 4.5. A Bayesian network was constructed for the 80 unique gene symbols with the smallest FDR, which were >1.5-fold up- or down- regulated between HT− and the transcomplented LCL conditions. Three expression states were specified for each gene (up or down), and 100 bootstrap iterations were performed. The final network was obtained using a bootstrap confidence threshold of 0.7 and visualized using Cytoscape version 2.6.2.
Gene Set Enrichment Analysis.
GSEA was performed as described (49). Only genes currently annotated in KEGG were retained. Of these remaining genes, those which had multiple probe sets mapping to a single gene symbol were reduced to a single probe set with the smallest P value. This resulted in a data set with 4,559 gene symbols. Using gene sets defined by KEGG pathways, GSEA was run on a linear model with the clonal and EBNA3C activity covariates using 1,000 permutations.
Functional Enrichment Analysis.
Using Fisher's exact tests available in the GOstats Bioconductor R package, we tested for over-enrichment of Gene Ontology terms: Biological Process (BP), Molecular Function (MF), Cellular Component (CC), and KEGG pathway terms (71). With each annotation set, P values were adjusted using the Benjamini–Hochberg correction and statistical significance P < 0.01 was used to identify significant functional categories. For the analyses described in this paper, we made use of the annotation packages available in the latest release of Bioconductor (Biobase 2.6.0, hgu133Plus 2.db version 2.3.5, KEGG.db version 2.3.5).
Intersection.
The overlap between the EBNA3C 550 genes that were P < 0.01 and >1.5-fold up- or down-regulated and EBNA2- and EBNA3A-regulated genes was assessed using a Fisher's exact test. Three existing expression profiling studies had collectively identified 492 genes as being regulated by EBNA2 using Affymetrix HGU133A and Agilent G4122A 44K HD array. Similarly, 287 genes were found to be regulated by EBNA3A using the Affymetrix Human Genome U133A 2.0 array platform. Because these studies used different expression profiling technologies, we mapped all significant probe sets to unique gene symbol identifiers before calculating the degree of overlap between these gene sets. A total of 12,703 gene symbols were common to all platforms.
Supplementary Material
Acknowledgments
We thank Ellen Cahir-McFarland, Michael Calderwood, and Daniel Portal for helpful discussions. This research was supported by National Institutes of Health Grants R01CA047006 (to E.K.) and P50 HG004233 (to E.K. and J.Q.) and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to S.M.). B.E.G. is a fellow of the Leukemia and Lymphoma Society and the Grunebaum Foundation.
Footnotes
The authors declare no conflict of interest.
Data deposition: The dataset reported in this paper has been deposited in the Gene Expression Omnibus database (accession no. GSE24362).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017419108/-/DCSupplemental.
References
- 1.Rickinson AB, Kieff ED. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2655–2700. [Google Scholar]
- 2.Henle W, Diehl V, Kohn G, Zur Hausen H, Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967;157:1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- 3.Pope JH. Establishment of cell lines from peripheral leucocytes in infectious mononucleosis. Nature. 1967;216:810–811. doi: 10.1038/216810a0. [DOI] [PubMed] [Google Scholar]
- 4.Kieff ED, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2603–2654. [Google Scholar]
- 5.Robertson ES, et al. Epstein-Barr virus nuclear protein 3C modulates transcription through interaction with the sequence-specific DNA-binding protein J kappa. J Virol. 1995;69:3108–3116. doi: 10.1128/jvi.69.5.3108-3116.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson ES, Lin J, Kieff E. The amino-terminal domains of Epstein-Barr virus nuclear proteins 3A, 3B, and 3C interact with RBPJ(kappa) J Virol. 1996;70:3068–3074. doi: 10.1128/jvi.70.5.3068-3074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waltzer L, Perricaudet M, Sergeant A, Manet E. Epstein-Barr virus EBNA3A and EBNA3C proteins both repress RBP-J kappa-EBNA2-activated transcription by inhibiting the binding of RBP-J kappa to DNA. J Virol. 1996;70:5909–5915. doi: 10.1128/jvi.70.9.5909-5915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao B, Marshall DR, Sample CE. A conserved domain of the Epstein-Barr virus nuclear antigens 3A and 3C binds to a discrete domain of Jkappa. J Virol. 