Abstract
Position effects limit the curative potential of gene transfer strategies for the hemoglobinopathies by inducing clonal variability of transgene expression. We evaluated the mitigating effects of the chicken hypersensitivity site 4 (HS4) insulator among lentiviral vector-transduced human hematopoietic cells. We constructed various lentiviral vectors using a green fluorescent protein (GFP) reporter under the control of a reverse-oriented murine stem cell virus (MSCV)-long-term repeat (LTR) promoter or a reverse-oriented β-globin expression cassette. A full-length HS4, a tandem HS4 core, and a single core insulator were inserted into the 3′ LTR in both forward and reverse orientation. All but the reverse single core insulator significantly decreased titers. All reduced %GFP without increasing mean fluorescence intensity (MFI) among erythroid progeny of transduced human CD34+ cells. A lower coefficient of variation (CV) was observed only among progeny of the full-length vector-transduced cells, yet a fivefold reduction in transduction efficiency was observed. In xenografted mice, the single core insulator decreased both the %GFP and the MFI at 4 and 8 weeks after transplantation with no difference in CVs. These data demonstrate that the inclusion of HS4 insulator elements lowers viral titers, reduces efficiency of transduction, and produces minimal effects on transgene expression among human hematopoietic cells in vitro and in vivo.
Introduction
Hematopoietic stem cell (HSC)-targeted gene therapy using retrovirus-based vectors is potentially curative for hemoglobin disorders such as the β-thalassemias and sickle-cell disease. In order to be successful, gene addition strategies must achieve high-level β-globin gene expression from vector constructs among erythroid progeny of transduced HSCs, especially for sickle-cell disease in which the production of pathologic β-globin continues after gene transfer. Significant improvements in the level of β-globin expression from viral vectors have resulted from the inclusion of large fragments of the locus control region, a reverse-oriented β-globin gene including introns, and the β-globin 3′-untranslated region in HIV1-based lentiviral vector systems.1,2,3,4 This vector system stably transmits the human β-globin gene and corrects the β-thalassemia intermedia phenotype of Hbbth3/+ mice,2 and also proved sufficient for phenotypic correction of a novel thalassemia major mouse model.5 Confirmatory results were also obtained by others in both thalassemia and sickle-cell disease mouse models.4,6,7,8,9 These results, while encouraging, may prove difficult to duplicate in humans and the development of methods to improve upon expression among human blood cells remains a priority.
The site of vector integration can influence gene expression among the progeny of transduced HSCs. HIV1 vectors have a general tendency toward equal integration into activated genes in the genome.10 In blood cells that derive from transduced stem/progenitor cells, the chromosome regions in which the vector provirus integrates may close during subsequent differentiation, resulting in reduction of transgene expression termed chromosomal position effects. Because many variations of viral integration sites exist in transduced HSCs, the position effect has the potential to further introduce clonal variability of transgene expression.11
Position effects were previously shown to be overcome by inserting chromatin insulator elements, such as the chicken hypersensitivity site 4 (HS4). The HS4 insulator has two described functions: enhancer-blocking activity that reduces enhancing effects on a promoter when inserted between the two; and barrier activity that prevents chromosomal position effects when the gene expression cassette is flanked by the insulator.12 Within the 1.2-kb full-length HS4 insulator, a 250b core element appears the important regulatory sequence for insulator function, especially for enhancer-blocking activity.13 Recent work demonstrates that the 1.2 kb HS4 insulator element reduces position effects in mouse erythroleukemia (MEL) cells and mouse erythroid cells in vivo, resulting in a consistent high-level expression of an HIV1-based therapeutic β-globin gene.14 However, it remains unknown whether the HS4 insulator can perform similarly in transduced human HSCs.
The incorporation of insulator elements may also have untoward consequences. Insertion of insulator fragments into 3′ long-term repeat (LTR) to achieve flanking of the expression cassette after integration reduces viral titers because of restrictions in reverse transcription and increased homologous recombination.15 As the size of the inserted HS4 insulators increases, viral titers also decrease.14,16 These effects are especially problematic for β-globin vectors which are already encumbered by low titers and low transduction efficiency for human primary HSCs. We thus sought to determine the effects of HS4 insulators on lentiviral vector titer, transduction efficiency, and transgene expression among primary human blood cells, and to determine the utility of this approach.
Results
Insulators decreased vector titers except for reverse-oriented 250b HS4 insulator
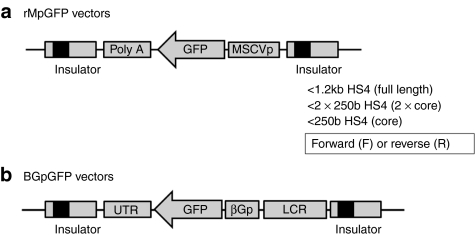
We constructed various types of insulated lentiviral vectors to evaluate the function of the HS4 insulator. We used a reverse-oriented green fluorescent protein (GFP) expression unit under the control of a murine stem cell virus (MSCV)-LTR promoter (rMpGFP vector) (Figure 1a), and a conventional reverse-oriented β-globin expression cassette in which the globin gene was changed to GFP complementary DNA (BGpGFP vector) (Figure 1b). This was done because the orientation of inserted HS4 insulator fragments changed the mean fluorescent intensity (MFI) of transgene expression from vectors that contained a forward-oriented GFP expression cassette (data not shown).16 A full-length HS4 insulator (1.2 kb HS4), a tandem HS4 core insulator (2 × 250b HS4), or a single core insulator (250b HS4) were inserted into the 3′LTR (Figure 1). We also constructed insulated vectors in which forward (F) and reverse (R) orientated insulator fragments were inserted into the LTR.
