Abstract
The transcription factor Kruppel-like Factor 2 (KLF2) has been proposed to regulate genes involved in both cell cycle entry and T cell trafficking: However, the physiological role of KLF2 expression in post-activated T cells is not well defined. Previous studies suggested that the cytokines IL-2 and IL-15 differentially regulate KLF2 re-expression in post-activation T cells, and that these cytokines also influence effector versus memory T cell differentiation. Using conditional and inducible KLF2 knockout model systems, we tested the specific role of KLF2 expression in activated CD8+ T cells cultured with these cytokines. KLF2 was required for effective transcription of S1P1 and CD62L in post-activation T cells. However, while different cytokines dramatically altered expression of cell cycle related genes, endogenous KLF2 had a minimal impact in this regulation. Correspondingly, KLF2-deficient T cells showed dysregulated trafficking but not altered proliferative characteristics following in vivo responses to antigen. Thus, our data help define KLF2-dependent and -independent aspects of activated CD8+ T cell differentiation, and argue against a physiological role in cell-cycle regulation.
Introduction
Kruppel-like Factor 2 (KLF2) is a member of a large family of Sp1-related transcription factors. Members of the mammalian KLF family have been shown to act as both transcriptional activators and repressors, and have been found to regulate tissue specific differentiation (1–4), but also in the maintenance of self-renewal and multi-potentiality in embryonic stem cells (5). In mice, KLF2-deficient T cells were found to exhibit a partially activated phenotype, and were reported to rapidly enter cell cycle and succumb to apoptotic death, leading to the proposal that KLF2 regulates proliferation (6). Further substantiating this model, KLF2 overexpression in the Jurkat T cell lines lead to the cells withdrawing from cell cycle being maintained as resting cells (7–9). Induction of KLF2 in these models caused decreased c-Myc transcription (7) and increased p21Cip1 transcription (8), which are positive and negative regulators of cell-cycle, respectively. These findings lead to an intriguing proposal that KLF2 acted as a quiescence factor, enforcing a resting state on T cells (6).
In addition to these findings, we and others reported that KLF2 regulates expression of T cell trafficking molecules. KLF2 enhanced expression of S1P1 and CD62L, involved in T cell egress from and access into lymphoid tissues, respectively (10, 11). Expression of β7-integrin is also reduced on KLF2-deficient mature thymocytes (10), while expression of CXCR3 is elevated (12, 13): However, we recently showed that altered expression of β7-integrin and CXCR3 are non-autonomous effects, arising from altered IL-4 production in the KLF2 deficient thymus (13, 14), while thymocyte expression of S1P1 and CD62L are under direct control of KLF2 (10, 11, 13, 14). In accordance with these findings, KLF2-deficient thymocytes show impaired thymic egress and, after adoptive transfer exhibit defective circulation through secondary lymphoid tissues (10). Hence, KLF2 appears to control sets of trafficking molecules, related to distinct migration patterns.
KLF2 is tightly regulated during T cell development and differentiation. It is not expressed in CD4+8+ thymocytes, but is induced in mature thymocytes and expression is maintained in naïve T cells. TCR activation leads to rapid and profound loss of KLF2 protein, evidently through active degradation, and reduced KLF2 transcription (6, 15). Re-expression of KLF2 occurs in vitro following the treatment with low dose IL-2 (16) IL-7 (11, 16) and IL-15 (17) which promote differentiation of CD8+ T cells toward memory-like cells. On the other hand KLF2 re-expression is inhibited by exposure to IL-4 (11), IL-12 (16) and high dose IL-2 (17), which are thought to sustain effector-like states. In agreement with this general model, KLF2 is strongly expressed in the memory T cell pool in vivo (16, 18, 19).
Together with the data discussed above, these findings suggest that cytokines inducing KLF2 re-expression would lead to reduced cell cycle entry and increased expression of “homeostatic” trafficking molecules such as S1P1 and CD62L, allowing memory cell trafficking through lymphoid tissues. Although appealing, this model has not been directly tested and it is unclear whether and how KLF2 re-expression influences the cell-cycle and trafficking properties of effector-versus memory-like cells induced by different cytokines. In particular, many previous studies tested the impact of KLF2 through over-expression and it is unclear whether these findings represent the activity of physiological levels of endogenous KLF2 (7–9, 11, 20).
In the current report, we use conditional and inducible knockout models of KLF2 to test the role of endogenous KLF2 in dictating the properties of in vitro activated CD8+ T cells driven toward effector-versus memory-like states by cytokines. While KLF2 has a direct and profound effect on expression of key trafficking molecules such as S1P1 and CD62L, transcription of cell-cycle related genes was only modestly influenced by endogenous KLF2. Correspondingly, proliferation and apoptosis of post-activated CD8+ T cells was unaffected by KLF2-deficiency. Furthermore, deletion of both KLF2 and KLF4 (a close homolog of KLF2) failed to dysregulate T cell quiescence. These data suggest that the physiological role of KLF2 in T cells relates primarily to its impact on T cell trafficking rather than its control of cell cycle.
Materials and Methods
Mice
B6, B6. PL (Thy1.1 congenic B6) and B6. SJL (CD45.1 congenic B6) mice were purchased from National Cancer Institute. KLF2-floxed mice have been described (13). CD4Cre Tg mice were purchased from Taconic Farms (Germantown, MD). ROSA26-floxSTOP-YFP mice were a kind gift of Dr. Frank Costantini, Columbia University. OT-I transgenic (Tg) mice with or without Rag−/− background were bred in our colony at the University of Minnesota. KLF2 null mice were previously described (10, 21). Mice were housed and bred in our animal facility in specific pathogen-free conditions with approval of the Institutional Animal Care and Use Committee at the University of Minnesota.
Cell culture
Spleen cells from Rag−/−OT-I mice were incubated with 10 nM ovalbumin peptide (OVAp, SIINFEKL) for 1 hour at 37 C. After intensive washing, cells were cultured in RPMI media (Cellgro, Herndon, VA), supplemented with 10% fetal calf serum (FCS) for stimulation. Polyclonal spleen cells and thymocytes were stimulated with plate-bound anti-CD3 antibody (145-2C11, BD PharMingen, San Diego, CA) at 1 μg/ml. To stimulate the thymocytes, irradiated (3000 rads) spleen cells from normal mice were also added to the culture. Two days later, viable cells were enriched by centrifugation with Cellgro lymphocyte separation medium (Mediatech, Herndon, VA), and then cultured in the presence of 20 ng/mL IL-2, IL-7 or IL-15 (R & D systems, Minneapolis, MN) for another 6 days. Cytokine-containing media were replaced every other day. Before isolating total RNA from cultured thymocytes, CD8+ cells were purified by positive selection with anti-CD8 magnetic beads following manufacturer’s directions (Myltenyi Biotec, Auburn, CA), which yielded >90% of CD8+CD4− cells. In some experiments, cells were subjected to sorting on FACSAria (Becton Dickenson, Mountain View, CA) at the end of the culture, yielding >90% purity of the desired target population.
