Abstract
Using a novel blinded intra-patient vehicle control design, we conducted a phase II study of topically-administered halofuginone, an angiogenesis inhibitor that inhibits collagen type-I and matrix metalloproteinases (MMPs), in patients with AIDS-related Kaposi’s sarcoma (KS). Serial KS biopsies assessed treatment effects on angiogenic factors and KSHV-LANA. We observed marked heterogeneity of KSHV-LANA expression. Although the small number of subjects whose response could be evaluated precluded definitive assessment of halofuginone’s efficacy, we observed a significant decrease in type-I collagen only in halofuginone-treated lesions, but no effect on MMP-2. The trial design is applicable to future studies of topical agents.
Keywords: Kaposi’s sarcoma, halofuginone, type-I collagen, angiogenesis, matrix metalloproteinases
INTRODUCTION
Kaposi’s sarcoma (KS), a multifocal angioproliferative disease, is driven by infection with the KS herpesvirus/human herpesvirus-8 (KSHV/HHV8) [1–3]. Various cytokines acting by autocrine and paracrine mechanisms establish an angiogenic milieu resulting in the vascular proliferation that characterizes KS lesions [4–8].
Angiogenesis involves local proliferation of endothelial cells (ECs), degradation of subendothelial capillary basement membrane (BM), migration of ECs and formation of vascular sprouts [9]. During angiogenesis, matrix metalloproteinases (MMPs) degrade BM, and MMP inhibitors inhibit angiogenesis. KSHV-infected cells release MMPs into culture medium [10]. The HIV Tat protein and basic fibroblast growth factor (bFGF) induce MMPs, and Tat-transgenic mice develop KS-like lesions [11]. Collagen type-I also plays a critical role in assembly of new vasculature and it too may be a potential target [12]. Thus, inhibitors of MMPs and collagen type-I are potential therapeutic agents for KS.
Halofuginone (Tempostatin™), a synthetic quinazolinone alkaloid derivative, induced anti-angiogenic, anti-metastatic and anti-proliferative effects in preclinical studies [13]. It exerts anti-proliferative effects on various cell types and lines, including bovine aortic ECs [14]. It also inhibits several essential stages of angiogenesis, namely, EC proliferation, MMP-2 expression, BM invasion and extracellular matrix deposition by newly formed vessels, and inhibits collagen type-I synthesis during angiogenic sprouting [13–16]. Inhibition of type-I collagen and transforming growth factor-β signaling may partly explain halofuginone’s anti-fibrotic properties in vitro and in vivo [17–19]. Halofuginone also inhibits bFGF-induced neovascularization [13]. These data suggested that halofuginone might have activity in KS. Therefore, the AIDS Malignancy Consortium undertook a phase II study of topical halofuginone in patients with AIDS-related KS using a novel design with blinded intra-patient vehicle control to evaluate its clinical activity and ability to inhibit its proposed targets.
PATIENTS AND METHODS
Patients
Eligible subjects were HIV-seropositive, ≥ 16 years old, with biopsy-proven KS, life expectancy ≥3 months, KPS≥60, and adequate hematologic, renal and liver function. Negative serum β-HCG and contraception was required for women of childbearing potential.
Subjects receiving antiretroviral therapy (ART) had to be on a stable regimen for ≥12 weeks without KS regression. At least 14 KS skin lesions were required, of which 12 needed to be bi-dimensionally measurable and ≥0.5cm in shortest dimension.
Subjects were excluded for: visceral KS or edema requiring chemotherapy; anti-neoplastic treatment within four weeks; local therapy of any treated lesion within 60 days; use of corticosteroids or investigational agents; active infections or serious medical illnesses within 14 days; pregnancy or breast feeding.
Study Drug
Halofuginone (NSC 713205) was supplied by Collgard Biopharmaceuticals Ltd (Atlanta, GA) to the National Cancer Institute (NCI) Division of Cancer Treatment and Diagnosis (DCTD) under a Cooperative Research and Development Agreement as a 0.01% w/w ointment and distributed by the Pharmaceutical Management Branch (PMB), Cancer Therapy Evaluation Program, DCTD, NCI. In addition to the active ingredient, the ointment contained beeswax, paraffin oil, white petrolatum, borax, and deionized water. The matching vehicle contained only the inactive ingredients. Ointment tubes were stored at 2–8°C.