Figure 1.
Lentiviral vectors to evaluate insulator functions in erythroid cells. (a) We constructed reverse-oriented GFP (rMpGFP) expression cassette under the control of an MSCV-LTR promoter or (b) β-globin (BGpGFP) expression cassette in which the globin gene was changed to GFP cDNA. A full-length HS4 insulator (1.2 kb HS4), a tandem HS4 core insulator (2 × 250b HS4), or a single core insulator (250b HS4) were inserted into the LTR, in forward or reverse orientations. GFP, green fluorescent protein; HS4, hypersensitivity site 4; LCR, locus control region; MSCVp, murine stem cell virus-long-term repeat promoter; Poly A, polyadenylation signal; UTR, untranslated region; βGp, β-globin promoter.
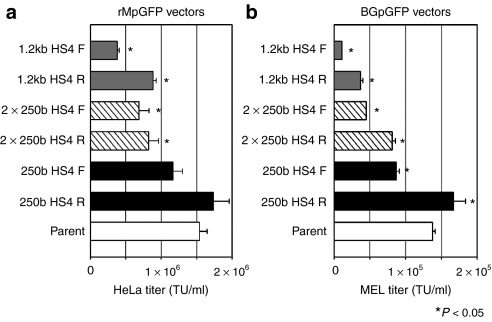
To evaluate the efficiency of vector preparation when insulator fragments were inserted into the LTR, we tittered the insulated vectors using the HeLa cell line for rMpGFP vectors (Figure 2a), and the MEL cell line for BGpGFP vectors (Figure 2b). Vector titers decreased with the insertion of the 1.2 kb HS4 and the 2 × 250b HS4 insulators in both orientations and both vector constructs in a size dependent manner, compared to the uninsulated vector (parent) [N = 3, P < 0.05 in one-way analysis of variance (ANOVA)]. Reverse-oriented insulator vectors showed higher titers than forward-oriented insulator vectors, except for the 250b HS4 in the rMpGFP vector (N = 3, P < 0.05 in t-test). The 250b HS4 R insulator preserved vector titers in both vector constructs, compared to the uninsulated vector.
Figure 2.
Viral titers among insulated vectors. Titers were evaluated in HeLa cell line for (a) insulated rMpGFP vectors and in (b) MEL cell line for insulated BGpGFP vectors. Titers were the lowest with the insertion of the largest insulators, compared to the uninsulated vector (parent) (N = 3, P < 0.05 in one-way ANOVA). The smallest 250b HS4 R insulator yielded the best vector titers in either vector constructs. Reverse-oriented insulators showed higher vector titers than the forward-oriented insulators, except the 2 × 250b HS4 in rMpGFP vectors. ANOVA, analysis of variance; HS4, hypersensitivity site 4; MEL, mouse erythroleukemia.
Insulated vectors transduce human HSCs less efficiently than MEL cell lines
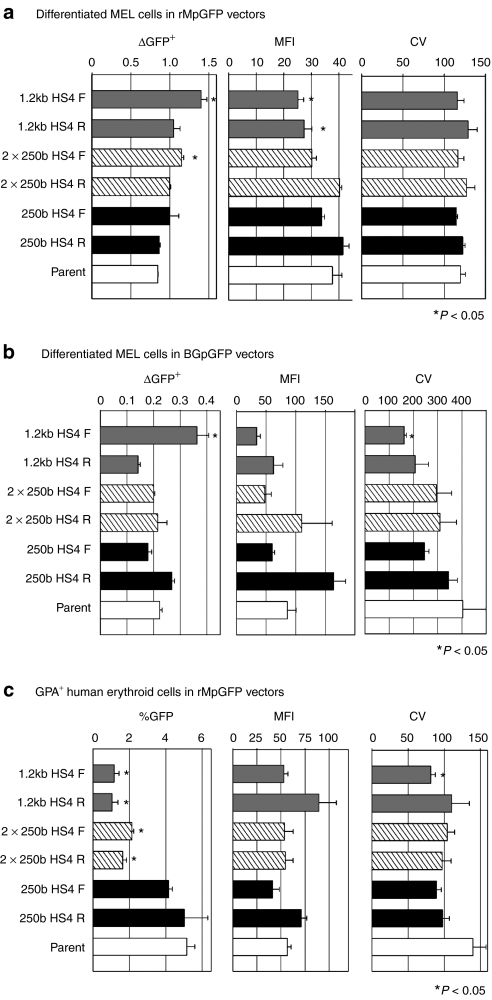
To evaluate the influence of various types of HS4 insulators on chromosomal position effects, we analyzed GFP expression in differentiated MEL cells after transduction with insulated rMpGFP and BGpGFP vectors before differentiation. Overall, in insulated rMpGFP vectors, the ratios of %GFP in differentiated cells to %GFP in undifferentiated cells (ΔGFP+) of insulated vectors were higher, but MFIs were lower, indicating that although there were more GFP+ cells, each differentiated cell had lower GFP expression levels. The 1.2 kb HS4 F and 2 × 250b HS4 F vectors yielded higher ΔGFP+ than that of the uninsulated vector (parent) (N = 3, P < 0.05 in one-way ANOVA). The MFIs of GFP for the 1.2 kb HS4 F and R vectors were inferior to that of the uninsulated vector (P < 0.05 in one-way ANOVA) (Figure 3a). The coefficient of variation (CV) showed no significant difference among insulated and uninsulated vectors (Figure 3a). In contrast, there were no clear trends in the insulated BGpGFP vectors. Only the 1.2 kb HS4 F insulated vector showed higher ΔGFP+, lower MFI, and lower CV compared to the parent vector (N = 3, P < 0.05 in one-way ANOVA) (Figure 3b).
