Abstract
Original reports of adeno-associated virus (AAV) vector-mediated gene transfer to the muscle resulted in high-level β-galactosidase (β-gal) expression and the promise of a viral vector that was largely nonimmunogenic. Subsequent attempts to utilize these vectors for genetic vaccination, however, demonstrated that it was possible to activate cellular and humoral immunity to AAV-encoded antigens. These findings fueled years of investigation into factors impacting the immunogenicity of recombinant AAV-mediated gene delivery, including route of administration, dose, host species, capsid serotype, and transgene product. In cases where AAV vectors could avoid transgene-directed immunity, it became clear that mechanisms of tolerance were at work, varying between ignorance, anergy/deletion, or active suppression. Here, we follow the field of AAV gene therapy from inception, as investigators have worked to understand the delicate balance between AAV-mediated tolerance and the activation of immunity. This review discusses our current appreciation of AAV vector immunology, with a specific focus on the transgene-specific T cell response.
Introduction
Over the past 20 years, the field of gene therapy has expanded in its attempts to treat monogenetic disease. To this end, numerous vectors, both viral and nonviral, have been developed to deliver therapeutic genes directly in vivo, with the basic objective of achieving sustained, high-level gene expression. In order to reach this goal, the vector of choice must be capable of transducing target cells with high efficiency, while simultaneously avoiding activation of deleterious immune responses to either the transgene product, or to the delivery vehicle itself.1 Historically, the use of nonviral gene therapy vectors, including DNA and liposomes, has resulted in suboptimal levels of transduction and transient expression in vivo.2,3 Initial attempts at viral vector-based gene therapy using retro- or adenoviruses (Ad), have been met with issues of toxicity, either through activation of immunity or genomic integration and tumor formation.4,5
In the search for a gene therapy vector for in vivo gene transfer, one virus emerged with a favorable profile of background properties. With its nonpathogenic and helper-dependent nature, adeno-associated virus (AAV), a small (~4.7 kb) single-stranded DNA virus, quickly came to the fore-front of the field. As a vector, AAV consists of only the inverted terminal repeats which are necessary for replication, packaging, and integration, while the viral coding sequences are entirely removed, rendering AAV vectors replication deficient.6 Similar to adenoviral vectors, AAV can transduce both dividing and nondividing cells; additionally, when delivered to postmitotic or slowly dividing target cells, AAV has the potential to facilitate long-term transgene expression in the absence of destructive T cell responses.7,8,9
This group of parvoviruses was identified over 30 years ago as contaminants in laboratory preparations of Ad.10,11 Six serotypes of AAV were originally identified, of which AAV serotype 2 was the most readily studied. Since then, an expanded family of AAVs has been isolated from humans and nonhuman primates based on recovery of latent forms of the genome using PCR techniques.12,13,14 To date, 11 serotypes and over 120 capsid variants have been categorized into six different phylogenetic clades representing a broad distribution of potential AAV biology.12,13,14,15,16,17,18,19 Application of these novel viruses as recombinant vectors for gene therapy has yielded impressive results, with transduction efficiencies superior to those achieved by vectors based on AAV serotypes 1–6. Encouraging preclinical data in mice has shown that recombinant AAV vectors are capable of generating stable, high-level gene expression for a variety of applications, including liver, lung, muscle, brain, and eye-directed gene transfer.20,21,22,23,24,25,26,27 In these cases, the stability of expression was facilitated by an apparent lack of immunogenicity to the transgene product. Although the molecular mechanisms are poorly understood, studies have confirmed that AAV is a poor activator of both innate and adaptive immunity when compared to other commonly used viral vectors, such as adenovirus.28
Despite its promise, investigators soon discovered that even AAV is not without its limitations: both from the very basic standpoint of its small packaging capacity, to the larger concern that immune responses to AAV vector-mediated gene delivery can develop under certain conditions. Due to natural infections or prior vector-administration, neutralizing antibodies can prevent vector readministration. Furthermore, cellular and humoral immune responses to the transgene product may result in inflammation, elimination of gene expression and destruction of transduced cells.29,30,31,32 Furthermore, as studies have progressed toward clinical trials, we have learned that while the threshold required for immune activation to AAV in mice is relatively high, immune responses following AAV gene delivery occur more readily in larger animal models and humans.33,34,35 As such, understanding the mechanism of immune activation to AAV and developing strategies to avoid or circumvent immunotoxicity are critical to the safe and efficacious use of AAV for gene delivery. This review discusses our current understanding of AAV immunology, with a specific focus on the generation or evasion of transgene-specific T cell responses. Here, we have taken a chronological approach that follows the development of the field over the past 20 years.
