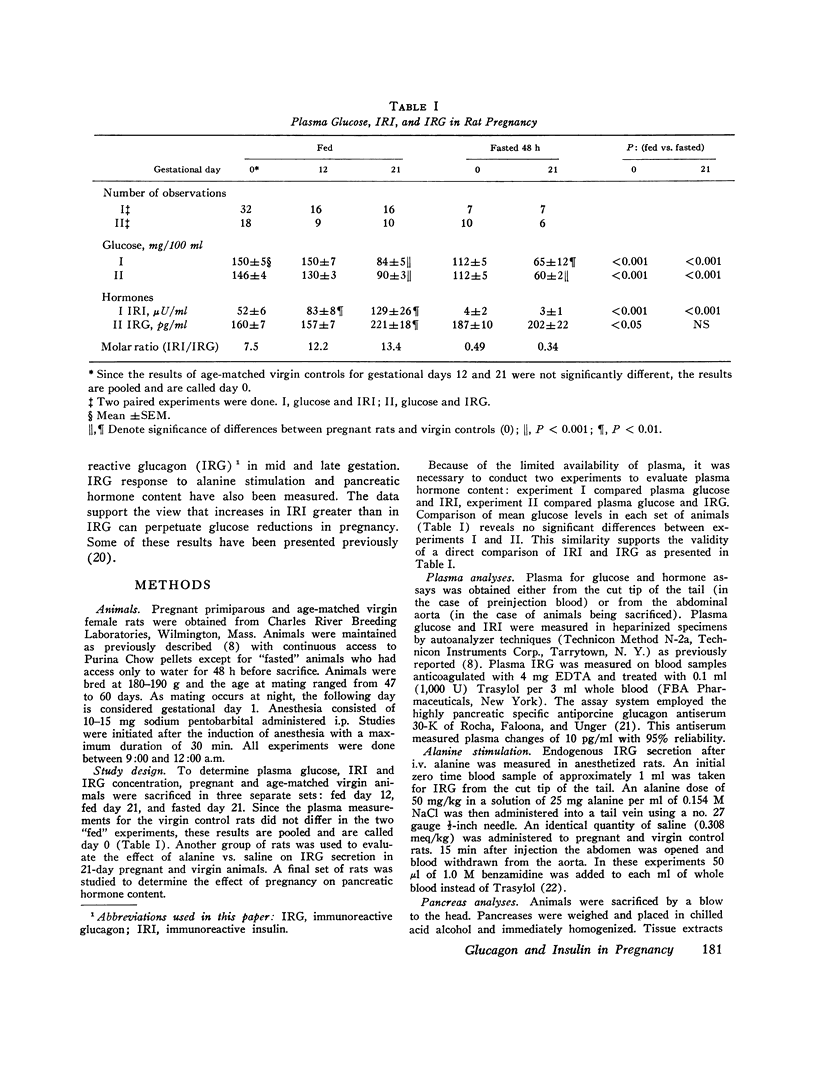
Abstract
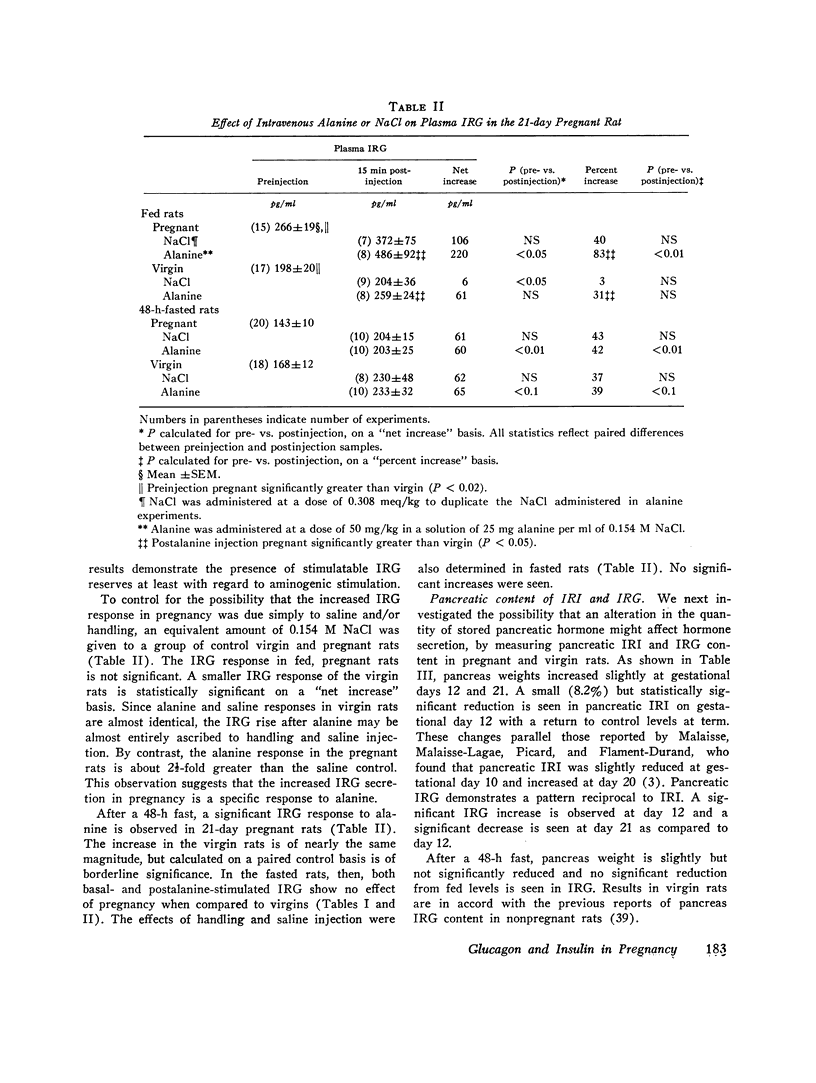
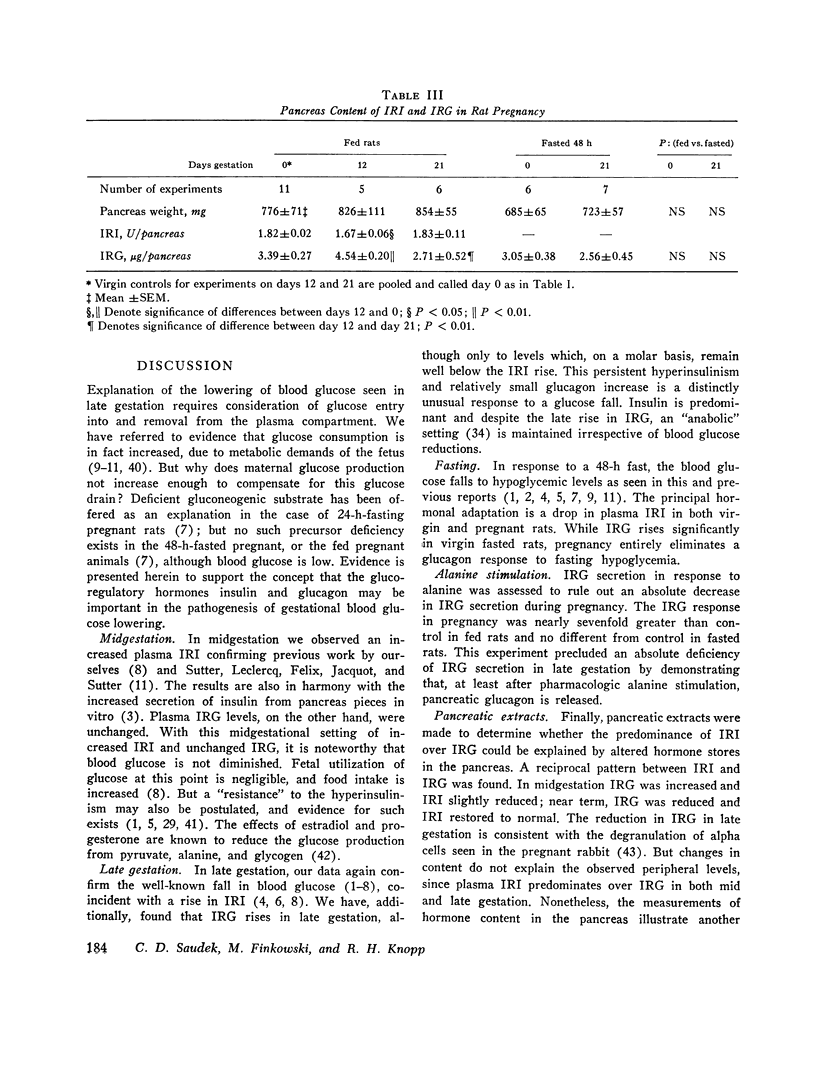
To determine if pancreatic glucoregulatory hormones can be implicated in the glucose fall of pregnancy, we have measured plasma immunoreactive insulin and glucagon (IRI and IRG) in rats. Fed rats in midgestation show a rise in IRI without a corresponding increase in IRG. In late gestation, IRG rises significantly, but only enough to keep pace with a further rise in IRI. On a molar basis, IRI remains the predominant hormone despite a marked fall in blood glucose. After a 48-h fast IRI falls to comparably low levels in pregnant and virgin rats. A small rise in IRG is seen in virgin but not in pregnant rats despite frank hypoglycemia in the latter. Thus, IRG secretion in pregnancy is diminished relative to IRI in the fed state and fails to increase in the fasted state despite the stimulus of a lower glucose in both instances. To evaluate IRG secretory reserve, the IRG response to i.v. alanine was assessed in late gestation. In fed rats a greater IRG increase is seen in pregnancy; after fasting no difference is seen between pregnant and virgin rats. These results preclude an absolute deficiency in glucagon secretion. Pancreas hormone stores were alos measured in an effort to explain the altered secretory state. We find reciprocal changes in IRI and IRG content favoring IRG in midgestation and IRI in late gestation. Thus, pancreas hormone storage is altered in pregnancy but does not account for the changes in hormone secretion. Rather, pregnancy exerts an effect on the islet secretory process itself. Release of IRI is enhanced relative to IRG regardless of the blood sugar level. These observations suggest that in the pregnant rat circulating levels of insulin and glucagon may act to limit hepatic glucose output. Available evidence from the literature supports the concept of restrained glucose production. It is proposed that a lower blood glucose production. It is proposed that a lower blood