Treatment
After obtaining written informed consent, 12 measurable lesions were divided into two groups of six, designated Group A and Group B. Subjects were supplied with diagrams and/or photographs to facilitate lesion identification. For the first 12 weeks, tubes designated A and B containing either halofuginone or vehicle ointments were supplied in a blinded fashion. Ointment A was applied to Group A lesions and ointment B was applied to Group B lesions twice daily. An option for 12 weeks of additional open-label halofuginone was available to subjects with stable or responding disease.
Criteria were included for treatment modification and dose-limiting toxicity if systemic adverse events (AEs) occurred. For ≥grade 3 local toxicity, treatment was held until the grade decreased to ≤1 and then resumed once-daily. If ≥grade 3 local toxicity recurred on the reduced-dose schedule, the subject was withdrawn.
Monitoring
Baseline and on-treatment measurements included CBC, serum chemistries, CD4 counts and percentages, HIV-1 and KSHV viral loads, vital signs, performance status and tumor assessments. In addition, 4mm punch biopsies of two KS lesions were obtained at baseline for measurement of secondary endpoints. On-study biopsies of one lesion each from Group A and Group B were performed at days 29 and 85.
KS Evaluations
To be evaluable for response, three 4-week cycles of treatment were required. KS response was evaluated at 4-week intervals using two assessment methods, Treatment Response (TR) and Overall Response (OR). TR evaluated only treated lesions, and measured local efficacy. Specifically, complete TR was total resolution of all lesions in a group. Partial TR (PR) was a ≥50% decrease in the sum of the products of the largest perpendicular diameters of the six lesions in each group, without an increase in the number of raised lesions, and/or a decrease in the number of raised lesions from ≥4 to ≤2, without an increase in the sum of the products of the largest perpendicular diameters of the lesions by ≥25% above baseline. Subjects with <4 raised lesions in a lesion group at baseline were not assessable for TR based on lesion flattening. Progression was ≥25% increase in the sum of the products of the largest perpendicular diameters of the lesions in a given treatment group and/or an increase in the number of raised lesions in a group by ≥2. Stable disease was any response not meeting criteria for response or progression. OR included assessments of lesions in addition to the 12 treated lesions, changes in tumor-associated edema and development of visceral disease using previously-described criteria[20]. OR was primarily intended to determine the need for alternative therapy, but could also detect treatment-induced systemic antitumor effects.
Biologic Endpoints
Formalin-fixed and paraffin embedded biopsy specimens were evaluated for collagen type-I mRNA expression by in situ hybridization and for MMP-2, VEGF and KSHV-LANA expression by immunohistochemistry (IHC) (see Methods, Supplemental Digital Content 1).
Pharmacokinetic Evaluations
Halofuginone plasma concentrations were measured in 10 patients remaining on treatment at week 8 immediately before the first daily ointment application (trough), and 1, 2 and 4 hours after application. We used a validated liquid chromatography-tandem mass spectrometric assay that can detect ≥1ng/mL [21].
Statistical Considerations
The study was designed to detect a difference in TR rate between 15% in vehicle-treated and 35% in halofuginone-treated lesions. To detect this difference at the one-sided 0.05 significance level with 0.78 power using McNemar’s chi-square test, assuming ≤25% of the patients had discordant results, required 30 patients.
Patients were randomly assigned to arm 1 (A=Halofuginone, B=Vehicle) or arm 2 (A=Vehicle, B=Halofuginone). Pre-labeled ointment tubes were shipped by PMB to the sites per randomization assignment. Vehicle and halofuginone response rates were compared using McNemar’s chi-square test. Changes from baseline to weeks 4 and 12 in MMP-2, VEGF and collagen type-I were compared for halofuginone- and vehicle-treated lesions using the Wilcoxon signed rank test. The Spearman rank correlation coefficient was used to assess correlations between normalized number of LANA-positive cells and absolute CD4 count or viral loads. Baseline KSHV viral loads in responders and non-responders were compared with the Wilcoxon rank sum test.
RESULTS
Patient Characteristics
Twenty-six patients enrolled; three withdrew before receiving treatment. Because of slow accrual, the study was stopped before the planned 30 patients enrolled. Table 1 summarizes baseline patient characteristics.
Table 1.