Figure 3.
Function of forward- and reverse-oriented insulators in differentiated MEL and human erythroid cells. To evaluate whether insulated vectors could escape from position effects, we evaluated GFP expression by ΔGFP+ (%GFP in differentiated cells divided by %GFP in undifferentiated cells), mean fluorescent intensity (MFI), and coefficient of variability (CV). In (a) rMpGFP vectors, insulated vectors had higher ΔGFP+, lower MFI, and similar CV (N = 3, P < 0.05 in one-way ANOVA). In (b) BGpGFP vectors, insulated vectors had similar ΔGFP+, MFI, and CV, except for the vectors containing 1.2k HS4 F (N = 3, P < 0.05 in one-way ANOVA). In (c) GPA+ human erythroid cells, which originated from transduced CD34+ cells, the two larger insulators yielded lower %GFP (N = 3, P < 0.05 in one-way ANOVA). Although the insulated vectors appeared to have lower CVs, there was no significant difference with the exception of the 1.2k HS4 F vector in BGpGFP vectors and GPA+ cells (N = 3, P < 0.05 in one-way ANOVA). ANOVA, analysis of variance; GFP, green fluorescent protein; HS4, hypersensitivity site 4; MEL, mouse erythroleukemia.
To evaluate the function of various types of HS4 insulators in primary human hematopoietic cells, we analyzed GFP expression in glycophorin A (GPA)+ cells originating from human CD34+ cells that were transduced with insulated rMpGFP vectors at a multiplicity of infection (MOI) of 3. On day 16 after erythroid differentiation, the inclusion of 1.2 kb HS4 and 2 × 250b HS4 insulators in both orientations decreased %GFP compared to the uninsulated vector (parent) (N = 3, P < 0.05 in one-way ANOVA) (Figure 3c). The MFIs from the insulated vectors were similar to those of the uninsulated vector. All insulated vectors had a tendency toward lower CVs, but only the 1.2 kb HS4 F insulated vector showed a significant difference (N = 3, P < 0.05 in one-way ANOVA) (Figure 3c). These data suggest that HS4 insulators have minimal effects on transgene expression levels, and insulators of 1.2 kb HS4 and 2 × 250b HS4 reduce transduction efficiency in human erythroid cells, even when MOIs were matched.
Insulated vectors showed modest enhancer-blocking activity
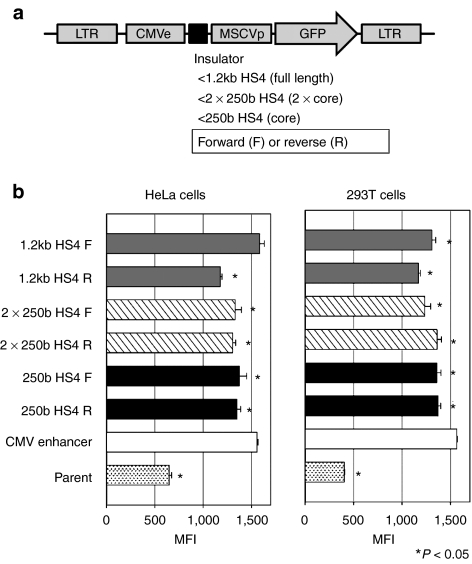
We next evaluated enhancer-blocking activity among various types of HS4 insulators that were inserted between a cytomegalovirus (CMV) enhancer and a MSCV-LTR promoter (Figure 4a). In transduced HeLa and 293T cell lines, almost all the HS4 insulator types significantly blocked CMV enhancer activity (N = 3, P < 0.05 in one-way ANOVA with the exception of 1.2 kb HS4 F in HeLa cells), but could not eliminate enhancer activity completely (0–42% reduction rates) (Figure 4b).
Figure 4.
Enhancer-blocking activity of various insulators. (a) We constructed lentiviral vector plasmids expressing GFP under the control an MSCV-LTR promoter (MSCVp) with CMV enhancer (CMVe), in which various types of HS4 insulator fragments were inserted between the CMV enhancer and the MSCV-LTR promoter. (b) MFIs of GFP expression were evaluated in HeLa cell line and 293T cell line. Almost all types of HS4 insulator reduced enhancement of GFP expression (N = 3, P < 0.05 in one-way ANOVA with the exception of 1.2 kb HS4 F in HeLa cells), showing enhancer-blocking activity of HS4 insulators. CMV enhancer, CMVe-enhancing vector that did not include HS4; GFP, green fluorescent protein; HS4, hypersensitivity site 4; LTR, long-terminal repeat; MFI, mean fluorescent intensity; MSCV, murine stem cell virus; parent, parent vector not including CMVe and HS4.