glucose in rat pregnancy may be a lesser liability teleologically than would be the obligate nitrogen wasting which accompanies gluconeogenesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER N., SHIPLEY R. A., CLARK R. E., INCEFY G. E. C14 studies in carbohydrate metabolism: glucose pool size and rate of turnover in the normal rat. Am J Physiol. 1959 Feb;196(2):245–252. doi: 10.1152/ajplegacy.1959.196.2.245. [DOI] [PubMed] [Google Scholar]
- BEATON G. H., BEARE J., RYU M. H., McHENRY E. W. Protein metabolism in the pregnant rat. J Nutr. 1954 Oct 11;54(2):291–304. doi: 10.1093/jn/54.2.291. [DOI] [PubMed] [Google Scholar]
- BEATON G. H. Urea formation in the pregnant rat. Arch Biochem Biophys. 1957 Mar;67(1):1–9. doi: 10.1016/0003-9861(57)90240-0. [DOI] [PubMed] [Google Scholar]
- BLEICHER S. J., O'SULLIVAN J. B., FREINKEL N. CARBOHYDRATE METABOLISM IN PREGNANCY. V. THE INTERRELATIONS OF GLUCOSE, INSULIN AND FREE FATTY ACIDS IN LATE PREGNANCY AND POST PARTUM. N Engl J Med. 1964 Oct 22;271:866–872. doi: 10.1056/NEJM196410222711702. [DOI] [PubMed] [Google Scholar]
- CURRY D. M., BEATON G. H. Cortisone resistance in pregnant rats. Endocrinology. 1958 Aug;63(2):155–161. doi: 10.1210/endo-63-2-155. [DOI] [PubMed] [Google Scholar]
- Costrini N. V., Kalkhoff R. K. Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J Clin Invest. 1971 May;50(5):992–999. doi: 10.1172/JCI106593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant Y. Z., Shafrir E. Enzymes of carbohydrate and lipid metabolism in the placenta and liver of pregnant rats. Biochim Biophys Acta. 1972 Oct 25;279(3):424–430. doi: 10.1016/0304-4165(72)90163-8. [DOI] [PubMed] [Google Scholar]
- Driskell J. A., Wiley J. H., Kirksey A. Alanine aminotransferase activity in liver and erythrocytes of pregnant and nonpregnant rats fed different levels of pyridoxine. J Nutr. 1971 Jan;101(1):85–91. doi: 10.1093/jn/101.1.85. [DOI] [PubMed] [Google Scholar]