Real-time PCR analysis
Total RNA was extracted from the indicated cell populations using the RNeasy kit (Qiagen, Valencia, CA) and cDNA was synthesized with SuperScript III RT-PCR kit (Invitrogen, Carlsbad, CA), according to manufacturers’ instructions. The cDNA was amplified on a SmartCycler (Cepheid, Sunnyvale, CA) by PCR with FastMaster SYBR Green Master (Roche, Basel, Swizerland) and the following primers: CD62L, 5′-CTAATTTCCCCTCGCTGATTCAT-3′ and 5′-GCATTAGCTTCTGTGCTGAATTGA-3′; c-Myc, 5′-TGAAGGCTGGATTTCCTTTG-3′ and 5′-TTCTCTTCCTCGTCGCAGAT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-TGGCCTACATGGCCTCCAA-3′ and 5′-TCCCTAGGCCCCTCCTGTTAT-3′; KLF2, 5′-CGCCTCGGGTTCATTTC-3′ and 5′-AGCCTATCTTGCCGTCCTTT-3′; p21Cip1, 5′-TTGCACTCTGGTGTCTGAGC-3′ and 5′-TCTGCGCTTGGAGTGATAGA-3′; S1P1, 5′-GTGTAGACCCAGAGTCCTGCG-3′ and 5′-AGCTTTTCCTTGGCTGGAGAG-3′. RNA levels of all samples were normalized to the detected amount of GAPDH mRNA.
Apoptosis and proliferation analysis
To examine the apoptosis and proliferation induced by T cell activation, thymocytes were placed in plates that were previously coated with anti-CD3 (145-2C11) at 1.0 μg/ml and anti-CD28 (37.51, BD Pharmingen) at 20 μg/ml. To detect apoptotic cells, cells were analyzed by flow cytometry for FITC-Annexin V binding (BD Pharmingen). Specific apoptosis induced by TCR stimulation was calculated according to a previous report (22). To evaluate the apoptosis of in vitro-induced memory-like cells, viable cells were enriched by Ficoll 6 days after starting the treatment of activated T cells with IL-15 (20 ng/ml) as described above. Cells were stained with Annexin V before (0 hour) and after 24 hour-culture in the presence of IL-15 (20 ng/ml). The percentage of cells that underwent apoptosis during the 24 hour period was calculated as follows: (Annexin V+ cell % at 24 hour) − (Annexin V+ cell % at 0 hour). During this 24 hour-period, complication with cell proliferation could be excluded, as dilution of 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) was not seen. To assess proliferation, cells were labeled with CFSE at 1.7 μM for 10 min at 37°C, and dilution of CFSE was analyzed by flow cytometry after culture for 24–72 hours.
Trans-activator of transcription (TAT) Cre treatment
TATCre was a kind gift from Dr. Donna Farber, University of Maryland. Viable cells separated from spleen cells stimulated for 48 hours with plate-bound anti-CD3 were intensively washed in Hyclone ADCF-MAb serum free media (Logan, UT) and re-suspended in the media at 5 × 106 cells/ml containing TATCre (50 μg/ml) and incubated for 45 min at 37 C. After washing with RPMI media supplemented with 10% FCS, cells were subjected to the culture with cytokines for additional 6 days as described above.
Vaccinia virus infection
Fetal liver chimeras were generated from the neonates of time-mated KLF2+/− breeders at day 12.5 of gestation as previously described (10). Thy1.1+CD8+CD4− thymocytes from KLF2−/+ or KLF2−/− OT-I Tg fetal liver chimeras were transferred to B6 mice (0.75 × 105 cells/mouse). One day later, the mice were injected i.p. with 5 × 106 PFU of vaccinia virus expressing ovalbumin (OVA). Five days after infection, donor cells in various tissues were analyzed by flow cytometry with Thy1 congenic markers.
Listeria monocytogenes infection
Thymocytes were obtained from KLF2fl/flCD4Cre (Thy1.1+Thy1.2+) and wild-type (Thy1.1+Thy1.2−) OT-I Tg mice. Cells were analyzed and mixed so that 2 × 104 mature CD8 T cells from each donor was transferred (by iv injection) into normal B6 mouse recipients. One day later, the host mice were injected i.p. with an attenuated (ActA−) strain of L. monocytogenes expressing OVA (3 × 106 CFU/mouse) (23). At different time points after infection, donor cells in various tissues were analyzed by flow cytometry with Thy1 congenic markers and the binding of OVAp/Kb tetramer.
Flow cytometry
Cells were resuspended in phosphate-buffered saline plus 1% fetal calf serum and incubated with fluorochrome-labeled antibodies: anti-CD4 (L3T4), CD8 (53-6.7), CD24 (M1/69) and Qa-2 (1-1-2) purchased from BD PharMingen; anti-CD25 (PC61.5) CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), Thy1.1 (HIS51) and Thy1.2 (53-2.1) from eBioscience (San Diego, CA). OVAp/Kb tetramer was prepared as previously described (24). Cells were analyzed using FACScalibur or LSR II flow cytometer (Becton Dickenson) and the data were processed using FlowJo software (Tree star, San Carlos, CA).
In vivo migration assay
To evaluate the T cell migration to secondary lymphoid organs and the blood (Fig. 4C), we applied the protocol previously reported with small modifications (25) (26). Equal number (5 × 106) of IL-15-treated cells (CD45.2+Thy1.2+) and naïve splenocytes from B6.PL mice (CD45.2+Thy1.1+) were mixed and injected intravenously into B6.SJL mice (CD45.1+Thy1.2+). At 24 hours after transfer, peripheral (axillary, inguinal, and submandibular) lymph nodes, mesenteric lymph nodes, spleens and the blood were harvested from recipients, and were analyzed for the frequency of CD45.2+Thy1.1−YFP+CD8+ cells (test %) and CD45.2+Thy1.1+CD8+ cells (internal control %) by flow cytometry. Homing index was calculated as follows, according to the previous report (25). Test %/internal control % before transfer = R0. Test %/internal control % after transfer = R1. Homing index = R1/R0.
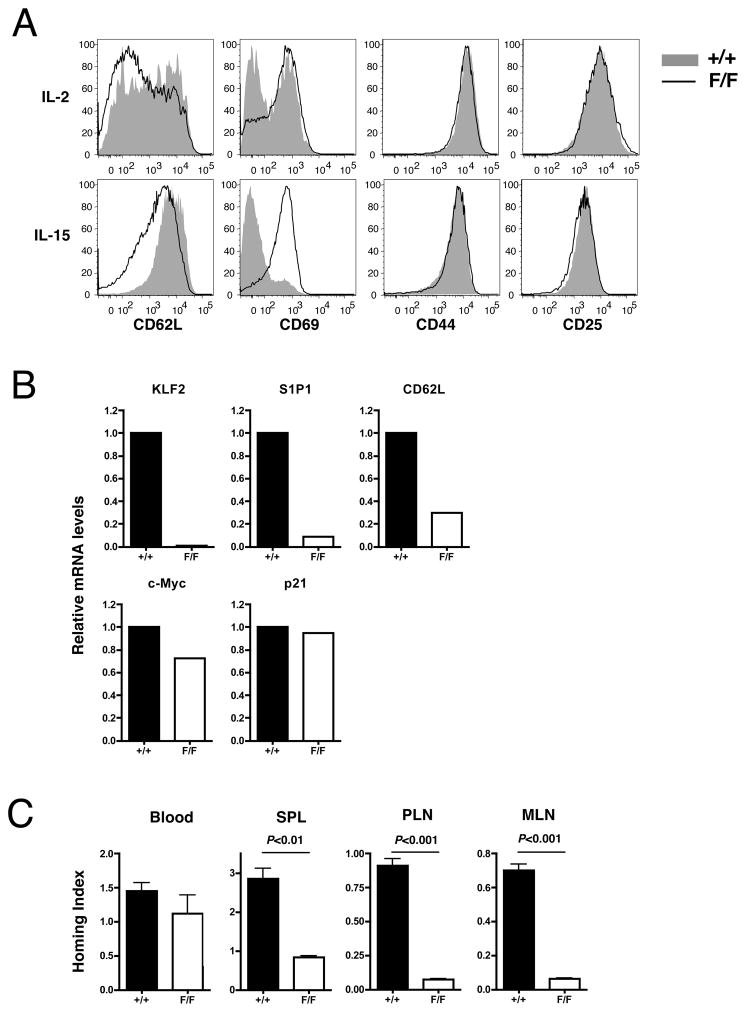
Figure 4. Inducible loss of KLF2 after activation impairs the expression of trafficking- but not quiescence-associated molecules.
