Abstract
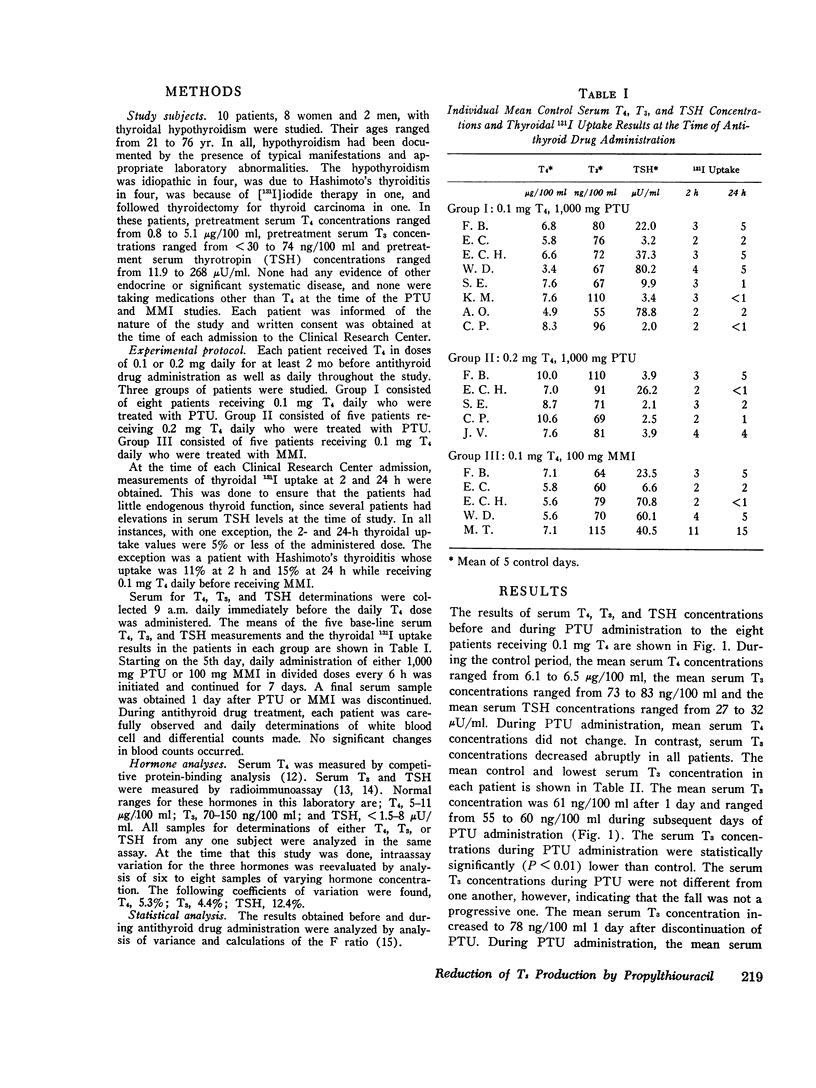
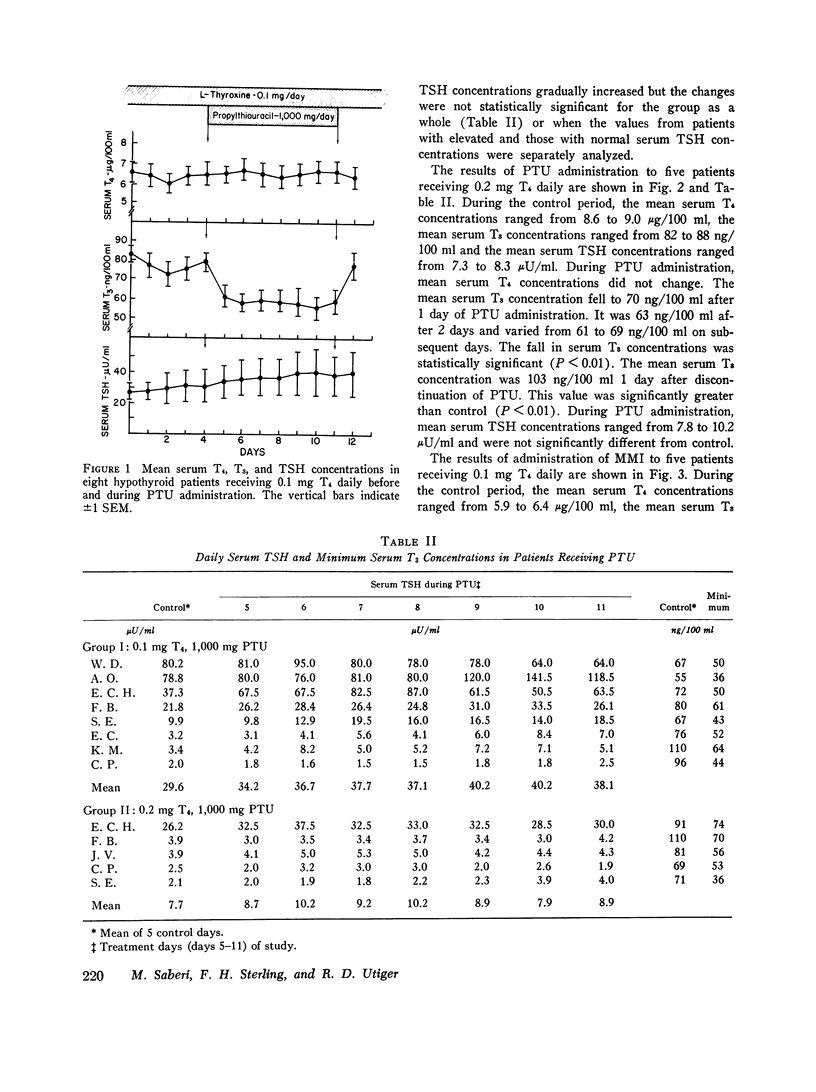
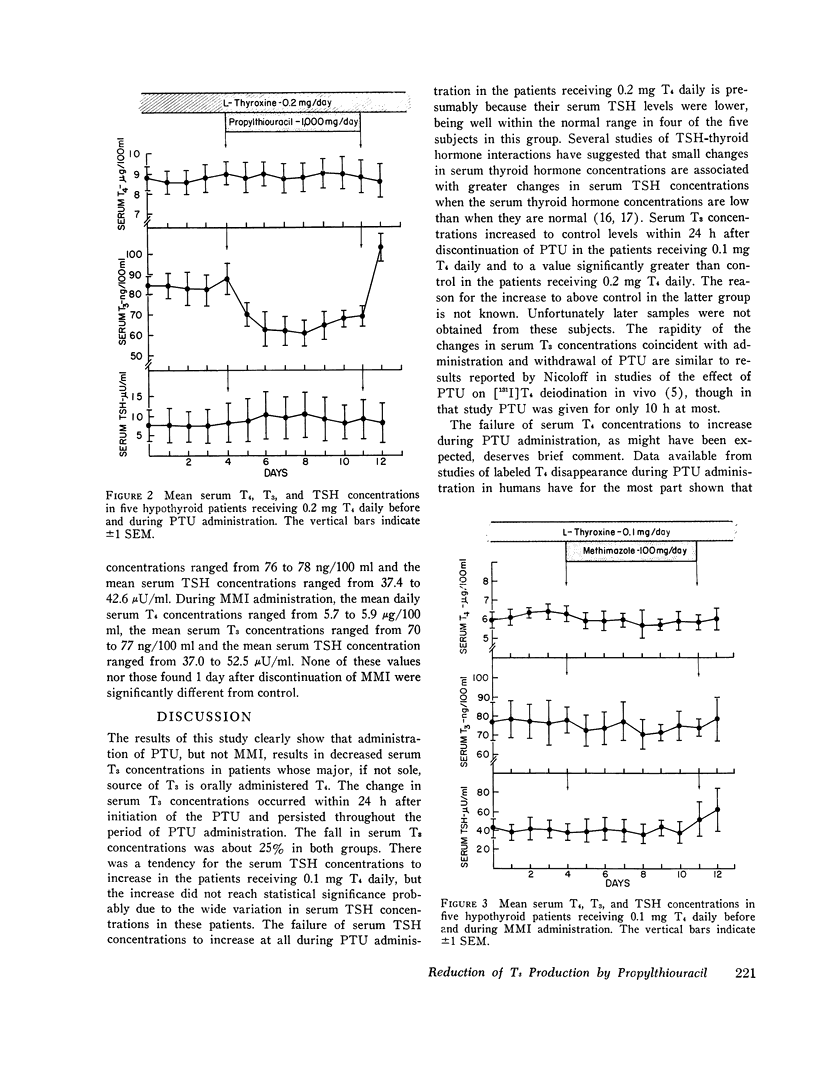
To determine if propylthiouracil (PTU) inhibited extrathyroidal thyroxine (T4) to triiodothyronine (T3) conversion in man, PTU was administered to T4-treated hypothyroid patients and serial measurements of T4, T3, and thyrotropin (TSH) carried out. All patients had proven thyroidal hypothyroidism and had been receiving 0.1 or 0.2 mg T4 daily for at least 2 mo before study. Hormone measurements were made for 5 consecutive days before and daily during a 7-day treatment period with PTU, 1,000 mg/day. In eight patients receiving 0.1 mg T4 daily, administration of PTU resulted in a prompt fall in mean serum T3 concentrations from 78 plus or minus 6 ng/100 ml (SEM) to 61 plus or minus 3 ng/100 ml after 1 day. The mean serum T3 concentrations ranged from 55 to 60 ng/100 ml during the remainder of the PTU treatment period (P less than 0.01). The mean control serum TSH concentration was 29.6 muU/ml and it increased to a peak of 40 muU/ml on the 5th and 6th days. In five patients receiving 0.2 mg T4 daily, the mean control serum T3 concentration was 84 plus or minus 7 NG/100ML. It fell to 70 plus or minus 5 ng/100 ml after 1 day and 63 plus or minus 7 ng/100 ml after 2 days of PTU administration and thereafter ranged from 6) to 69 ng/100 ml (P LESS THAN 0.01). Serum TSH concentrations did not increase. No changes in serum T4 concentrations were found in either group. In five patients who received 100 mg methimazole (MMI) daily for 7 days there were no changes in serum T4, T3, or TSH concentrations. These results indicate that PTU, but not MMI, produces a prompt and sustained, albeit modest, reduction in serum T3 concentrations in patients whose sole or major source of T3 is ingested T4. These findings most likely result from inhibition of extrathyroidal formation of T3 from T4.