Demographics and Selected Baseline Characteristics
| Male, number (%) | 23 (100) |
| Race/Ethnicity, number (%) | |
| Hispanic, white | 3 (13) |
| Non-Hispanic, white | 13 (57) |
| Non-Hispanic, black | 6 (26) |
| Ethnicity unknown, black | 1 (4) |
| Age | |
| Mean (± SD), years | 43.1 (8.7) |
| Range | 23 – 62 |
| Absolute CD4 count | |
| N | 21 |
| Mean (± SD), cells/μl | 301.5 (178.3) |
| Range | 2 – 693 |
| Plasma HIV RNA level undetectable | 15/22 (68%) |
| Prior KS Treatment, number (%) | 21 (91) |
| Local Therapy | 8 (35) |
| Interferon | 4 (17) |
| Systemic Chemotherapy | 13 (57) |
| Investigational Therapy | 12 (52) |
| Receiving Antiretroviral Therapy, number (%) | 21 (91) |
| Protease inhibitor-based regimen | 10 (43.5) |
| NNRTI-based regimen | 10 (43.5) |
| Other | 1 (4) |
| KSHV viral load, median copies/mL (range) | 34,800 (0 – 2870000) |
Adverse Events (AEs)
Treatment was well-tolerated. Twelve patients reported 109 AEs; all but four were grade 1 (66 events) or 2 (39 events). Only nine AEs (6 grade 1) were considered at least possibly related to study drug; five were local reactions, one of which led to patient withdrawal.
Kaposi’s Sarcoma Response
Fourteen subjects completed 12 weeks of therapy. Three terminated for KS progression. Six of 23 patients terminated for reasons other than progression before 12 weeks and were not assessable for TR.
Four of 17 patients who were assessable for TR showed PR in halofuginone-treated lesions only, two showed PR in vehicle-treated lesions only, and two showed PR in both lesion groups. The respective response rates were 35% (95% CI, 14–62%) and 23.5% (95% CI, 7–50%) in halofuginone- and vehicle-treated lesions, p=0.683.
Of the 23 patients assessed for OR, seven (30%) showed PR (95% CI, 13–53%), 11 showed stable disease as the best response, 4 showed KS progression and one was treated for only 2 weeks and was not evaluable. There was no significant difference between responders and non-responders with respect to baseline KSHV viral load (data not shown).
Pharmacokinetics
None of 10 subjects in whom plasma halofuginone was assayed had detectable drug concentrations at any timepoint.
Biological Responses
Collagen type-I mRNA decreased significantly in halofuginone-treated lesions at week 4 and showed a similar trend at week 12, whereas vehicle-treated lesions showed no change. VEGF decreased significantly only in vehicle-treated lesions and only at week 4. There were no differences in MMP-2 and VEGF protein levels between halofuginone-and vehicle-treated lesions at any timepoint (see Table, Supplemental Digital Content 2, which summarizes changes from baseline in expression of angiogenic markers).
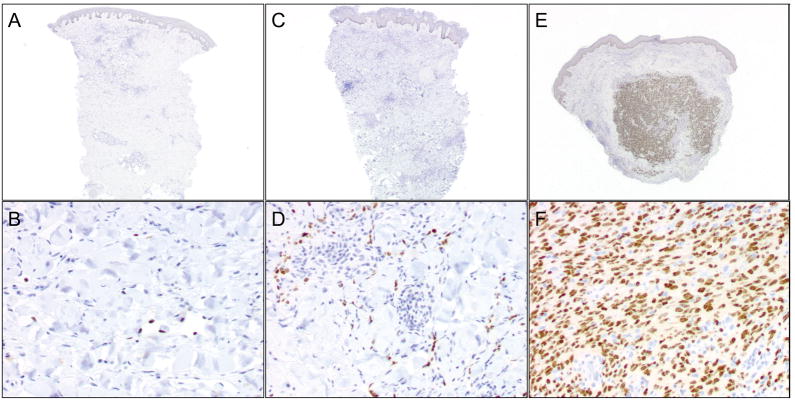
The number of LANA-positive cells ranged from 13 to >4000, and tended to be higher in lesions with more cellularity and spindle cell density (Figure 1). The percentage of LANA-positive cells ranged from 0.49 to 15%. Total numbers of LANA-positive cells normalized to both total area and number of nuclei were compared at baseline and weeks 4 and 12 and between halofuginone- and vehicle-treated lesions. No differences were observed over time or between treatments. Adjustment of collagen type-I, MMP-2 and VEGF expression data for LANA expression did not influence results for those parameters. Furthermore, the average number of LANA-positive cells at baseline for each patient did not correlate with CD4 counts or with either KSHV or HIV viral load. Given data suggesting that halofuginone inhibits the TH17 lymphocyte subset [22], we investigated CD4 counts and HIV viral loads, but observed no changes during the study.