Spleen cells from KLF2fl/fl (F/F) or KLF2+/+ (+/+) mice bearing ROSA26-floxSTOP-YFP allele were stimulated with plate-bound anti-CD3 for 2 days, then cultured with Tat-Cre for 45 minutes. Viable cells were cultured in the presence of IL-2 or IL-15 for additional 6 days. (A) Phenotype of CD8+YFP+ cells at the end of the culture. (B) CD8+YFP+CD44hi cells were sorted from the IL-15-treated group. RNA was extracted and used for the template of the real-time PCR analysis for the indicated genes. Expression levels are shown relative to WT group defined as 1.0. (C) IL-15-treated F/F or +/+ cells (CD45.2+Thy1.2+) were mixed with equal number (5 × 106) of naïve splenocytes from B6. PL mice (CD45.2+Thy1.1+) and injected intravenously into B6. SJL mice (CD45.1+Thy1.2+). Twenty-four hours after adoptive transfer, the blood, spleen (SPL), peripheral lymph nodes (PLN), mesenteric lymph nodes (MLN) from recipients were analyzed for the frequency of CD45.2+Thy1.1-YFP+CD8+ cells (test cells) and CD45.2+Thy1.1+CD8+ cells (internal control). Homing index was calculated as described in materials and methods. Representative of 2–3 independent experiments with similar results.
Statistical analysis
Statistical significance was determined by unpaired (or, for Fig. 5, paired) Student’s t-test with two-tailed distributions. P values less than 0.05 were considered significant.
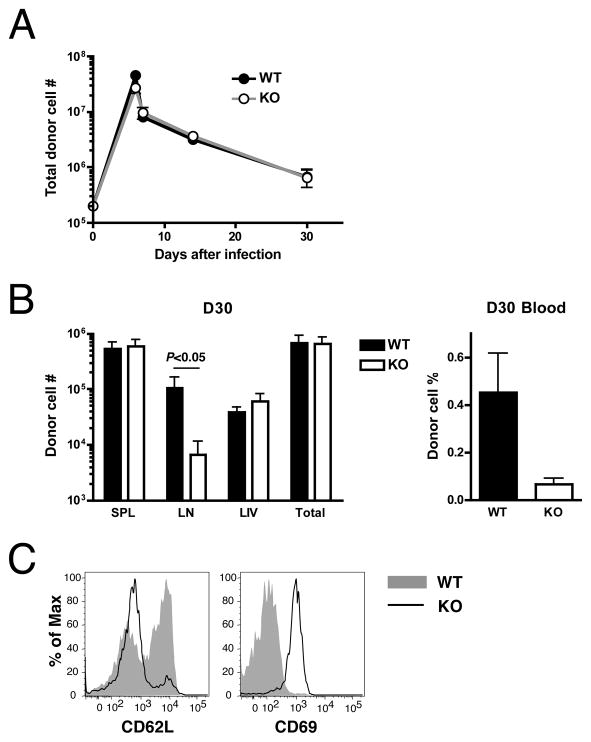
Figure 5. KLF2-deficient T cells respond normally to infection but show aberrant distribution.
CD8SP thymocytes from KLF2fl/flCD4Cre OT-I Tg (KO) or control OT-I Tg (WT) mice were mixed at equal number and co-transferred to B6 mice (2 × 104 cells from each group/recipient). One day later, recipient mice were injected i.p. with 3 × 106 CFU of attenuated L. monocytogenes expressing OVA. (A) Changes of donor CD8+ T cell numbers over time following infection in the indicated tissues. Total number indicates the sum of donor cell counts in the spleen, lymph nodes, and the liver. (B) Comparison of the KO and WT cell numbers (left graph) in the spleen (SPL), lymph nodes (LN) and the liver (LIV) or the percentages in the live cell gate in the blood (right graph) at day 30 after infection. (C) Histograms show the expression of CD62L and CD69 on the surface of donor CD8+ T cells at day 30 after infection. Representative of 3 independent experiments with similar results.
Results
Expression of KLF2-related molecules in different CD8+ T cell stages
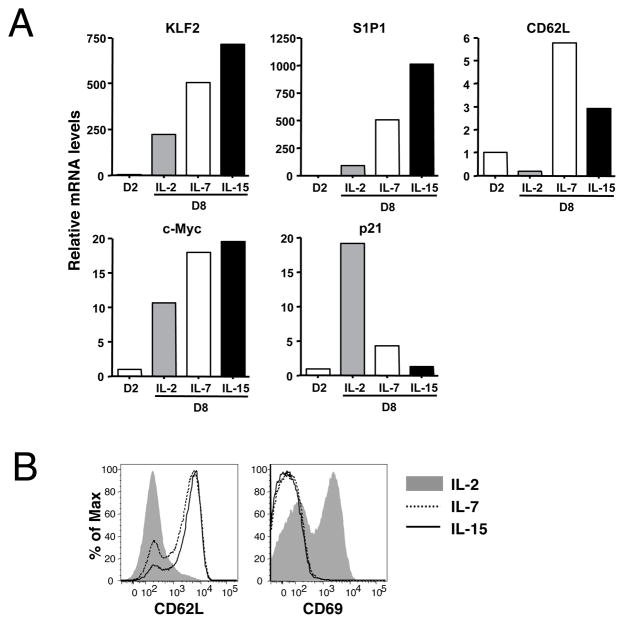
Previous studies reported that activated CD8+ T cells cultured with high dose IL-2 differentiate into a state which phenotypically and functionally mimics effector cells, whereas treatment with IL-7 or IL-15 induces central memory-like cells (27) (28, 29). To begin assessing the role of KLF2 in regulating the differentiation status of peripheral T cells, we characterized how culture in IL-2, IL-7 or IL-15 affected mRNA expression of KLF2 and its proposed targets. Real-time RT-PCR analysis showed that KLF2 mRNA is abundantly expressed in naïve OT-I T cells but significantly decreased upon stimulation with OVA peptide (data not shown), corresponding to the findings in previous reports (16, 18). Subsequent culture with IL-2 for 6 additional days maintained these low levels of KLF2 mRNA, while IL-7- or IL-15-treated cells displayed a substantial recovery of KLF2 mRNA expression (Fig 1A), consistent with previous studies (16),(17). Differentiation of T cells from naïve to effector and memory cells is characterized by changes in expression of the trafficking molecules S1P1 and CD62L. These are expressed in naïve and central memory cells, but not in effector cells, and their expression has been shown to be KLF2-dependent, at least in part (10, 11). Paralleling expression of KLF2, transcripts of genes encoding S1P1 and CD62L were low in activated T cells, and were induced by culture with IL-7 or IL-15, but not with IL-2 (Fig 1A). As expected based on previous studies (27, 28), CD62L expression correlated with CD62L transcription (Fig. 1B). Elevated expression of CD69, which is generally inversely expressed with cell surface S1P1 (30, 31), was also observed for IL-2 but not IL-7 or IL-15 cultured cells (Fig. 1B).