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuid J., Larsen P. R. Triiodothyronine and thyroxine in hyperthyroidism. Comparison of the acute changes during therapy with antithyroid agents. J Clin Invest. 1974 Jul;54(1):201–208. doi: 10.1172/JCI107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuid J., Stinson D. A., Larsen P. R. Serum triiodothyronine and thyroxine in the neonate and the acute increases in these hormones following delivery. J Clin Invest. 1973 May;52(5):1195–1199. doi: 10.1172/JCI107286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantine J. J., Oliner L. Failure of methimazole to affect peripheral thyroid indices in man. Proc Soc Exp Biol Med. 1968 Feb;127(2):388–391. doi: 10.3181/00379727-127-32697. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton G. E., Gorman C. A., Mayberry W. E. Suppression of thyrotropin (h-TSH) in serums of patients with myxedema of varying etiology treated with thyroid hormones. N Engl J Med. 1971 Sep 2;285(10):529–533. doi: 10.1056/NEJM197109022851001. [DOI] [PubMed] [Google Scholar]
- Eastman E. J., Corcoran J. M., Jequier A., Ekins R. P., Williams E. S. Triiodothyronine concentration in cord and maternal sera at term. Clin Sci Mol Med. 1973 Aug;45(2):251–255. doi: 10.1042/cs0450251. [DOI] [PubMed] [Google Scholar]