1996;70:4228–4236. doi: 10.1128/jvi.70.7.4228-4236.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper A, et al. EBNA3A association with RBP-Jkappa down-regulates c-myc and Epstein-Barr virus-transformed lymphoblast growth. J Virol. 2003;77:999–1010. doi: 10.1128/JVI.77.2.999-1010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordadze AV, et al. Notch1IC partially replaces EBNA2 function in B cells immortalized by Epstein-Barr virus. J Virol. 2001;75:5899–5912. doi: 10.1128/JVI.75.13.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomkinson B, Robertson E, Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J Virol. 1993;67:2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maruo S, Johannsen E, Illanes D, Cooper A, Kieff E. Epstein-Barr Virus nuclear protein EBNA3A is critical for maintaining lymphoblastoid cell line growth. J Virol. 2003;77:10437–10447. doi: 10.1128/JVI.77.19.10437-10447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruo S, et al. Epstein-Barr virus nuclear protein 3A domains essential for growth of lymphoblasts: Transcriptional regulation through RBP-Jkappa/CBF1 is critical. J Virol. 2005;79:10171–10179. doi: 10.1128/JVI.79.16.10171-10179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maruo S, et al. Epstein-Barr virus nuclear protein EBNA3C is required for cell cycle progression and growth maintenance of lymphoblastoid cells. Proc Natl Acad Sci USA. 2006;103:19500–19505. doi: 10.1073/pnas.0604919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruo S, et al. Epstein-Barr virus nuclear protein EBNA3C residues critical for maintaining lymphoblastoid cell growth. Proc Natl Acad Sci USA. 2009;106:4419–4424. doi: 10.1073/pnas.0813134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, et al. Epstein-Barr virus nuclear protein 3C domains necessary for lymphoblastoid cell growth: Interaction with RBP-Jkappa regulates TCL1. J Virol. 2009;83:12368–12377. doi: 10.1128/JVI.01403-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen A, Divisconte M, Jiang X, Quink C, Wang F. Epstein-Barr virus with the latent infection nuclear antigen 3B completely deleted is still competent for B-cell growth transformation in vitro. J Virol. 2005;79:4506–4509. doi: 10.1128/JVI.79.7.4506-4509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A, Zhao B, Kieff E, Aster JC, Wang F. EBNA-3B- and EBNA-3C-regulated cellular genes in Epstein-Barr virus-immortalized lymphoblastoid cell lines. J Virol. 2006;80:10139–10150. doi: 10.1128/JVI.00854-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allday MJ, Farrell PJ. Epstein-Barr virus nuclear antigen EBNA3C/6 expression maintains the level of latent membrane protein 1 in G1-arrested cells. J Virol. 1994;68:3491–3498. doi: 10.1128/jvi.68.6.3491-3498.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allday MJ, Crawford DH, Thomas JA. Epstein-Barr virus (EBV) nuclear antigen 6 induces expression of the EBV latent membrane protein and an activated phenotype in Raji cells. J Gen Virol. 1993;74:361–369. doi: 10.1099/0022-1317-74-3-361. [DOI] [PubMed] [Google Scholar]
- 21.Zhao B, Sample CE. Epstein-barr virus nuclear antigen 3C activates the latent membrane protein 1 promoter in the presence of Epstein-Barr virus nuclear antigen 2 through sequences encompassing an spi-1/Spi-B binding site. J Virol. 2000;74:5151–5160. doi: 10.1128/jvi.74.11.5151-5160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao B, et al. Transcriptional regulatory properties of Epstein-Barr virus nuclear antigen 3C are conserved in simian lymphocryptoviruses. J Virol. 2003;77:5639–5648. doi: 10.1128/JVI.77.10.5639-5648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiménez-Ramírez C, et al. Epstein-Barr virus EBNA-3C is targeted to and regulates expression from the bidirectional LMP-1/2B promoter. J Virol. 2006;80:11200–11208. doi: 10.1128/JVI.00897-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Johannsen E, Robertson E, Kieff E. Epstein-Barr virus nuclear antigen 3C putative repression domain mediates coactivation of the LMP1 promoter with EBNA-2. J Virol. 2002;76:232–242. doi: 10.1128/JVI.76.1.232-242.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang F, et al. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J Virol. 