AAV Generates Stable Transgene Expression
In the 1990s, when limitations to nonviral and adenoviral vector-based gene therapies began to surface, efforts were directed to developing AAV as a viral vector because it was capable of generating prolonged expression and avoiding immune activation. In the mid to late 90's, three laboratories gave the first reports of long-term, high level expression of an Escherichia coli β-galactosidase (β-gal) transgene following intramuscular (i.m.) administration.7,8,9 The use of other vector systems to target the muscle for somatic gene delivery failed to generate stable expression due to either transgene silencing or an immune response against transduced cells. For the first time, the labs of Byrne, Samulski, and Wilson provided evidence that with the use of an AAV vector, immunity to a highly expressed, nonself transgene could be avoided, allowing for prolonged and robust expression in mice.7,8,9
To summarize, after a single i.m. administration of recombinant AAV2/2 into BALB/c mice, Kessler et al. demonstrated histochemical expression of β-gal in situ, out to 32 weeks postinjection.7 Xiao et al. simultaneously confirmed this result, following mice out to 1.5 years post-i.m. administration of AAV2/2.LacZ.8 This group also showed a direct comparison to an adenoviral vector expressing the identical LacZ transgene. Compared to AAV2/2, Ad.LacZ demonstrated inefficient transduction of mature myocytes, accompanied by a large degree of cellular infiltration in the muscle 4 days postinjection. This cellular response cleared after 3 weeks, at a time when Ad.LacZ positive muscle fibers were no longer present. In contrast, AAV2/2 generated very minimal and self-limiting cellular infiltration, which had no effect on the efficiency or stability of transduction. Fisher et al. confirmed the persistence of high-level β-gal expression in the muscle of C57BL/6 mice following a single i.m. injection of AAV2/2.LacZ.9
In an attempt to further define the mechanism involved in avoiding immune activation to β-gal, Fisher et al. analyzed lymphocytes harvested from inguinal lymph nodes for cytolytic activity and cytokine production.9 As expected from its transient expression, Ad.LacZ generated strong cellular and humoral responses to both adenoviral proteins and β-gal, including antigen-induced secretion of interferon-γ and interleukin (IL)-10. Conversely, AAV2/2.LacZ was unable to elicit antibodies or cytotoxic T lymphocytes (CTLs) and showed little to no activation of CD4+ T cell subsets to the β-gal transgene. Th2-type CD4+ T cell responses were activated to the AAV viral proteins, resulting in a neutralizing antibody response to the vector capsid, however, this was unable to block readministration of the vector in this study. Analysis of the AAV genome demonstrated efficient incorporation into nuclei of differentiated muscle fibers where head-to-tail concatamers with variable inverted terminal repeat deletions appeared to persist. These were the first reports of achieving stable transduction and long-term gene expression in vivo without the need for immunosuppression or modulation.
Around this time, reports of stable expression with AAV2/2 vectors expressing alternative transgenes emerged. In fact, in their original report, Kessler et al. documented the stability of AAV2/2.Epo, which resulted in a dose-dependent secretion of human erythropoietin and corresponding increases in red blood cell production that persisted for up to 40 weeks after a single i.m. injection into BALB/c mice.7 The Fisher et al. study also showed stability of human β-glucuronidase in the muscle of this small animal model.9 Over the years, preclinical data have shown that recombinant AAV vectors can generate stable expression of numerous self and nonself transgenes, not only in the muscle, but in tissues ranging from liver, lung, and heart to the brain and eye.20,21,22,23,24,25,26,27 These results can also extend beyond murine models into higher order species. Stability of human or canine blood coagulation factor IX (hFIX or cFIX, respectively) expression in the liver, for example, has been reported in mice, hemophilic dogs and rhesus macaques.20,36,37,38,39 It is important to note that findings of stable transgene expression in larger animal models are not consistently demonstrated, as will be discussed further in greater detail.
Stable Transgene Expression Involves Lack of Antigen-Presenting Cell Transduction: Evidence for Immunological Tolerance
Recombinant AAV vectors are nonreplicating and lack viral open reading frames; however, the encoded (nonself) transgene product and viral capsid proteins are still viable targets for host immune responses. As such, investigators began to question how AAV expressing β-gal could efficiently transduce muscle fibers without activating cellular or humoral immunity, especially considering that expression of the identical transgene in an adenoviral vector resulted in robust capsid and transgene T cell responses capable of clearing transduced cells.40 How does AAV evade immune activation? Does AAV avoid T cell priming entirely? Are T cells primed improperly leading to tolerance? Or, does AAV transduction render peripheral target cells unrecognizable to CTLs?