- FISHER D. A., LEHMAN H., LACKEY C. PLACENTAL TRANSPORT OF THYROXINE. J Clin Endocrinol Metab. 1964 May;24:393–400. doi: 10.1210/jcem-24-5-393. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H., Erenberg A., Lam R. W. Thyroxine and triiodothyronine metabolism in maternal and fetal sheep. Pediatr Res. 1972 Dec;6(12):894–899. doi: 10.1203/00006450-197212000-00007. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H., Hobel C. J., Lam R. Serum and thyroid gland triiodothyronine in the human fetus. J Clin Endocrinol Metab. 1973 Feb;36(2):397–400. doi: 10.1210/jcem-36-2-397. [DOI] [PubMed] [Google Scholar]
- Furth E. D., Rives K., Becker D. V. Nonthyroidal action of propylthiouracil in euthyroid, hypothyroid and hyperthyroid man. J Clin Endocrinol Metab. 1966 Mar;26(3):239–246. doi: 10.1210/jcem-26-3-239. [DOI] [PubMed] [Google Scholar]
- GRUMBACH M. M., WERNER S. C. Transfer of thyroid hormone across the human placenta at term. J Clin Endocrinol Metab. 1956 Oct;16(10):1392–1395. doi: 10.1210/jcem-16-10-1392. [DOI] [PubMed] [Google Scholar]
- HERSHMAN J. M. EFFECT OF 5- AND 6-PROPYLTHIOURACIL ON THE METABOLISM OF L-THYROXINE IN MAN. J Clin Endocrinol Metab. 1964 Feb;24:173–179. doi: 10.1210/jcem-24-2-173. [DOI] [PubMed] [Google Scholar]
- HERSHMAN J. M. Effect of various compounds on the binding of thyroxine to serum proteins in the rat. Endocrinology. 1963 May;72:799–803. doi: 10.1210/endo-72-5-799. [DOI] [PubMed] [Google Scholar]
- Lieblich J. M., Utiger R. D. Triiodothyronine in cord serum. J Pediatr. 1973 Feb;82(2):290–292. doi: 10.1016/s0022-3476(73)80172-6. [DOI] [PubMed] [Google Scholar]
- Lieblich J., Utiger R. D. Triiodothyronine radioimmunoassay. J Clin Invest. 1972 Jan;51(1):157–166. doi: 10.1172/JCI106786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL M. L., O'ROURKE M. E., HARDEN A. B. Resin uptake of radiothyroxine from serum in thyroid disease and in pregnancy. J Clin Endocrinol Metab. 1961 Nov;21:1448–1454. doi: 10.1210/jcem-21-11-1448. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G., Escobar del Rey F. Extrathyroid effects of some antithyroid drugs and their metabolic consequences. Recent Prog Horm Res. 1967;23:87–137. doi: 10.1016/b978-1-4831-9826-2.50006-4. [DOI] [PubMed] [Google Scholar]