1990;64:2309–2318. doi: 10.1128/jvi.64.5.2309-2318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paschos K, et al. Epstein-barr virus latency in B cells leads to epigenetic repression and CpG methylation of the tumour suppressor gene Bim. PLoS Pathog. 2009;5:e1000492. doi: 10.1371/journal.ppat.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall D, Sample C. Epstein-Barr virus nuclear antigen 3C is a transcriptional regulator. J Virol. 1995;69:3624–3630. doi: 10.1128/jvi.69.6.3624-3630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radkov SA, et al. Epstein-Barr virus EBNA3C represses Cp, the major promoter for EBNA expression, but has no effect on the promoter of the cell gene CD21. J Virol. 1997;71:8552–8562. doi: 10.1128/jvi.71.11.8552-8562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radkov SA, et al. Epstein-Barr virus nuclear antigen 3C interacts with histone deacetylase to repress transcription. J Virol. 1999;73:5688–5697. doi: 10.1128/jvi.73.7.5688-5697.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grundhoff AT, et al. Characterization of DP103, a novel DEAD box protein that binds to the Epstein-Barr virus nuclear proteins EBNA2 and EBNA3C. J Biol Chem. 1999;274:19136–19144. doi: 10.1074/jbc.274.27.19136. [DOI] [PubMed] [Google Scholar]
- 31.Touitou R, Hickabottom M, Parker G, Crook T, Allday MJ. Physical and functional interactions between the corepressor CtBP and the Epstein-Barr virus nuclear antigen EBNA3C. J Virol. 2001;75:7749–7755. doi: 10.1128/JVI.75.16.7749-7755.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subramanian C, et al. Epstein-Barr virus nuclear antigen 3C and prothymosin alpha interact with the p300 transcriptional coactivator at the CH1 and CH3/HAT domains and cooperate in regulation of transcription and histone acetylation. J Virol. 2002;76:4699–4708. doi: 10.1128/JVI.76.10.4699-4708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaul R, Murakami M, Lan K, Choudhuri T, Robertson ES. EBNA3C can modulate the activities of the transcription factor Necdin in association with metastasis suppressor protein Nm23-H1. J Virol. 2009;83:4871–4883. doi: 10.1128/JVI.02286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight JS, Lan K, Subramanian C, Robertson ES. Epstein-Barr virus nuclear antigen 3C recruits histone deacetylase activity and associates with the corepressors mSin3A and NCoR in human B-cell lines. J Virol. 2003;77:4261–4272. doi: 10.1128/JVI.77.7.4261-4272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian C, Robertson ES. The metastatic suppressor Nm23-H1 interacts with EBNA3C at sequences located between the glutamine- and proline-rich domains and can cooperate in activation of transcription. J Virol. 2002;76:8702–8709. doi: 10.1128/JVI.76.17.8702-8709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irizarry RA, et al. Multiple-laboratory comparison of microarray platforms. Nat Methods. 2005;2:345–350. doi: 10.1038/nmeth756. [DOI] [PubMed] [Google Scholar]
- 38.Irizarry RA, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 40.Kaiser C, et al. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J Virol. 1999;73:4481–4484. doi: 10.1128/jvi.73.5.4481-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao B, et al. RNAs induced by Epstein-Barr virus nuclear antigen 2 in lymphoblastoid cell lines. Proc Natl Acad Sci USA. 2006;103:1900–1905. doi: 10.1073/pnas.0510612103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gualco G, Weiss LM, Barber GN, Bacchi CE. T-cell leukemia 1 expression in nodal Epstein-Barr virus-negative diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma. Hum Pathol. 2010;41:1238–1244. doi: 10.1016/j.humpath.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Teitell MA, et al. TCL1 expression and Epstein-Barr virus status in pediatric Burkitt lymphoma. Am J Clin Pathol. 2005;124:569–575. doi: 10.1309/77V7U4E03V69QHRR. [DOI] [PubMed] [Google Scholar]
- 44.Kauffmann-Zeh A, et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed NN, Grimes HL, Bellacosa A, Chan TO, Tsichlis PN. Transduction of interleukin-2 antiapoptotic and proliferative signals via Akt protein kinase. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Djebbari A, Quackenbush J. Seeded Bayesian Networks: Constructing genetic networks from microarray data. BMC Syst Biol. 2008;2:57. doi: 10.1186/1752-0509-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Z, Gentleman R. Extensions to gene set enrichment. Bioinformatics. 2007;23:306–313. doi: 10.1093/bioinformatics/btl599. [DOI] [PubMed] [Google Scholar]