To address this, in 1998 Jooss et al. made an interesting discovery relating to the ability of AAV2/2 to avoid β-gal transgene-specific immune responses in the muscle.40 This group demonstrated that while AAV2/2.LacZ alone resulted in stable expression and no T cell activation, coinjection with an Ad.LacZ vector in the contralateral leg resulted in substantial inflammation, T cell infiltration, and loss of β-gal protein expression in both the Ad and AAV-transduced muscles. This confirmed that muscle fibers transduced with AAV were suitable targets for CTL-mediated destruction, but that AAV transduction alone was unable to prime functional, antigen-specific T cells. The authors went on to show that adoptive transfer of ex vivo Ad.LacZ transduced antigen-presenting cells (APCs) into AAV2/2.LacZ injected mice was sufficient to support the priming of strong CD4+ and CD8+ T cell responses that effectively cleared AAV-transduced muscle fibers. However, APCs exposed to AAV2/2.LacZ were not sufficient to prime a CTL response following adoptive transfer into AAV2/2.LacZ-injected mice. Furthermore, while transduction of APCs with Ad.LacZ showed LacZ transgene expression in dendritic cells (DCs) and macrophages, LacZ expression was undetectable in APCs exposed to AAV2/2.LacZ. Taken together, these results demonstrate that APC transduction and presentation of endogenously produced antigen are crucial in the successful development of β-gal-specific T cell responses, as seen with Ad.LacZ. However, AAV vectors show inefficient transduction and antigen presentation by APCs, allowing AAV to evade the generation of a cytotoxic T cell response.
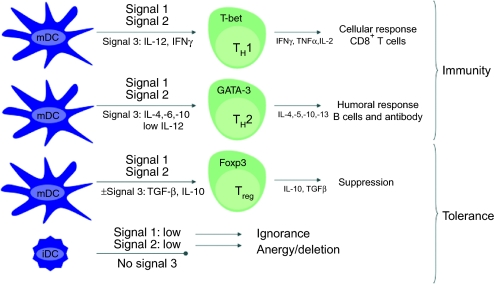
Two years later, Zhang et al. went on to show that if enough DCs are recruited and transduced by AAV2/2.LacZ ex vivo, adoptive transfer into AAV2/2.LacZ-injected mice could result in the development of β-gal-specific T cells and a marked reduction in transgene expression in the muscle.41 However, AAV2/2.LacZ transduction was only capable ex vivo, in immature DCs (iDCs) but not mature DCs. Even then, transduction of iDCs with AAV could not induce DC maturation, as the iDCs did not upregulate surface expression of major histocompatibility complex (MHC), CD86, and CD40 molecules, as opposed to stark upregulation of these DC activation markers by Ad.LacZ transduction. Presentation of antigen by iDCs has been shown to favor the induction of tolerance over immunity (Figure 1). In summary, the loss of transgene expression occurred only by adoptive transfer of iDCs infected ex vivo (not by direct i.m. injection of AAV). This study confirms that direct i.m. injection of AAV2/2.LacZ does not provide sufficient DC transduction and antigen presentation to facilitate an adaptive response.
Figure 1.
The activation status of APCs influences the immunogenic or tolerogenic nature of the adaptive response. The type of pathogen and its interaction with pattern recognition receptors (PRRs) on the surface of the dendritic cell (DC) influences the maturation of different subsets of either immunogenic or tolerogenic DCs. Each type of mature DC (mDC) has a unique cytokine profile which acts as signal 3 during T cell priming. If signal 3 consists of the proinflammatory cytokines IL-12 and IFN-γ, a Th1 response is primed, which in turn provides help in priming functional CD8+ T cell effectors. In the presence of IL-4, IL-6, and IL-10, mature DCs prime Th2-type CD4+ T cells, which favor a humoral response. Regulatory T cells (Treg) can be primed by mDCs which are either unable to secrete cytokines, or secrete the anti-inflammatory cytokine TGF-β. Immature DCs (iDC) have a reduced T cell stimulatory capacity, which also leads to tolerance. APC, antigen-presenting cell; IFN, interferon; IL, interleukin; TGF-β, transforming growth factor-β TNF-α, tumor necrosis factor α.