- Eisenstein A. B., Strack I., Steiner A. Glucagon stimulation of hepatic gluconeogenesis in rats fed a high-protein, carbohydrate-free diet. Metabolism. 1974 Jan;23(1):15–23. doi: 10.1016/0026-0495(74)90099-7. [DOI] [PubMed] [Google Scholar]
- Ensinck J. W., Shepard C., Dudl R. J., Williams R. H. Use of benzamidine as a proteolytic inhibitor in the radioimmunoassay of glucagon in plasma. J Clin Endocrinol Metab. 1972 Sep;35(3):463–467. doi: 10.1210/jcem-35-3-463. [DOI] [PubMed] [Google Scholar]
- Felig P., Kim Y. J., Lynch V., Hendler R. Amino acid metabolism during starvation in human pregnancy. J Clin Invest. 1972 May;51(5):1195–1202. doi: 10.1172/JCI106913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Lynch V. [Starvation in human pregnancy: hypoglycemia, hypoinsulinemia, and hyperketonemia]. Science. 1970 Nov 27;170(3961):990–992. doi: 10.1126/science.170.3961.990. [DOI] [PubMed] [Google Scholar]
- Girard J. R., Cuendet G. S., Marliss E. B., Kervran A., Rieutort M., Assan R. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest. 1973 Dec;52(12):3190–3200. doi: 10.1172/JCI107519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinsmann W. H., Mortimore G. E. Influence of glucagon and 3', 5'-AMP on insulin responsiveness of the perfused rat liver. Am J Physiol. 1968 Sep;215(3):553–559. doi: 10.1152/ajplegacy.1968.215.3.553. [DOI] [PubMed] [Google Scholar]
- Gresham E. L., Simons P. S., Battaglia F. C. Maternal-fetal urea concentration difference in man: metabolic significance. J Pediatr. 1971 Nov;79(5):809–811. doi: 10.1016/s0022-3476(71)80396-7. [DOI] [PubMed] [Google Scholar]
- HAGEN A. Blood sugar findings during pregnancy in normals and possible prediabetics. Diabetes. 1961 Nov-Dec;10:438–444. doi: 10.2337/diab.10.6.438. [DOI] [PubMed] [Google Scholar]
- HAGERMAN D. D. Metabolism of tissues from pregnant, diabetic rats in vitro. Endocrinology. 1962 Jan;70:88–94. doi: 10.1210/endo-70-1-88. [DOI] [PubMed] [Google Scholar]
- Hager D., Georg R. H., Leitner J. W., Beck P. Insulin secretion and content in isolated rat pancreatic islets following treatment with gestational hormones. Endocrinology. 1972 Oct;91(4):977–981. doi: 10.1210/endo-91-4-977. [DOI] [PubMed] [Google Scholar]