Figure 1. Immunohistochemical staining for KSHV LANA shows significant heterogeneity among different patients.
A and B: a representative biopsy with a scant number of LANA-positive spindle cells. C and D: a biopsy from another patient with a moderate amount of LANA positivity. E and F: numerous LANA positive cells in a biopsy that showed a nodule with a dense concentration of spindle cells. (A,C,E: LANA, 2 × magnification; B,D,F: LANA, 20 × magnification).
DISCUSSION
Inhibition of angiogenic pathways has shown promise for KS treatment in several clinical trials. Targeted, orally-bioavailable agents including COL-3 and imatinib have influenced KS development pathways and induced therapeutic effects [23, 24]. However, responses were rarely complete, only a subset of patients responded, and systemic side-effects were observed, supporting evaluation of topical agents that could potentially influence pathways involved in KS development.
Because of its critical roles in blood vessel assembly, we hypothesized that collagen type-I inhibition might induce KS regression. Although halofuginone inhibited collagen type-I in KS lesions, it had no effect on MMP-2 expression and no clear clinical benefits resulted. The decrease in VEGF expression observed in vehicle-treated lesions at week 4 is unexplained, but needs to be interpreted in the context of a small study with relatively few samples. The significance of the heterogeneity in the number of KSHV-infected cells in KS lesions is unclear, as it did not correlate with HIV viral loads or CD4+ cell counts. Nevertheless, this observation could have important pathobiological implications, in that infection of rare cells within KS lesions is sufficient for lesions to develop.
A potential limitation of our study is that subjects could have applied both ointments to all their lesions to maximize potential therapeutic effects, obscuring any differences. Our finding that collagen type-I expression decreased only in halofuginone-treated lesions argues against this explanation. A more likely explanation is that collagen type-I inhibition is insufficient to inhibit angiogenesis. In addition, although most patients had been on ART for ≥12 weeks, it is likely that at least some responses reflect delayed antitumor effects of ART. Finally, because accrual fell short of the intended goal and the response of many patients could not be evaluated, the study lacked power to make definitive statements about efficacy.
Despite its limitations, this study presents a novel design applicable to future trials of topical, non-absorbed agents in which the patient could serve as his own control. Such trials must include biologic endpoints that would not only address drug mechanisms but could also help assess compliance for drugs with known targets.
Supplementary Material
Acknowledgments
Support: Supported by a grant from the Department of Health and Human Services and the National Institutes of Health, UO1 CA121947, to the AIDS Malignancy Consortium.
We would like to acknowledge the support of the Pathology and Laboratory Medicine Translational Research Program of Weill Cornell Medical College; Yi-Fand Liu (Weill Cornell) for technical assistance with LANA immunohistochemistry; Robert Kim (Weill Cornell) for image analysis; Drs. William Wachsman and Erin Reid (University of California, San Diego), Dr. Ronald T. Mitsuyasu (University of California, Los Angeles), Dr. Zale Bernstein (Roswell Park) and Dr. Manisha Shah (Ohio State University) for patient accrual; and Dr. Richard Ambinder (Johns Hopkins University) for measurement of KSHV viral loads.
Footnotes
Presented in part at the XII International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, Bethesda, MD, April 26–27, 2010.