Figure 1. Differential cytokine effects on post-activated CD8+ T cells.
Spleen cells from Rag−/−OT-I mice were stimulated with OVAp for 2 days. Viable cells were subsequently cultured in the presence of IL-2, IL-7 or IL-15 for another 6 days. (A) RNA samples were obtained at day 2 or 8 of the stimulation culture and subjected to real time PCR analysis of indicated genes. (B) Expression of CD62L and CD69 on CD8+ T cells at the end of culture (day 8). Representative of 2–4 independent experiments with similar results.
We next assessed expression of cell cycle regulatory genes. Forced expression of KLF2 has been reported to induce exit from the cell cycle by inhibiting the transcription of c-Myc and elevating expression of the negative regulator p21Cip1 (7, 8). Despite the rapid initial proliferation of IL-2 cultured cells, expression of c-Myc mRNA was lower in this population than in IL-7- or IL-15-treated cells (Fig 1A). Similarly, mRNA expression of p21Cip1, a cell growth inhibitor, was significantly increased with IL-2 treatment but not with IL-7 and IL-15. These results are consistent with other studies showing that activated CD8 T cells began to exit cell cycle around 6–8 days of culture in IL-2, while proliferation was sustained in IL-15 containing media (29). Importantly, these data did not correspond to the model previously suggested, which would predict KLF2 re-expression would lead to reduced c-myc transcription and elevated p21Cip1 transcription (7, 8). Overall then, KLF2 expression in post-activated CD8 T cell populations correlated with transcriptional regulation of genes controlling lymphocyte trafficking but not cellular quiescence.
KLF2-deficient thymocytes are functionally mature and have normal proliferative capacity
We next wished to test the impact of KLF2 deficiency on post-activaation T cell gene expression. However, it was unclear whether the function of KLF2 deficient T cells may be compromised. When CD4 or CD8 single positive (SP) thymocytes are produced in the thymus they are not fully mature, but rather continue to functionally develop. For example, “semi-mature” CD4 SP remain susceptible to apoptosis when stimulated via TCR, whereas fully mature CD4 SP are competent to proliferate (32). This delay in maturation presumably facilitates negative selection by tissue specific antigens encountered in the medulla. The change from apoptosis susceptibility to resistance is associated with cell surface phenotype changes such as downregulation of CD24 and CD69, and upregulation of CD62L and Qa2 (33). We previously reported that KLF2-deficient SP thymocytes do not have profound survival defect in vivo, but it was unclear whether these cells achieved full maturity, since they fail to downregulate CD69 and upregulate CD62L (10). However, KLF2 deficiency did not prevent downregulation of CD24 or upregulation of Qa2 on SP thymocytes (Fig. 2A). Indeed, the regulation of these markers is even more dramatic in KLF2-deficient SP than wild-type SP, suggesting that KLF2-deficient SP thymocytes are older than their WT counterparts, consistent with their thymic retention (10, 33). Given this mixed phenotype, we sought to test directly whether KLF2 was required for complete functional maturation of thymocytes.
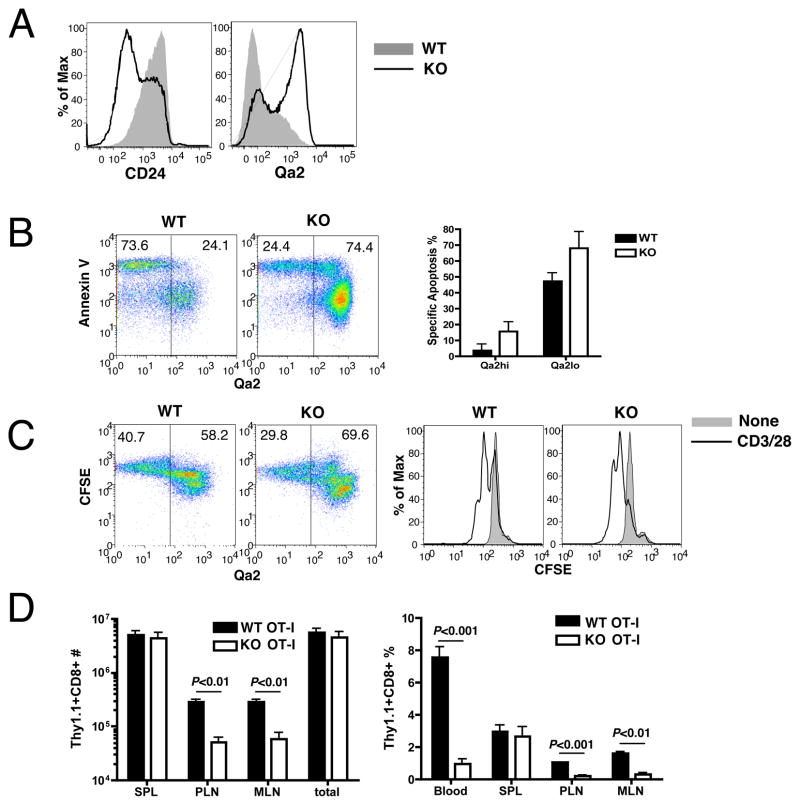
Figure 2. KLF2-deficient cells are functionally mature.
(A) Phenotype of naïve CD4SP thymocytes from KLF2fl/flCD4Cre (KO) or control (WT) mice. (B and C). KLF2fl/flCD4Cre (KO) or control (WT) thymocytes were stimulated with anti-CD3 and anti-CD28 in vitro. In (B) the dot plots shows Qa2 expression and Annexin V binding (18 hours after stimulation) on CD4SP cells. The bar graph shows quantification of specific apoptosis in Qa2hi versus Qa2lo CD4SP cells. In (C) Thymocytes were labeled with CFSE prior to stimulation. The dot plots show Qa2 expression and CFSE (48 hours after stimulation) on CD4SP gate. Histograms show CFSE dilution of Qa2hiCD4SP cells cultured with or without anti-CD3 and anti-CD28. (D) Thy1.1+CD8+CD4− thymocytes from OT-I KLF2−/+ (WT) or KLF2−/− (KO) fetal liver chimeras were transferred to B6 mice (0.75 × 105 cells/mouse). Recipient mice were injected i.p. with 5 × 106 PFU of vaccinia virus expressing OVA. Graphs show the number and frequency (in the live cell gate) of donor cells in different organs 5 days after infection. Representative of 2–3 independent experiments with similar results.