The reverse-oriented 250b HS4 insulator showed lower %GFP and MFI in human GPA+ cells in erythroid culture and human white blood cells in xenografted mice
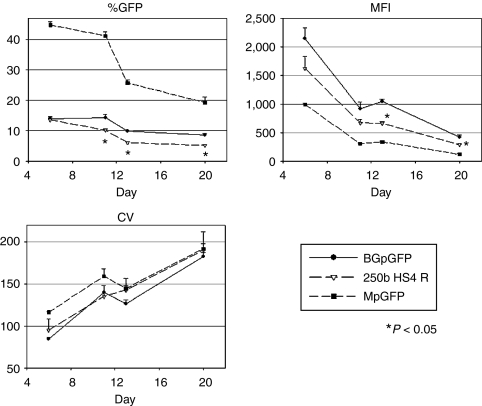
Given the minimal effects of HS4 insulators and lower vector titers with globin vectors, we chose the 250b HS4 R insulator, which had the highest titer, to evaluate transgene expression in human erythroid cells. We transduced human CD34+ cells with the uninsulated BGpGFP vector, the 250b HS4 R insulated BGpGFP vector, and the standard MpGFP vector containing a forward-oriented GFP expression cassette under the control of a MSCV-LTR promoter as a maximal transduction control (all at MOI = 20). We then followed the time course of GFP expression among GPA+ cells in erythroid culture (Figure 5). As expected, %GFP and MFIs decreased and CVs increased in GPA+ cells transduced with MpGFP, demonstrating a chromosomal position effect. However, the insulated vector containing 250b HS4 R showed lower %GFP (N = 3, P < 0.05 in t-test on days 11, 13, and 20) and lower MFIs (N = 3, P < 0.05 in t-test on days 13 and 20), compared to those of the uninsulated BGpGFP vector. There was no significant difference in CVs. These data show that this 250b HS4 R insulator decreased transduction efficiency and transgene expression level in human erythroid cells and did not protect the transgene from position effects in short-term ex vivo erythroid culture.
Figure 5.
GFP expression in human erythroid cells after transduction with an insulated BGpGFP vector. To evaluate whether an HS4 insulator can protect differentiation-dependent reduction of transgene expression (position effect), human CD34+ cells were transduced with MpGFP (forward-oriented GFP-expressing vector under the control of an MSCV-LTR promoter), BGpGFP (reverse-oriented GFP-expressing vector under the control of a β-globin promoter), and BGpGFP vector containing a reverse orientation HS4 core insulator (250b HS4 R), all at MOI of 20. GFP expression in GPA+ cells was serially analyzed over days of culture. %GFP and MFIs decreased and CVs increased from cells transduced with the control MpGFP vector, showing position effects. The 250b HS4 R insulator showed lower %GFP and lower MFI (N = 3, P < 0.05 in t-test on days 13 and 20), compared to those of the uninsulated vector BGpGFP. There was no difference in CVs. CV, coefficient of variation; GFP, green fluorescent protein; HS4, hypersensitivity site 4; LTR, long-terminal repeat; MFI, mean fluorescent intensity; MSCV, murine stem cell virus.
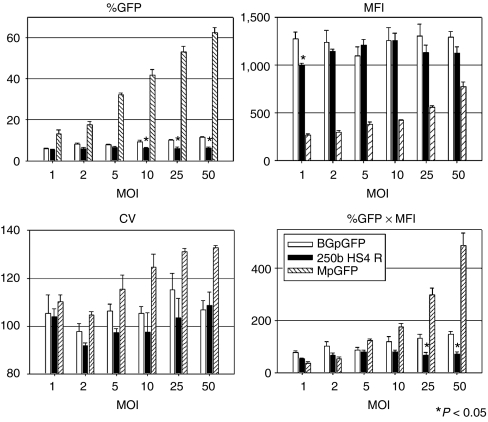
We then transduced human CD34+ cells with escalating MOIs of the 250b HS4 R insulated vector, and evaluated GFP expression of GPA+ cells at day 13 of erythroid culture (Figure 6). The insulated vector (250b HS4 R) showed lower %GFP at MOI 10, 25, and 50 (N = 3, P < 0.05 in t-test) and lower overall GFP expression (%GFP × MFI) at MOI 25 and 50 (N = 3, P < 0.05 in t-test) compared to the uninsulated BGpGFP vector. These results demonstrate that the decrease in GFP expression associated with the inclusion of HS4 insulator elements was not overcome by increasing MOI, despite having comparable infectious and p24 titers (Supplementary Figure S1).
Figure 6.
Titration of BGpGFP vectors in human erythroid culture. GFP expression of GPA+ cells was evaluated with escalating MOI of BGpGFP vectors on day 13 of human erythroid culture. The insulated vector (250b HS4 R) showed lower %GFP in MOI 10, 25, and 50 (N = 3, P < 0.05 in t-test) and lower overall GFP expression (%GFP × MFI) at MOI 25 and 50 (N = 3, P < 0.05 in t-test), compared to the uninsulated BGpGFP vector, demonstrating that the inclusion of HS4 insulator elements decreases GFP expression, and could not be overcome by increasing MOI. GFP, green fluorescent protein; HS4, hypersensitivity site 4; MFI, mean fluorescence intensity; MOI, multiplicity of infection.
To evaluate CpG methylation status in the MSCV-LTR promoter, we used an AscI restriction enzyme that is blocked by CpG methylation. Using this enzyme, we can detect methylated provirus as a 2.8 kb band and unmethylated provirus as a 2.0 kb band. Human CD34+ cells were transduced with parent MpGFP vector and 250b HS4 R insulated vector, and these cells were differentiated to erythroid cells for 17 days. The parent vector and 250b HS4 R vector were detected at 2.0 kb as an unmethylated signal (Supplementary Figure S2).