- Nicoloff J. T. A new method for the measurement of acute alterations in thyroxine deiodination rate in man. J Clin Invest. 1970 Feb;49(2):267–273. doi: 10.1172/JCI106236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell W. D., Wilber J. F., Utiger R. D. Studies of thyrotropin physiology by means of radioimmunoassay. Recent Prog Horm Res. 1967;23:47–85. doi: 10.1016/b978-1-4831-9826-2.50005-2. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. An explanation of the antithyroxine effect of propylthiouracil and evidence supporting the concept that triiodothyronine is the active thyroid hormone. J Clin Invest. 1972 Sep;51(9):2493–2497. doi: 10.1172/JCI107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman C. S., Chambers J. B., Jr, Read V. H. The extrathyroidal conversion rate of thyroxine to triiodothyronine in normal man. J Clin Invest. 1971 Jun;50(6):1187–1196. doi: 10.1172/JCI106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichlin S., Utiger R. D. Regulation of the pituitary-thyroid axis in man: relationship of TSH concentration to concentration of free and total thyroxine in plasma. J Clin Endocrinol Metab. 1967 Feb;27(2):251–255. doi: 10.1210/jcem-27-2-251. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANARSDEL P. P., Jr, WILLIAMS R. H. Effect of propylthiouracil on degradation of I 131-labeled thyroxine and triiodothyronine. Am J Physiol. 1956 Sep;186(3):440–444. doi: 10.1152/ajplegacy.1956.186.3.440. [DOI] [PubMed] [Google Scholar]