To address functional competence, we stimulated CD4SP thymocytes obtained from KLF2fl/flCD4Cre and control mice with anti-CD3 and anti-CD28. Apoptosis was measured by Annexin V binding at 18 hrs and proliferation measured by CFSE dye dilution at 48 hrs. In the control cultures, semi-mature (Qa2 low) SP thymocytes were most susceptible to apoptosis (Fig. 2B), whereas proliferation was detected only in the mature (Qa2 hi) SP subset (Fig. 2C). This distinction was maintained in KLF2-deficient cells (Fig. 2B,C). Even though KLF2 mutant thymocytes contained a smaller proportion of Qa2 low CD4 SP cells, such cells preferentially underwent apoptosis (Fig. 2B, bar graph). In addition, the prominent pool of KLF2-deficient Qa2 hi cells appeared to be functionally competent, since such cells divided and underwent minimal apoptosis following activation (Fig. 2B and C). Likewise, we saw similar responses by WT and KLF2−/− CD8 SP thymocytes in these assays, although there was considerable variability in the frequency and susceptibility to spontaneous apoptosis of semi-mature (Qa-2low, CD24high) CD8 SP (data not shown), making this analysis more difficult to interpret. Importantly, we found that KLF2 deficiency did not lead to spontaneous proliferation in either the CD4 or CD8 SP pools (Fig 2C and data not shown) in contrast to the dysregulated proliferation previously suggested following analysis of T cells isolated from the periphery of KLF2-deficient mice (6). It is possible that the few KLF2-deficient T cells that are found in peripheral tissues are abnormal, due to their impaired thymic egress and/or the profound T cell lymphopenia in such animals.
Next, we tested the ability of KLF2-deficient T cells to undergo activation and expansion in vivo. KLF2−/− and KLF2+/− OT-I TCR transgenic animals were generated and used to make fetal liver radiation chimeras (10). Thymocytes from these chimeras were transferred into congenic recipients that were then infected with a vaccinia virus strain expressing OVA. The total expansion of OT-I T cells at the peak of the response (day 5) was similar between KLF2−/− and KLF2+/− controls (Fig. 2D). Notably, however, the distribution of KLF2−/− T cells in recipients was distinct, being relatively low in blood and lymph nodes, but similar to controls in the spleen. These data suggested the KLF2-deficient T cells exhibited a normal initial proliferative responses to antigen in vivo, but showed defective trafficking, similar to that observed for naïve T cells (10).
KLF2 is required for expression of S1P1 and CD62L but not c-myc or p21cip1 in post-activated T cells
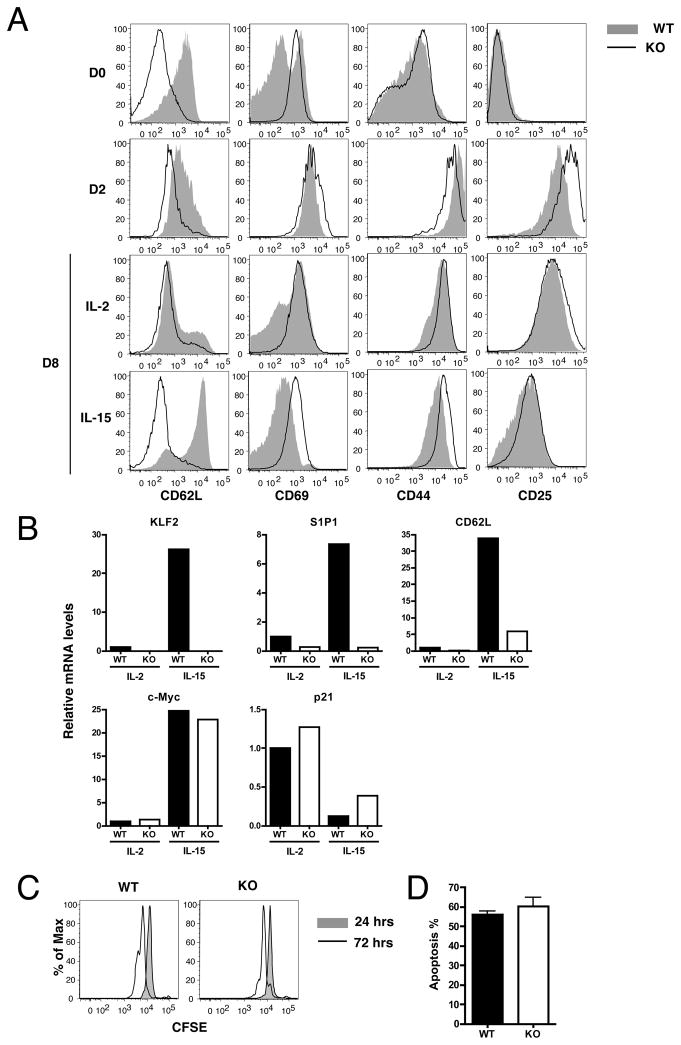
Our studies on gene expression by in vitro activated CD8 T cells suggested a correlation between KLF2 re-expression and induction of suitable trafficking (but not cell cycle) regulatory genes (Fig 1). However, these studies did not determine whether KLF2 re-expression was required for this expression of trafficking molecules. Furthermore, it was possible that KLF2 did control transcription of c-myc or p21Cip1, but not in the way initially predicted. Having established that KLF2-deficient T cells are functionally competent, we sought to explore these issues by analyzing gene expression by in vitro activated control and KLF2 deficient T cells. As mature KLF2 T cells cannot exit the thymus, we again used thymocytes from KLF2fl/flCD4Cre and KLF2fl/fl control mice, subjecting these cells to in vitro activation and subsequent culture with IL-2 or IL-15. Two days after stimulation, KLF2-deficient and control CD8SP cells were similar phenotypically, showing typical activation characteristics, including upregulation of CD44, CD25, and CD69. Expression of CD62L was still lower in KLF2-deficient than in control cells, but the difference between two groups was smaller after activation compared to the naïve populations. The two groups were also similar following subsequent IL-2 treatment, both pools exhibiting a CD62Llo, CD69hi phenotype. On the other hand, following IL-15 culture, KLF2-deficient CD8SP cells showed defective upregulation of CD62L and sustained CD69 expression, resulting in reduced frequency of (central) memory-like cells compared to control cells. After these cytokine treatments, expression of CD25 and CD44 were similar in both groups.
These phenotypic differences of CD69 and CD62L expression after IL-15 treatment were in accordance with real-time PCR results (Fig. 3B). As seen earlier, KLF2 mRNA was induced by IL-15 treatment, and this correlated with S1P1 and CD62L mRNA expression (Fig 3B): However, IL-15 treatment of KLF2 deficient cells resulted in minimal upregulation of S1P1 and CD62L mRNA (Fig. 3B). On the other hand, we observed no substantial differences between KLF2-deficient and control cells in expression of c-Myc and p21Cip1 mRNA (Fig 3B).
Figure 3. KLF2 deficiency does not affect the quiescence of post-activated CD8+ T cells.
Thymocytes were obtained from WT (KLF2fl/flCD4Cre) and KO (KLF2fl/fl) mice and were stimulated with anti-CD3 and irradiated splenocytes for 2 days. Viable cells were cultured in the presence of IL-2 or IL-15 for additional 6 days. (A) Shows changes of CD8+ T cell phenotype at various time points after activation. (B) At the end of culture (day 8), CD8+ cells were enriched by magnetic beads, and RNA was extracted. Expression of indicated genes were evaluated by real time PCR. Values are relative to the level of IL-2-treated WT group which was defined 1.0. (C) At day 8, cells were labeled with CFSE and further cultured with IL-15 for additional 24 and 72 hours. Histograms show the CFSE dilution in CD8SP gate. (D) At day 8, viable cells were enriched and Annexin V staining was examined before (0 hour) and after the 24-hour-culture in the presence of IL-15. Apoptosis during these 24 hours was calculated as described in materials and methods. Representative of 2–4 independent experiments with similar results.