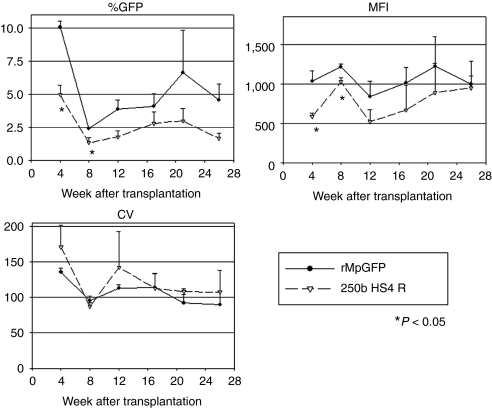
To evaluate transgene expression from the insulated vector in vivo, we transplanted human CD34+ cells into NOD/SCID/IL2Rγnull mice following transduction with the 250b HS4 R insulated rMpGFP vector or the control. We then followed GFP expression among human CD45+ cells in the peripheral blood of the transplanted mice for 27 weeks (Figure 7). The insulated vector showed lower %GFP and MFIs among human CD45+ cells in vivo, 4 and 8 weeks after the transplantation (P < 0.05 in t-test). There was no significant difference in CVs between insulated and uninsulated vectors at all time points. When humanized murine bone marrow cells were evaluated by linear amplification-mediated PCR, the results demonstrated multiple integration bands (Supplementary Figure S3). These data revealed that the 250 HS4 R insulator lowered transduction efficiency and transgene expression levels in human white blood cells in vivo, while providing a minimal protective effect on the chromosomal position effects encountered in the transduction of human hematopoietic cells.
Figure 7.
Insulator functions in human WBCs in xenograft mice. Human CD34+ cells were transduced with rMpGFP with and without 250b HS4 R insulator, and infused in NOD/SCID/IL2Rγnull mice after peritoneal injection of busulfan (35 mg/kg). GFP expression of the human differentiated blood cells in vivo was compared by flow cytometry. In the human CD45+ fraction of murine peripheral blood cells, the insulator element decreased %GFP and MFIs in 4 and 8 weeks after transplantation (P < 0.05 in t-test). There was no significant difference of CVs among the insulated and uninsulated (rMpGFP) vectors in all time points. GFP, green fluorescent protein; MFI, mean fluorescent intensity; WBC, white blood cell.
Discussion
For a therapeutic effect in hemoglobin disorders, HSC gene transfer requires high-level expression of the β-globin gene per transduced cell. Current therapeutic vectors combining a large-sized locus control region, intron-dependent globin gene, and the 3′ untranslated region produced in an HIV1-based vector can achieve globin expression levels approaching those sufficient for amelioration of the hemoglobinopathies in humans. One of the remaining obstacles is the potential for reduction of transgene expression over time.
In rhesus gene transfer preclinical studies as well as human gene therapy trials, transgene expression rates among peripheral blood cells generally increase for the first month post-transplant, decrease over the next 2–3 months, and finally plateau at low levels around 6 months after transplantation.17,18,19 One explanation for these observations is that committed progenitors reconstituted peripheral blood cells for the first 3 months, and stem cells reconstituted at 6 months and beyond.20 Alternatively, lower long-term transgene expression can result from transgene methylation, histone acetylation, or vector integration sites changing from euchromatin (open) to heterochromatin (closed) following HSC differentiation (chromosomal position effects). These changes in the proviral sequences can occur at any time and may influence eventual transgene expression.
We thus designed a series of experiments testing whether methylation or position effects was more dominant in reducing transgene expression. We first evaluated the effects of the HS4 insulators on vector production. Viral titer is one of the most important factors in clinical human gene therapy applications targeting globin disorders because current therapeutic vectors already have 10–100 fold lower titers compared to gene marking vectors. In the present work, we demonstrated that incorporation of a full-length 1.2 kb HS4 insulator led to a five to tenfold reduction in viral titers (Figure 2), which would render its inclusion impractical for clinical use. On the other hand, insertion of a reverse-oriented 250b HS4 core insulator did not decrease viral titers. As such, we further tested this 250b HS4 R insulator among in vitro differentiated human erythroid cells and in a human xenograft model.
When we transduced HEL cell line with 1.2 k HS4 F insulated or uninsulated MpGFP vector, and cultured long-term after single-cell sorting, GFP expression did not decrease in both insulated and uninsulated vectors up to 6 months (data not shown). This lack of change in %GFP and MFI led us to other experiments using differentiated MEL cell lines and human CD34+ primary cells. Insulated and uninsulated vectors showed lower MFIs or %GFP expression following differentiation, suggesting a position effect (Figures 3 and 5). In a separate erythroid culture of transduced human CD34+ cells, there was no CpG methylation of the viral internal promoter 17 days after differentiation (Supplementary Figure S2). These data suggest that the gradual reduction of GFP expression was more dependent on chromosomal position effects, rather than on DNA methylation in this ex vivo differentiation culture.