- Harding H. R., Rosen F., Nichol C. A. Effects of pregnancy on several cortisol-responsive enzymes in rat liver. Am J Physiol. 1966 Dec;211(6):1361–1365. doi: 10.1152/ajplegacy.1966.211.6.1361. [DOI] [PubMed] [Google Scholar]
- Heath D. F., Rose J. G. The distribution of glucose and [14C]glucose between erythrocytes and plasma in the rat. Biochem J. 1969 Apr;112(3):373–377. doi: 10.1042/bj1120373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E., Knopp R. A. Pentose monophosphate shunt dehydrogenases and fatty acid synthesis in late rat pregnancy. Experientia. 1972 Jun 15;28(6):646–647. doi: 10.1007/BF01944953. [DOI] [PubMed] [Google Scholar]
- Herrera E., Knopp R. H., Freinkel N. Carbohydrate metabolism in pregnancy. VI. Plasma fuels, insulin, liver composition, gluconeogenesis, and nitrogen metabolism during late gestation in the fed and fasted rat. J Clin Invest. 1969 Dec;48(12):2260–2272. doi: 10.1172/JCI106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoff R. K., Jacobson M., Lemper D. Progesterone, pregnancy and the augmented plasma insulin response. J Clin Endocrinol Metab. 1970 Jul;31(1):24–28. doi: 10.1210/jcem-31-1-24. [DOI] [PubMed] [Google Scholar]
- Knopp R. H., Herrera E., Freinkel N. Carbohydrate metabolism in pregnancy. 8. Metabolism of adipose tissue isolated from fed and fasted pregnant rats during late gestation. J Clin Invest. 1970 Jul;49(7):1438–1446. doi: 10.1172/JCI106361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp R. H., Ruder H. J., Herrera E., Freinkel N. Carbohydrate metabolism in pregnancy. VII. Insulin tolerance during late pregnancy in the fed and fasted rat. Acta Endocrinol (Copenh) 1970 Oct;65(2):352–360. [PubMed] [Google Scholar]
- Knopp R. H., Saudek C. D., Arky R. A., O'Sullivan J. B. 2 phases of adipose tissue metabolism in pregnancy: maternal adaptations for fetal growth. Endocrinology. 1973 Apr;92(4):984–988. doi: 10.1210/endo-92-4-984. [DOI] [PubMed] [Google Scholar]