In recent studies, we reported that some trafficking molecule, including β7-integrin and CXCR3, are dysregulated in KLF2 deficient animals due to non-autonomous, bystander effects in the thymus (13, 14). Hence, we might expect expression of these molecules in post-activated T cells to be independent of KLF2. Indeed, our analysis showed that CXCR3 and β7-integrin expression was similar on KLF2−/− and WT cells following in vitro stimulation and cytokine culture (Supplementary Figure 1).
IL-15 sustains the turn-over of memory CD8+ T cells, and this proliferation is at least partially dependent on c-Myc (34). Therefore, we then examined the effect of KLF2 deficiency on the proliferation of in-vitro induced memory-like cells in response to IL-15. As shown in Fig. 3C, there was no significant difference between KLF2-deficient and control cells at 72 hrs. In addition, the frequency of the cells undergoing to apoptosis also appeared to be equivalent between two groups (Fig. 3D). Together, these data suggest that post-activated KLF2 deficient thymocytes show a defect in expression of key trafficking molecules but not in transcription of cell cycle regulatory genes.
Impact of KLF2 gene deletion in post-activated T cells
Although T cell-specific deletion of floxed KLF2 gene by CD4Cre produced functionally competent mature thymocytes, it was difficult to exclude the possibility that alterations in development and their prolonged thymic retention might influence gene expression following activation. Furthermore, as we recently reported, IL-4 produced by the KLF2-deficient thymocytes can alter the phenotype and potentially the function of bystander cells (13, 14). Therefore we chose to use an inducible KLF2 knockout system, allowing us to study naïve peripheral T cells acutely deleted of the KLF2 gene. Splenocytes were obtained from KLF2fl/fl and control KLF2+/+ mice and, following in vitro activation for 48 hours, the cells were treated with TATCre, which penetrates into the cell nuclei in a dose dependent manner (35, 36). These cells were also transgenic for a YFP reporter locus (in which YFP expression is induced by Cre-mediated elimination of a floxed STOP cassette) allowing flow cytometric identification of cells which had acquired Cre during this in vitro culture. Following activation, the T cells were cultured in the presence of IL-2 or IL-15 to induce effector-like or memory-like CD8 T cells, respectively. As reported in similar TATCre systems (37), we saw expression of the YFP reporter on 40–60% of treated CD8+ T cells during our cultures, and this correlated with loss of KLF2 (see below).
As expected, YFP+CD8+ T cells showed reduction of CD62L and increase of CD69 in KLF2fl/fl compared to KLF2+/+ group following culture with IL-15 but not IL-2 (Fig. 4A). However, the defect in CD62L cell surface expression by induced KLF2-deficient cells was not as profound as that seen on conditional KLF2-deficient thymocytes (Fig 3A). Whether this difference is a consequence of the timing of KLF2 deletion (relative to initial expression of the CD62L gene) or the differentiation state of cell (thymocyte versus peripheral T cell) is not yet clear. On the other hand, expression of CD44 and CD25 was not affected by inducible KLF2 deletion. We then sorted YFP+CD8+ T cells from cells cultured with IL-15, and examined the mRNA expression by quantitative real-time PCR. As expected, KLF2 mRNA was lost in the YFP+ve KLF2fl/fl cells, but not YFP+ve KLF2+/+, suggesting efficient inducible deletion of KLF2 in this system. In addition, mRNA levels of S1P1 and CD62L was considerably lower in YFP+ve KLF2fl/fl cells compared to the KLF2+/+ group. Low S1P1 expression offers a likely explanation for the elevated CD69 protein expression levels on KLF2-deleted T cells, due to the mutual antagonism of CD69 and S1P1 for surface expression (30, 31). In contrast, the effect of KLF2 depletion on mRNA expression of the cell cycle regulators c-Myc and p21 was moderate at best. These results were similar to those in KLF2-deficient mature thymocytes from conditional knockout mice (Fig. 3A and B), supporting the relevance of former experiments to the function of KLF2 in peripheral T cells.
To examine the physiological significance of phenotypic changes caused by acute KLF2 deletion, we treated KLF2fl/fl and KLF2+/+ cells with Tat-Cre and cultured them with IL-15 before testing their homing capacity in vivo. In this experiment, IL-15-cultured memory-like CD8+ T cells were adoptively co-transferred with naive CD8+ T cells from the spleen of congenic normal mice that provide an internal control. Migration efficiency of these internal control cells was defined as 1.0 in Figure 4C. Twenty-four hours after transfer to normal mice, migration to the blood was similar between KLF2fl/fl and KLF2+/+ cells. In contrast, KLF2fl/fl cells migrated less efficiently than KLF2+/+ cells to secondary lymphoid organs, including the spleen and lymph nodes. In lymph nodes, migration of KLF2+/+ memory-like cells was similar to that of internal control naïve CD8+ T cells, as indicated by the homing index near 1.0 (Fig. 4C). On the other hand, KLF2+/+ memory-like cells migrated to the spleen 3-times more efficiently than internal control cells, while KLF2fl/fl memory-like cells showed splenic homing capability equivalent to control cells. Thus, KLF2 appeared important for normal homing to secondary lymphoid organs of post-activated T cells, whereas the quiescence of cells did not seem to be perturbed. The presence of KLF2-deficient cells in the blood was surprising, but could relate to the fact that these cells (unlike their WT counterparts) are denied access to lymph nodes, and so may remain in the circulation after iv transfer for the short duration of these studies.
These studies using an inducible KLF2 deletion approach argue that the requirement for KLF2 in induction of S1P1 and CD62L (but not c-Myc or p21Cip1) gene expression is not related to possible effects of KLF2 loss during thymic development.
KLF2 deficiency affects the cell distribution but not number after infection
To investigate the involvement of endogenous KLF2 in T cell quiescence and migration in vivo, we next examined the immune response of KLF2-deficient CD8+ T cells against infection and subsequent contraction and differentiation into memory cells. While some of the phenotypic abnormalities of thymocytes in polyclonal KLF2-deficient mice are due to non-autonomous bystander effects(13, 14), OT-I TCR Tg cells were spared from this bystander effect, providing a good tool to examine the direct effects of KLF2 deficiency(14). Equivalent numbers of KLF2-deficient and control OT-I Tg thymocytes (distinguished by congenic markers) were co-transferred into B6 mice, and the recipients infected with L. monocytogenes. As shown in Fig. 5A, in terms of total cell number, the kinetics of T cell response were very similar between the WT and KLF2-deficient groups, including the initial expansion, subsequent contraction and survival for memory differentiation. Once again, these data suggest that a loss of KLF2 does not lead to a dramatic defect in cell cycle regulation. However, KLF2-deficient cells were under represented compared to control cells in the lymph nodes and the blood (although the statistical significance between two groups was not obtained in the blood) (Fig. 5B). Similar to our in vitro studies (Fig. 3A and 4A), we found that KLF2-deficient donor cells showed decreased expression of CD62L and increased expression of CD69 at the memory stage (Fig. 5C). Thus, whereas there is no significant abnormality in the total cell number upon infection, tissue distribution of memory T cells was strikingly affected by KLF2 deficiency. A potential caveat for these studies is that the developmental “age” of WT versus KLF2−/− OT-I thymocytes is different, due to retention of KLF2−/− cells in the thymus. In preliminary studies we sorted CD24high, Qa-2low CD8 SP cells from WT and KLF2−/− OT-I thymocytes prior to adoptive transfer and LM-OVA, and we observed similar results to those obtained with bulk OT-I CD8 SP thymocytes (data not shown).