Generally, higher MOIs lead to higher transduction rates reaching up to 100%. However, human CD34+ cells revealed dose-dependent rates of transduction at lower MOIs only, and these transduction rates plateaued at below 100% at higher MOIs.17 These observations suggest that human CD34+ cells have some specific regulatory factors for viral transduction and this regulation is saturated by a large amount of viral vectors. In GPA+ erythroid cells after differentiation from transduced CD34+ cells, the normal MpGFP vectors increased transduction rates with escalating MOIs (up to 50), but BGpGFP vectors did not (Figure 6). We then measured p24 titers and infectious titers in transduced HeLa cells, and both titers were similar in forward-oriented vectors (Supplementary Figure S1). However, the reverse-oriented vectors (rMpGFP, which was used as a surrogate for BGpGFP) had high p24 titers but significantly lower infectious titer (Supplementary Figure S1). This discrepancy suggested that around tenfold more viral particles of reverse-oriented vectors were used for CD34+ cell transduction and erythroid differentiation, compared to those of forward-oriented vectors at MOIs which were calculated by infectious titers. Therefore, BGpGFP vectors of low MOIs (calculated by infectious titers) might saturate some regulation factors of viral transduction with noninfectious particles and therefore not increase transduction rates even with higher MOIs. To test whether such noninfectious viral particles can block viral transduction, we performed additional experiments by transducing human CD34+ cells with a forward-oriented vector (MpGFP) at MOI 10 along with escalating MOI of a reverse-oriented vector (rMpGFP), because rMpGFP vector included more noninfectious viral particles than MpGFP vectors (Supplementary Figure S1). Addition of the rMpGFP vector at MOI 50 reduced total GFP expression rates of both the MpGFP vector and the rMpGFP vector, compared to those of same MOI of MpGFP vector alone (Supplementary Figure S4). These data suggest that a large amount of noninfectious viral particles might saturate some regulation factors and block viral transduction for human CD34+ cells. Possible reasons include that a large amount of viral particles might saturate vesicular stomatitis virus glycoprotein receptors (since vesicular stomatitis virus glycoprotein envelop was used for all lentiviral vectors),21 many viral RNAs might enhance innate immunity reaction,22,23,24 or a large amount of preintegration complex might saturate nuclear transport factors.25,26
Whether to include HS4 insulators in therapeutic vectors depends upon both major properties of HS4 insulators. A lack of efficacy in improving transgene expression could be balanced by efficacy in enhancer-blocking ability. In a clinical study of gamma retroviral gene therapy for X-linked severe combined immunodeficiency disease, four successfully treated patients developed T-cell type acute lymphoblastic leukemia by insertional mutagenesis.27,28,29 Although the vector design of a self-inactivating-LTR and a lineage-specific promoter may significantly reduce the risk of insertional mutagenesis,30,31 we reasoned that the insertion of the HS4 insulator into 3′LTR may further reduce risk by the enhancer-blocking activity in the LTRs after integration. Our results demonstrated a modest enhancer-blocking effect when HS4 insulators are placed between a strong enhancer and a constitutively active promoter (Figure 4), and thus provide a minor improvement over self-inactivating-lentiviral vectors with a lineage-specific promoter.
Another clinical gene therapy trial, but for β-thalassemia, is now ongoing in France.32,33 Two patients received autologous bone marrow transplantation in which enriched CD34+ cells were transduced with HIV1-based lentiviral vector containing a β-globin gene under the control of component of the β-globin locus control region, β-globin promoter, and 3′ untranslated region. This vector included tandem 250b chicken HS4 insulator elements in the LTR. The first patient had prolonged post-transplant cytopenia and eventually received backup CD34+ cells for hematopoietic rescue. The second patient with HbE/β-thalassemia had hematopoietic reconstitution 5 weeks after transplantation, and became transfusion independent with a hemoglobin level above 9 g/dl around 1 year later. However, a “relative clonal dominance” was found: around 10% of genetically modified cells were derived from one clone, identified by integration in the HMGA2 gene. The HMGA2 gene is a chromatin-binding protein, which interacts with transcription factors to regulate gene expression. The presence of HMGA2 clone may be explained by engraftment from a small number of transduced HSCs, or by the 250b HS4 insulator fragments being insufficient to prevent insertional mutagenesis, but longer follow-up and more in depth analysis will be necessary to fully understand this observation.
In summary, the full-length HS4 insulator was proved undesirable for in vivo gene transfer experiments because of prohibitively low viral titers. While reverse-oriented 250b HS4 core insulators demonstrated no reduction in titers, transduction efficiency was reduced in human GPA+ cells (ex vivo erythroid culture) and in engrafted human cells (immunodeficient mice). Together these data and the available clinical trial observations suggest that HS4 insulated vectors produce minimal effects on expression and offer modest enhancer-blocking activity at a cost of reduced titers and reduced transduction efficiency when tested in human primary cells both in vitro and in vivo.
Materials and Methods
Plasmid construction. We constructed various types of insulated lentiviral vectors to evaluate HS4 insulator function (Figure 1). HIV1-based lentiviral vector plasmids were kindly provided by Dr Arthur Nienhuis.34 We used a reverse-oriented GFP expression cassette under the control of a MSCV-LTR promoter (rMpGFP) (Figure 1a) or a conventional reverse-oriented β-globin expression cassette, in which the β-globin gene was changed to GFP complementary DNA (pCL20c BGpGFP) (Figure 1b). The β-globin-expressing lentiviral plasmid was kindly provided by Dr Michel Sadelain.2 A full-length HS4 insulator (1.2 kb HS4), a tandem HS4 core insulator (2 × 250b HS4), and a single core insulator (250b HS4) were inserted into the deleted U3 region of 3′LTR. The insulator elements were inserted in both forward (F) and reverse (R) orientations.
To evaluate enhancer-blocking activity, we constructed a forward-oriented lentiviral vector plasmid expressing GFP under the control of an MSCV-LTR promoter with a CMV enhancer. Forward and reverse orientations of the 1.2 kb HS4, 2 × 250b HS4, and 250b HS4 insulators were inserted between the CMV enhancer and MSCV-LTR promoter (Figure 4a). A detail of plasmid construction is shown in the Supplementary Materials and Methods.