- Lopez-Quijada C., Gomez-Acebo J., Candela J. L. Decrease in the insulin of rabbit pancreas in late pregnancy. Diabetologia. 1967 Oct;3(5):435–442. doi: 10.1007/BF01228079. [DOI] [PubMed] [Google Scholar]
- Mackrell D. J., Sokal J. E. Antagonism between the effects of insulin and glucagon on the isolated liver. Diabetes. 1969 Nov;18(11):724–732. doi: 10.2337/diab.18.11.724. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Picard C., Flament-Durand J. Effects of pregnancy and chorionic growth hormone upon insulin secretion. Endocrinology. 1969 Jan;84(1):41–44. doi: 10.1210/endo-84-1-41. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Wright P. H. Effect of fasting upon insulin secretion in the rat. Am J Physiol. 1967 Oct;213(4):843–848. doi: 10.1152/ajplegacy.1967.213.4.843. [DOI] [PubMed] [Google Scholar]
- Matute M. L., Kalkhoff R. K. Sex steroid influence on hepatic gluconeogenesis and glucogen formation. Endocrinology. 1973 Mar;92(3):762–768. doi: 10.1210/endo-92-3-762. [DOI] [PubMed] [Google Scholar]
- Metzger B. E., Agnoli F. S., Hare J. W., Freinkel N. Carbohydrate metabolism in pregnancy. X. Metabolic disposition of alanine by the perfused liver of the fasting pregnant rat. Diabetes. 1973 Aug;22(8):601–612. doi: 10.2337/diab.22.8.601. [DOI] [PubMed] [Google Scholar]
- Metzger B. E., Hare J. W., Freinkel N. Carbohydrate metabolism in pregnancy. IX. Plasma levels of gluconeogenic fuels during fasting in the rat. J Clin Endocrinol Metab. 1971 Nov;33(5):869–872. doi: 10.1210/jcem-33-5-869. [DOI] [PubMed] [Google Scholar]
- Morishige W. K., Pepe G. J., Rothchild I. Serum luteinizing hormone, prolactin and progesterone levels during pregnancy in the rat. Endocrinology. 1973 May;92(5):1527–1530. doi: 10.1210/endo-92-5-1527. [DOI] [PubMed] [Google Scholar]
- O'Sullivan J. B., Snyder P. J., Sporer A. C., Dandrow R. V., Jr, Charles D. Intravenous glucose tolerance test and its modification by pregnancy. J Clin Endocrinol Metab. 1970 Jul;31(1):33–37. doi: 10.1210/jcem-31-1-33. [DOI] [PubMed] [Google Scholar]