Discussion
KLF2 has been suggested to be a prototypical quiescence factor for T cells, affecting the expression of cell cycle regulators, including c-Myc and p21Cip1 (6–8, 38). However, this model largely relies on the findings from retroviral transfection into T cell lines, in which the resulting halt of autonomous tumor cell growth suggests that KLF2 can work as a quiescence factor when overexpressed (7, 8, 11). However, our previous study showed that KLF2-deficient mature thymocytes (which cannot leave the thymus) survive and persist after transfer to normal recipients even though exhibiting a trafficking defect (10). In addition, the proliferative response of these naïve thymocytes against TCR stimulation was quite normal both in vivo and in vitro (Figs. 2 and 5), suggesting the dispensability of KLF2 in naïve T cell quiescence.
In the present study, we examined the role of endogenous KLF2 in post-activated CD8+ T cells by cultured with IL-2 or IL-15, since these cytokines have strikingly-different effects on KLF2 re-expression in post-activated T cells (16),(17)(Fig. 1A). Expression of c-Myc and p21Cip1 in post-activated CD8+ T cells was also strongly influenced by the specific cytokines used for culture, but in contrast to the conclusion from previous studies (7, 8) this regulation was entirely independent of endogenous KLF2 (Fig. 1A, 3B and 4B). On the other hand, expression of the trafficking molecules S1P1 and CD62L was dramatically impaired by KLF2 deficiency in post-activated CD8 T cells (Figs. 3 and 4). Furthermore, the in vivo antigen specific response of KLF2 deficient CD8 T cells was characterized by defective trafficking but no change in expansion or contraction dynamics (Fig. 5). These findings reinforce the model that KLF2 is pivotal for normal T cell migration, but dispensable for T cell quiescence.
Recent studies reported the involvement of the signaling through phosphatidylinositol-3-OH kinase (PI3K) - Akt signaling pathway in negatively regulating KLF2 expression in T cells. One group showed that mammalian target of rapamycin (mTOR) suppresses KLF2 expression downstream of PI3K and Akt (17), while another group suggested that the Akt regulated transcription factor Foxo1 induces KLF2 transcription (39). In either case, differential expression of KLF2 by IL-2 versus IL-15 in post-activated T cells could be attributed to the fact that IL-2, but not IL-15, sustains high PI3K-Akt signaling (17). Cantrell and colleagues recently reported that optimal activation of PI3K-Akt pathway with functional PDK1 is required for the downregulation of KLF2, CD62L and S1P1 (40). In contrast, proliferation of the T cells after TCR stimulation was not affected even with the suboptimal activation PI3K-Akt pathway with PDK1 mutation, indicating the proliferation in primary antigen stimulation is independent of KLF2 loss (40). This is in accordance with our overall observation that the expression of trafficking molecules but not cell cycle regulatory genes was influenced by endogenous KLF2 expression.
Molecular mechanism of homing to the spleen remains unclear, while the migration into the lymph nodes is quite well defined. It was previously reported that memory-like cells induced in vitro by antigen stimulation and subsequent IL-15 treatment show enhanced migration to the spleen compared to naïve T cells and IL-2-treated effector-like cells (25). Consistent with this finding, we observed WT IL-15-treated memory like CD8+ T cells accumulate in the spleen 3 times more efficiently than naïve CD8+ T cells did 24 hours after transfer into normal recipient mice (Fig. 4C). On the other hand, notably, KLF2-deficient memory-like cells migrated to the spleen only as well as naïve CD8 T cells, suggesting that efficient migration of wild-type IL-15-treated memory-like cells to the spleen is dependent on KLF2 (Fig. 4C). As has been reported, normal memory cells migrate to the spleen similarly to (or slightly less efficiently than) naïve cells (41–44), and indeed, KLF2-deficient and -sufficient memory cells generated in vivo after infection were present in the spleen at similar frequencies (Fig. 5B). Further analysis of KLF2-sufficient and deficient IL-15-treated cells will be useful to better understand the mechanisms regulating splenic migration.
It is possible that regulation of T cell quiescence is redundantly controlled by multiple KLF family members (of which there are many (3, 45–47)) besides KLF2. KLF4 is closely related to KLF2, and a recent study found that inducible deletion of KLF4 leads to enhanced proliferation of CD8+ T cells in response to primary TCR stimulation (48), although T cell function was evaluated at 9 months following the induction of KLF4 gene deletion in those studies, and it is not clear whether the effects were mediated primarily on naïve T cells. To further explore this issue, we analyzed gene expression following activation of T cells deficient for both KLF2 and KLF4 (data not shown), but observed minimal differences compared to T cells deficient for KLF2 alone (data not shown). Hence these data further argue against a key role for KLF2 (and similar factors) in physiological regulation of c-myc and p21Cip1 expression in post-activated T cells.
At the same time, our data do not exclude a model that KLF2 (and/or KLF4) may be capable of repressing c-Myc and inducing p21Cip1 under some situations of activation or during T cell development. Our studies focus on CD8 T cells stimulated with cognate antigen but other stimuli, such as lymphopenia driven proliferation, also induce naïve T cell expansion. Furthermore, c-Myc is induced at key proliferative steps of T cell development (49), and it is possible that KLF2 expression during T differentiation will be important for control of cell cycle progression. Nevertheless, our data suggest the expression of KLF2 is not critical for cell cycle restraint following mature CD8 T cell activation.
In conclusion, we show that endogenous KLF2 expression plays a non-redundant function in regulating the expression of trafficking molecules and controlling migration of post-activated CD8+ T cells, yet we were unable to demonstrate a critical role of KLF2 in regulating key cell-cycle T cell proliferation, our data might also argue that the documented impact of forced KLF2 expression on inducing cellular quiescence is a result of non-physiologically high expression levels of these transcription factors. Further studies will be needed to explore this finding further.
Supplementary Material
Acknowledgments
We thank Dr. Donna Farber (Univ. Maryland) for the generous gift of Tat-Cre, Dr. Frank Costantini (Columbia) for kindly providing key mouse strains, and Drs. John Harty (Univ. Iowa) and Hao Shen (Univ. Pennsylvania) for actA- LM-OVA. We appreciate the input of all the Jamequist lab members during design and analysis of these studies.
Abbreviations used in this article
- KLF
Kruppel-like factor
- KO
knockout
- OVAp
OVA peptide
- SP
single positive
- S1P1
sphingosine-1-phosphate receptor-1
- TAT
transactivator of transcription
- Tg
transgenic
- WT
wild-type
Footnotes
This work was supported by NIH grant R37AI38903 (to S.C.J.) and by a fellowship from the Japanese Society for the Promotion of Science (to K.T.).