Lentiviral vector preparation. Self-inactivating HIV1-based lentiviral vectors were prepared and tittered as previously described.16,17,35 Briefly, lentiviral vectors were prepared in 10 cm dishes by cotransfection of 293T cells with 10 µg of various vector plasmids, 6 µg of Gag/Pol plasmids (pCAG-KGP1.1R), 2 µg of Rev/Tat plasmids (pCAG4-RTR2), and 2 µg of vesicular stomatitis virus glycoprotein envelope plasmids (pCAGGS-vesicular stomatitis virus glycoprotein). The rMpGFP vectors were tittered on HeLa cells, and the BGpGFP vectors on MEL cells, as previously described.16,17,35 For triplicate evaluation of viral titers, 8 × 105 293T cells were cultured on 6-well dishes. Twenty-four hours later, 20 µg of plasmid mixtures were divided into three and these were added to 293T cells using calcium phosphate transfection methods.16,17,35
Transduction of cells and erythroid differentiation. The MEL cell line (D1B, TIB-56; ATCC, Manassas, VA) was transduced with various insulated vectors. These vectors were added to 12-well plates containing 1 × 105 cells per 1 ml of Roswell Park Memorial Institute 1640 medium containing 10% fetal bovine serum (FBS) and 8 µg/ml polybrene (hexadimethrine bromide; Sigma-Aldrich, St Louis, MO). Three or four days later, GFP expression was detected by flow cytometry analysis using FACSCalibur (BD Biosciences, Franklin Lakes, NJ). Transduced MEL cells were induced to differentiate in Dulbecco's modified Eagle medium, 10% FBS, and 5 mmol/l N,N′-hexamethylene bisacetamide (Sigma-Aldrich).14 Six days later, GFP expression was evaluated by FACSCalibur. HeLa and 293T cell lines were cultured in Dulbecco's modified Eagle medium containing 10% FBS and were transduced under the same conditions as the MEL cell line.
Human CD34+ cells were isolated by positive immunoselection from peripheral blood mononuclear cells that were harvested by leukapheresis after recombinant human granulocyte colony-stimulating factor injection under a protocol approved by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Institutional Review Board.17 The CD34+ cells were transduced with insulated or control lentiviral vectors in X-VIVO10 medium supplemented with stem cell factor, FMS-like tyrosine kinase 3 ligand, and thrombopoietin, as previously described.17 Transduced CD34+ cells were cultured in erythroid differentiation medium consisting of Dulbecco's modified Eagle medium containing 30% FBS, bovine serum albumin, β-mercaptoethanol, dexamethasone, holo-transferrin, stem cell factor, erythropoietin, and transforming growth factor-β.36 Human erythroid cells were detected using a human GPA—phycoerythrin conjugated antibody (clone GA-R2; BD Biosciences), and GFP expression in GPA+ cells was evaluated by flow cytometry using a FACSCalibur.
Human xenograft mouse model. Male NOD/SCID/IL2Rγnull mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; Jackson Laboratory, Bar Harbor, ME) that were 8-weeks old were used following the guidelines set out by the Public Health Services Policy on Humane Care and Use of Laboratory Animals under a protocol approved by the NIDDK Animal Care and Use Committee. Mice were intraperitoneally injected with 35 mg/kg busulfan (Busulfex; PDL BioPharma, Redwood City, CA) 2 days before transplantation.37 Human CD34+ cells (2 × 106 cells per mouse) were prestimulated for 24 hours and transduced with insulated (250b HS4 R) or uninsulated rMpGFP vectors at MOI 20 for 24 hours.17 Transduced human CD34+ cells were then injected into the tail vein of the mice. Following transplantation, we obtained peripheral blood of the mice to evaluate GFP expression in human CD45+ cells (human CD45-phycoerythrin antibody; clone HI30, BD Biosciences) over 6 months post-transplantation.
Statistical analysis. Statistical analyses were performed using JMP 7 software (SAS Institute, Cary, NC). In vitro experiments comparing various types of insulated vectors to the uninsulated vector were analyzed using Dunnett's test (one-way ANOVA). In vivo experiments comparing the 250b HS4 R insulated vector and the uninsulated vector were analyzed using the Student's t-test. A P value of <0.05 was deemed significant. SEs of the mean are shown as error bars in all figures.
SUPPLEMENTARY MATERIAL Figure S1. Comparison of infectious titers with p24 titers. Figure S2. Evaluation of CpG methylation status by AscI enzyme activity in the MSCV promoter. Figure S3. Multiple integration patterns evaluated by LAM-PCR. Figure S4. Effects of addition of rMpGFP vector on CD34+ cell transduction with MpGFP vector. Materials and Methods.
Acknowledgments
This work was supported by the intramural research program of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) at the National Institutes of Health.
Supplementary Material
Comparison of infectious titers with p24 titers.
Evaluation of CpG methylation status by AscI enzyme activity in the MSCV promoter.
Multiple integration patterns evaluated by LAM-PCR.
Effects of addition of rMpGFP vector on CD34+ cell transduction with MpGFP vector.