In Memorium
Dr. Merrill J. Egorin died on August 7, 2010. We will sorely miss his friendship, mentorship and passion and his generous collaboration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schalling M, Ekman M, Kaaya EE, et al. A role for a new herpes virus (KSHV) in different forms of Kaposi’s sarcoma. Nat Med. 1995;1(7):707–708. doi: 10.1038/nm0795-707. [DOI] [PubMed] [Google Scholar]
- 2.Dupin N, Fisher C, Kellam P, et al. Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci U S A. 1999;96(8):4546–4551. doi: 10.1073/pnas.96.8.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 4.Masood R, Cai J, Zheng T, et al. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci U S A. 1997;94(3):979–984. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miles SA, Rezai AR, Salazar-Gonzalez JF, et al. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci U S A. 1990;87(11):4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturzl M, Roth WK, Brockmeyer NH, et al. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci U S A. 1992;89(15):7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturzl M, Brandstetter H, Zietz C, et al. Identification of interleukin-1 and platelet-derived growth factor-B as major mitogens for the spindle cells of Kaposi’s sarcoma: a combined in vitro and in vivo analysis. Oncogene. 1995;10(10):2007–2016. [PubMed] [Google Scholar]
- 8.Skobe M, Brown LF, Tognazzi K, et al. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi’s sarcoma. J Invest Dermatol. 1999;113(6):1047–1053. doi: 10.1046/j.1523-1747.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 10.Qian LW, Xie J, Ye F, et al. Kaposi’s sarcoma-associated herpesvirus infection promotes invasion of primary human umbilical vein endothelial cells by inducing matrix metalloproteinases. J Virol. 2007;81(13):7001–7010. doi: 10.1128/JVI.00016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toschi E, Barillari G, Sgadari C, et al. Activation of matrix-metalloproteinase-2 and membrane-type-1-matrix-metalloproteinase in endothelial cells and induction of vascular permeability in vivo by human immunodeficiency virus-1 Tat protein and basic fibroblast growth factor. Mol Biol Cell. 2001;12(10):2934–2946. doi: 10.1091/mbc.12.10.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seandel M, Noack-Kunnmann K, Zhu D, et al. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97(8):2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- 13.Elkin M, Miao HQ, Nagler A, et al. Halofuginone: a potent inhibitor of critical steps in angiogenesis progression. Faseb J. 2000;14(15):2477–2485. doi: 10.1096/fj.00-0292com. [DOI] [PubMed] [Google Scholar]
- 14.Nagler A, Miao HQ, Aingorn H, et al. Inhibition of collagen synthesis, smooth muscle cell proliferation, and injury-induced intimal hyperplasia by halofuginone. Arterioscler Thromb Vasc Biol. 1997;17(1):194–202. doi: 10.1161/01.atv.17.1.194. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Sekine S, Goto Y, et al. Halofuginone inhibits neointimal formation of cultured rat aorta in a concentration-dependent fashion in vitro. Heart Vessels. 1998;13(1):18–23. doi: 10.1007/BF02750639. [DOI] [PubMed] [Google Scholar]
- 16.Gavish Z, Pinthus JH, Barak V, et al. Growth inhibition of prostate cancer xenografts by halofuginone. Prostate. 2002;51(2):73–83. doi: 10.1002/pros.10059. [DOI] [PubMed] [Google Scholar]
- 17.Granot I, Halevy O, Hurwitz S, et al. Halofuginone: an inhibitor of collagen type I synthesis. Biochim Biophys Acta. 1993;1156(2):107–112. doi: 10.1016/0304-4165(93)90123-p. [DOI] [PubMed] [Google Scholar]
- 18.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85(2):47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnainsky Y, Kushnirsky Z, Bilu G, et al. Gene expression during chemically induced liver fibrosis: effect of halofuginone on TGF-beta signaling. Cell Tissue Res. 2007;328(1):153–166. doi: 10.1007/s00441-006-0330-1. [DOI] [PubMed] [Google Scholar]
- 20.Cianfrocca M, Cooley TP, Lee JY, et al. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi’s sarcoma: a phase I AIDS malignancy consortium study. J Clin Oncol. 2002;20(1):153–159. doi: 10.1200/JCO.2002.20.1.153. [DOI] [PubMed] [Google Scholar]
- 21.Parise RA, Sparrow BR, Merrill JW, et al. Liquid chromatography-electrospray tandem mass spectrometric assay suitable for quantitation of halofuginone in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810(1):35–40. doi: 10.1016/j.jchromb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Sundrud MS, Koralov SB, Feuerer M, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324(5932):1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dezube BJ, Krown SE, Lee JY, et al. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi’s sarcoma: an AIDS Malignancy Consortium Study. J Clin Oncol. 2006;24(9):1389–1394. doi: 10.1200/JCO.2005.04.2614. [DOI] [PubMed] [Google Scholar]
- 24.Koon HB, Bubley GJ, Pantanowitz L, et al. Imatinib-induced regression of AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2005;23(5):982–989. doi: 10.1200/JCO.2005.06.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.