- 50.Piovan E, et al. Chemokine receptor expression in EBV-associated lymphoproliferation in hu/SCID mice: Implications for CXCL12/CXCR4 axis in lymphoma generation. Blood. 2005;105:931–939. doi: 10.1182/blood-2004-03-0799. [DOI] [PubMed] [Google Scholar]
- 51.Spender LC, et al. Cell target genes of Epstein-Barr virus transcription factor EBNA-2: Induction of the p55alpha regulatory subunit of PI3-kinase and its role in survival of EREB2.5 cells. J Gen Virol. 2006;87:2859–2867. doi: 10.1099/vir.0.82128-0. [DOI] [PubMed] [Google Scholar]
- 52.Maier S, et al. Cellular target genes of Epstein-Barr virus nuclear antigen 2. J Virol. 2006;80:9761–9771. doi: 10.1128/JVI.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hertle ML, et al. Differential gene expression patterns of EBV infected EBNA-3A positive and negative human B lymphocytes. PLoS Pathog. 2009;5:e1000506. doi: 10.1371/journal.ppat.1000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bain M, Watson RJ, Farrell PJ, Allday MJ. Epstein-Barr virus nuclear antigen 3C is a powerful repressor of transcription when tethered to DNA. J Virol. 1996;70:2481–2489. doi: 10.1128/jvi.70.4.2481-2489.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hickabottom M, Parker GA, Freemont P, Crook T, Allday MJ. Two nonconsensus sites in the Epstein-Barr virus oncoprotein EBNA3A cooperate to bind the co-repressor carboxyl-terminal-binding protein (CtBP) J Biol Chem. 2002;277:47197–47204. doi: 10.1074/jbc.M208116200. [DOI] [PubMed] [Google Scholar]
- 56.Cludts I, Farrell PJ. Multiple functions within the Epstein-Barr virus EBNA-3A protein. J Virol. 1998;72:1862–1869. doi: 10.1128/jvi.72.3.1862-1869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Q, et al. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci USA. 2009;106:8683–8688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mutlu AD, et al. In vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: A cell and animal model of virally induced Kaposi's sarcoma. Cancer Cell. 2007;11:245–258. doi: 10.1016/j.ccr.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petti LM, Ricciardi EC, Page HJ, Porter KA. Transforming signals resulting from sustained activation of the PDGFbeta receptor in mortal human fibroblasts. J Cell Sci. 2008;121:1172–1182. doi: 10.1242/jcs.018713. [DOI] [PubMed] [Google Scholar]
- 60.Doreau A, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 61.Cahir-McFarland ED, Davidson DM, Schauer SL, Duong J, Kieff E. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc Natl Acad Sci USA. 2000;97:6055–6060. doi: 10.1073/pnas.100119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ahr B, Denizot M, Robert-Hebmann V, Brelot A, Biard-Piechaczyk M. Identification of the cytoplasmic domains of CXCR4 involved in Jak2 and STAT3 phosphorylation. J Biol Chem. 2005;280:6692–6700. doi: 10.1074/jbc.M408481200. [DOI] [PubMed] [Google Scholar]
- 63.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 64.Pello OM, et al. SOCS up-regulation mobilizes autologous stem cells through CXCR4 blockade. Blood. 2006;108:3928–3937. doi: 10.1182/blood-2006-02-006353. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalez SL, Stremlau M, He X, Basile JR, Münger K. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J Virol. 2001;75:7583–7591. doi: 10.1128/JVI.75.16.7583-7591.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker GA, et al. Epstein-Barr virus nuclear antigen (EBNA)3C is an immortalizing oncoprotein with similar properties to adenovirus E1A and papillomavirus E7. Oncogene. 1996;13:2541–2549. [PubMed] [Google Scholar]
- 67.Knight JS, Sharma N, Robertson ES. Epstein-Barr virus latent antigen 3C can mediate the degradation of the retinoblastoma protein through an SCF cellular ubiquitin ligase. Proc Natl Acad Sci USA. 2005;102:18562–18566. doi: 10.1073/pnas.0503886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brazma A, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 69.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saeed AI, et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 71.Wixon J, Kell D. The Kyoto encyclopedia of genes and genomes—KEGG. Yeast. 2000;17:48–55. doi: 10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.