REFERENCES
- Sadelain M, Wang CH, Antoniou M, Grosveld F., and, Mulligan RC. Generation of a high-titer retroviral vector capable of expressing high levels of the human β-globin gene. Proc Natl Acad Sci USA. 1995;92:6728–6732. doi: 10.1073/pnas.92.15.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, et al. Therapeutic haemoglobin synthesis in β-thalassaemic mice expressing lentivirus-encoded human β-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- Miller AD, Bender MA, Harris EA, Kaleko M., and, Gelinas RE. Design of retrovirus vectors for transfer and expression of the human β-globin gene. J Virol. 1988;62:4337–4345. doi: 10.1128/jvi.62.11.4337-4345.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestina TI, Hargrove PW, Jay D, Gray JT, Boyd KM., and, Persons DA. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol Ther. 2009;17:245–252. doi: 10.1038/mt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S, May C, Chadburn A, Rivière I., and, Sadelain M. A novel murine model of Cooley anemia and its rescue by lentiviral-mediated human β-globin gene transfer. Blood. 2003;101:2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- Persons DA, Allay ER, Sabatino DE, Kelly P, Bodine DM., and, Nienhuis AW. Functional requirements for phenotypic correction of murine β-thalassemia: implications for human gene therapy. Blood. 2001;97:3275–3282. doi: 10.1182/blood.v97.10.3275. [DOI] [PubMed] [Google Scholar]
- Imren S, Payen E, Westerman KA, Pawliuk R, Fabry ME, Eaves CJ, et al. Permanent and panerythroid correction of murine β thalassemia by multiple lentiviral integration in hematopoietic stem cells. Proc Natl Acad Sci USA. 2002;99:14380–14385. doi: 10.1073/pnas.212507099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveetil G, Scholes J, Carbonell D, Qureshi N, Xia P, Zeng L, et al. Successful correction of the human β-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104:3445–3453. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- Malik P, Arumugam PI, Yee JK., and, Puthenveetil G. Successful correction of the human Cooley's anemia β-thalassemia major phenotype using a lentiviral vector flanked by the chicken hypersensitive site 4 chromatin insulator. Ann N Y Acad Sci. 2005;1054:238–249. doi: 10.1196/annals.1345.030. [DOI] [PubMed] [Google Scholar]
- Wu X, Li Y, Crise B., and, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- Rivella S., and, Sadelain M. Genetic treatment of severe hemoglobinopathies: the combat against transgene variegation and transgene silencing. Semin Hematol. 1998;35:112–125. [PubMed] [Google Scholar]
- Gaszner M., and, Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- Chung JH, Bell AC., and, Felsenfeld G. Characterization of the chicken β-globin insulator. Proc Natl Acad Sci USA. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam PI, Scholes J, Perelman N, Xia P, Yee JK., and, Malik P. Improved human β-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Urbinati F, Arumugam P, Higashimoto T, Perumbeti A, Mitts K, Xia P, et al. Mechanism of reduction in titers from lentivirus vectors carrying large inserts in the 3'LTR. Mol Ther. 2009;17:1527–1536. doi: 10.1038/mt.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Hanawa H, Dan K, Inokuchi K., and, Shimada T. Leukemogenesis of b2a2-type p210 BCR/ABL in a bone marrow transplantation mouse model using a lentiviral vector. J Nippon Med Sch. 2009;76:134–147. doi: 10.1272/jnms.76.134. [DOI] [PubMed] [Google Scholar]
- Uchida N, Washington KN, Hayakawa J, Hsieh MM, Bonifacino AC, Krouse AE, et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol. 2009;83:9854–9862. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EM, Choi U, Theobald N, Linton G, Long Priel DA, Kuhns D, et al. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood. 2010;115:783–791. doi: 10.1182/blood-2009-05-222760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kim YS, Larochelle A, Renaud G, Wolfsberg TG, Adler R, et al. Sustained high-level polyclonal hematopoietic marking and transgene expression 4 years after autologous transplantation of rhesus macaques with SIV lentiviral vector-transduced CD34+ cells. Blood. 2009;113:5434–5443. doi: 10.1182/blood-2008-10-185199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Zickler P, Hoffmann G, Haas S, Wissler M, Muessig A, et al. Polyclonal long-term repopulating stem cell clones in a primate model. Blood. 2002;100:2737–2743. doi: 10.1182/blood-2002-02-0407. [DOI] [PubMed] [Google Scholar]
- Guibinga GH, Miyanohara A, Esko JD., and, Friedmann T. Cell surface heparan sulfate is a receptor for attachment of envelope protein-free retrovirus-like particles and VSV-G pseudotyped MLV-derived retrovirus vectors to target cells. Mol Ther. 2002;5 5 Pt 1:538–546. doi: 10.1006/mthe.2002.0578. [DOI] [PubMed] [Google Scholar]
- Mariani R, Chen D, Schröfelbauer B, Navarro F, König R, Bollman B, et al. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell. 2003;114:21–31. doi: 10.1016/s0092-8674(03)00515-4. [DOI] [PubMed] [Google Scholar]
- Sheehy AM, Gaddis NC, Choi JD., and, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P., and, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L., and, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Aida Y., and, Matsuda G. Role of Vpr in HIV-1 nuclear import: therapeutic implications. Curr HIV Res. 2009;7:136–143. doi: 10.2174/157016209787581418. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Evans-Galea MV, Gray JT, Bodine DM, Persons DA., and, Nienhuis AW. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111:1866–1875. doi: 10.1182/blood-2007-04-085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, et al. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA. Gene therapy continues to mature and to face challenges. Mol Ther. 2009;17:1305–1306. doi: 10.1038/mt.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons DA. Hematopoietic stem cell gene transfer for the treatment of hemoglobin disorders. Hematology Am Soc Hematol Educ Program. 2009. pp. 690–697. [DOI] [PubMed]
- Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE, et al. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Kelly PF, Nathwani AC, Persons DA, Vandergriff JA, Hargrove P, et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- Bhanu NV, Trice TA, Lee YT., and, Miller JL. A signaling mechanism for growth-related expression of fetal hemoglobin. Blood. 2004;103:1929–1933. doi: 10.1182/blood-2003-05-1624. [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Hsieh MM, Uchida N, Phang O., and, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem Cells. 2009;27:175–182. doi: 10.1634/stemcells.2008-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of infectious titers with p24 titers.
Evaluation of CpG methylation status by AscI enzyme activity in the MSCV promoter.
Multiple integration patterns evaluated by LAM-PCR.
Effects of addition of rMpGFP vector on CD34+ cell transduction with MpGFP vector.