- Rishi S., Golob E. K., Becker K. L., Shah N. Pancreatic insulin content of nonpregnant, pregnant and postpartum rats and the developing rat fetus. Diabetes. 1969 May;18(5):268–272. doi: 10.2337/diab.18.5.268. [DOI] [PubMed] [Google Scholar]
- Roberge A., Charbonneau R., Berlinguet L. Variation of the enzymes of the urea cycle and aspartate transcarbamylase in liver of pregnant rats. Can J Biochem. 1967 Sep;45(9):1371–1374. doi: 10.1139/o67-161. [DOI] [PubMed] [Google Scholar]
- Rocha D. M., Faloona G. R., Unger R. H. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972 Sep;51(9):2346–2351. doi: 10.1172/JCI107046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., BRINLEY M. S. HYPERLIPEMIA AND KETOSIS IN THE PREGNANT RAT. Am J Physiol. 1964 Apr;206:796–804. doi: 10.1152/ajplegacy.1964.206.4.796. [DOI] [PubMed] [Google Scholar]
- SILVERSTONE F. A., SOLOMONS E., RUBRICIUS J. The rapid intravenous glucose tolerance test in pregnancy. J Clin Invest. 1961 Dec;40:2180–2189. doi: 10.1172/JCI104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Daughaday W. H., Kipnis D. M. Regulation of immunoreactive growth hormone secretion in male rats. Endocrinology. 1971 Apr;88(4):909–917. doi: 10.1210/endo-88-4-909. [DOI] [PubMed] [Google Scholar]
- Tyson J. E., Rabinowitz D., Merimee T. J., Friesen H. Response of plasma insulin and human growth hormone to arginine in pregnant and postpartum females. Am J Obstet Gynecol. 1969 Feb 1;103(3):313–319. doi: 10.1016/0002-9378(69)90488-8. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Müller W. A., Faloona G. R. Insulin glucagon ration. Trans Assoc Am Physicians. 1971;84:122–129. [PubMed] [Google Scholar]
- Wiest W. G. Progesterone and 20-alpha-hydroxypregn-4-en-3-one in plasma, ovaries and uteri during pregnancy in the rat. Endocrinology. 1970 Jul;87(1):43–48. doi: 10.1210/endo-87-1-43. [DOI] [PubMed] [Google Scholar]
- Wuttke W., Gelato M., Meites J. Mechanisms of pentobarbital actions on prolactin release. Endocrinology. 1971 Nov;89(5):1191–1194. doi: 10.1210/endo-89-5-1191. [DOI] [PubMed] [Google Scholar]