References
- 1.Suzuki T, Aizawa K, Matsumura T, Nagai R. Vascular implications of the Kruppel-like family of transcription factors. Arterioscler Thromb Vasc Biol. 2005;25:1135–1141. doi: 10.1161/01.ATV.0000165656.65359.23. [DOI] [PubMed] [Google Scholar]
- 2.Haldar SM, Ibrahim OA, Jain MK. Kruppel-like Factors (KLFs) in muscle biology. J Mol Cell Cardiol. 2007;43:1–10. doi: 10.1016/j.yjmcc.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczynski J, Cook T, Urrutia R. Sp1- and Kruppel-like transcription factors. Genome Biol. 2003;4:206. doi: 10.1186/gb-2003-4-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner J, Crossley M. Mammalian Kruppel-like transcription factors: more than just a pretty finger. Trends Biochem Sci. 1999;24:236–240. doi: 10.1016/s0968-0004(99)01406-1. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, Robson P, Zhong S, Ng HH. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 6.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 7.Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc--dependent pathway. Nat Immunol. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Lingrel JB. KLF2 inhibits Jurkat T leukemia cell growth via upregulation of cyclin-dependent kinase inhibitor p21WAF1/CIP1. Oncogene. 2004;23:8088–8096. doi: 10.1038/sj.onc.1207996. [DOI] [PubMed] [Google Scholar]
- 9.Haaland RE, Yu W, Rice AP. Identification of LKLF-regulated genes in quiescent CD4+ T lymphocytes. Mol Immunol. 2005;42:627–641. doi: 10.1016/j.molimm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 11.Bai A, Hu H, Yeung M, Chen J. Kruppel-like factor 2 controls T cell trafficking by activating L-selectin (CD62L) and sphingosine-1-phosphate receptor 1 transcription. J Immunol. 2007;178:7632–7639. doi: 10.4049/jimmunol.178.12.7632. [DOI] [PubMed] [Google Scholar]
- 12.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]
- 13.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endrizzi BT, Jameson SC. Differential role for IL-7 in inducing lung Kruppel-like factor (Kruppel-like factor 2) expression by naive versus activated T cells. Int Immunol. 2003;15:1341–1348. doi: 10.1093/intimm/dxg133. [DOI] [PubMed] [Google Scholar]
- 16.Schober SL, Kuo CT, Schluns KS, Lefrancois L, Leiden JM, Jameson SC. Expression of the transcription factor lung Kruppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J Immunol. 1999;163:3662–3667. [PubMed] [Google Scholar]
- 17.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grayson JM, Murali-Krishna K, Altman JD, Ahmed R. Gene expression in antigen-specific CD8+ T cells during viral infection. J Immunol. 2001;166:795–799. doi: 10.4049/jimmunol.166.2.795. [DOI] [PubMed] [Google Scholar]
- 19.Riou C, Yassine-Diab B, Van grevenynghe J, Somogyi R, Greller LD, Gagnon D, Gimmig S, Wilkinson P, Shi Y, Cameron MJ, Campos-Gonzalez R, Balderas RS, Kelvin D, Sekaly RP, Haddad EK. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Zhu Y, Huang Y, McAvoy S, Johnson WB, Cheung TH, Chung TK, Lo KW, Yim SF, Yu MM, Ngan HY, Wong YF, Smith DI. Transcriptional repression of WEE1 by Kruppel-like factor 2 is involved in DNA damage-induced apoptosis. Oncogene. 2005;24:3875–3885. doi: 10.1038/sj.onc.1208546. [DOI] [PubMed] [Google Scholar]
- 21.Wani MA, Means RT, Jr, Lingrel JB. Loss of LKLF function results in embryonic lethality in mice. Transgenic Res. 1998;7:229–238. doi: 10.1023/a:1008809809843. [DOI] [PubMed] [Google Scholar]
- 22.Villunger A, V, Marsden S, Zhan Y, Erlacher M, Lew AM, Bouillet P, Berzins S, Godfrey DI, Heath WR, Strasser A. Negative selection of semimature CD4(+)8(−)HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7052–7057. doi: 10.1073/pnas.0305757101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haring JS, Corbin GA, Harty JT. Dynamic regulation of IFN-gamma signaling in antigen-specific CD8+ T cells responding to infection. J Immunol. 2005;174:6791–6802. doi: 10.4049/jimmunol.174.11.6791. [DOI] [PubMed] [Google Scholar]
- 24.Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med. 2009;206:2253–2269. doi: 10.1084/jem.20082553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weninger W, Crowley MA, Manjunath N, von Andrian UH. Migratory properties of naive, effector, and memory CD8(+) T cells. J Exp Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iezzi G, Scheidegger D, Lanzavecchia A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J Exp Med. 2001;193:987–993. doi: 10.1084/jem.193.8.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 29.Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 30.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 31.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 33.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi T, Gasser S, Trumpp A, MacDonald HR. c-Myc acts downstream of IL-15 in the regulation of memory CD8 T-cell homeostasis. Blood. 2006;107:3992–3999. doi: 10.1182/blood-2005-09-3851. [DOI] [PubMed] [Google Scholar]
- 35.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 36.Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4489–4494. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 39.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waugh C, Sinclair L, Finlay D, Bayascas J, Cantrell D. PI(3,4,5)P3 binding to Phosphoinositide dependent kinase 1 regulates a Protein Kinase B/Akt signalling threshold that dictates T cell migration not proliferation. Mol Cell Biol. 2009 doi: 10.1128/MCB.00585-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerwenka A, Morgan TM, Dutton RW. Naive, effector, and memory CD8 T cells in protection against pulmonary influenza virus infection: homing properties rather than initial frequencies are crucial. J Immunol. 1999;163:5535–5543. [PubMed] [Google Scholar]
- 42.Tietz W, Hamann A. The migratory behavior of murine CD4+ cells of memory phenotype. European journal of immunology. 1997;27:2225–2232. doi: 10.1002/eji.1830270916. [DOI] [PubMed] [Google Scholar]
- 43.Williams MB, Butcher EC. Homing of naive and memory T lymphocyte subsets to Peyer’s patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
- 44.Bradley LM, Harbertson J, Watson SR. Memory CD4 cells do not migrate into peripheral lymphnodes in the absence of antigen. European journal of immunology. 1999;29:3273–3284. doi: 10.1002/(SICI)1521-4141(199910)29:10<3273::AID-IMMU3273>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Fruman DA. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr Opin Immunol. 2004;16:314–320. doi: 10.1016/j.coi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol Rev. 2000;176:216–246. doi: 10.1034/j.1600-065x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 47.Teague TK, Hildeman D, Kedl RM, Mitchell T, Rees W, Schaefer BC, Bender J, Kappler J, Marrack P. Activation changes the spectrum but not the diversity of genes expressed by T cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:12691–12696. doi: 10.1073/pnas.96.22.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada T, Park CS, Mamonkin M, Lacorazza HD. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Kruppel-like factors KLF4 and KLF2. Nat Immunol. 2009;10:618–626. doi: 10.1038/ni.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang CY, Bredemeyer AL, Walker LM, Bassing CH, Sleckman BP. Dynamic regulation of c-Myc proto-oncogene expression during lymphocyte development revealed by a GFP-c-Myc knock-in mouse. European journal of immunology. 2008;38:342–349. doi: 10.1002/eji.200737972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.