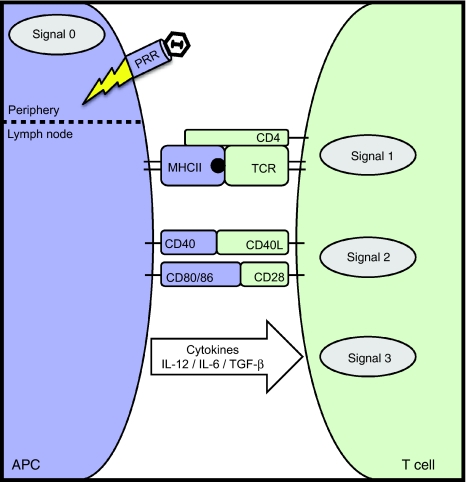
Along with APC transduction, the activation of innate immunity (signal 0) stimulates the maturation of APCs, characterized by the upregulation of MHCII, CD80/86, and CD40, and the release of proinflammatory cytokines and chemokines (Figure 2). Without these signals, APCs cannot properly migrate, take up, or present antigen (signal 1). Not surprisingly based on their ability to transduce APCs and prime T cells effectively, adenoviral vectors have been described as strong inducers of innate immunity. Zaiss et al. went on to show that AAV2/2 vectors, on the other hand, elicit significantly reduced expression of inflammatory cytokines and chemokines, including RANTES, IP-10, IL-8, MIP-1α and β, as compared to Ad.LacZ both in vitro and in vivo.28 In fact, the use of AAV2/2.LacZ in vitro showed no induction of innate immune markers above baseline, and the induction of chemokines in vivo was transient, returning to baseline 6 hours postinjection. Overall, their results suggest that AAV vectors, unlike Ad, are poor activators of innate immunity, which may prevent proper activation and performance of APCs.
Figure 2.
The APC—T cell synapse: three signal model for T cell activation. In the periphery, initial interaction of AAV with pattern recognition receptors (PRRs), such as TLRs, initiates the activation and maturation of APCs in a process called innate immunity, or signal 0. This allows APCs to upregulate the surface molecules and cytokines involved in signals 1–3, and promotes migration to the lymph node where APCs come into contact with naive T cells. In the lymph node, signal 1 activates the T cell through interaction of the T cell receptor (TCR) with antigen presented in the context of MHC molecules. MHCI binds the CD8 TCR; MHCII interacts with the CD4. Costimulation in the form of CD80/CD86 (also known as B7.1 and B7.2) and CD28 ligation provides the necessary second signal, promoting T cell function and survival. The CD40–CD40L (CD154) interaction is important during costimulation, as it further activates the APC, supporting upregulation of CD80/86. Cytokines, either pro- or anti-inflammatory in nature, provide signal 3, which dictates the differentiation status of the T cell. AAV, adeno-associated virus; APC, antigen-presenting cell; IL, interleukin; MHC, major histocompatibility complex; TGF-β, transforming growth factor-β.
These reports were the first attempts at describing the mechanism of immune activation, or rather immune evasion, by AAV. Taken together, they suggest that one factor contributing to the lack of immunogenicity following AAV vector-mediated gene delivery is immunological ignorance: a mechanism of tolerance which involves the lack of antigen presentation required to prime a cellular response (no signal 1) (Figure 1). A second factor appears to be insufficient activation of innate immunity (signal 0) to promote the upregulation of costimulatory molecules necessary for T cell priming (signal 2). This poor innate activation prevents proper maturation of APCs, limiting the ability of those cells to take up, process and present antigen. In combination, AAV's poor transduction of APCs provides even less of the antigen necessary to prime naive T cells. Ultimately, it appears that there is a threshold of innate immune activation and APC transduction that must be reached in order to elicit the downstream adaptive response to viral antigens. I.m. injection of AAV2/2.LacZ was simply not capable of reaching this threshold. This also suggests that under more inflammatory conditions, using alternative transgenes, adjuvants or routes of administration, AAV vectors may overcome this threshold, resulting in the priming of cellular immunity to encoded antigens. In fact, one study in later years suggests it may be possible to achieve some level of innate immune activation, even with AAV.42
Stability of Expression is Transgene- Dependent: Evidence of Immune Activation
It did not take long for investigators to discover conditions whereby AAV was capable of reaching the threshold required to activate immunity. In the late 90's, Manning et al. was the first to report that i.m. injection of AAV2/2 encoding transgenes other than β-gal were able to generate both CTL and humoral responses in mice.31 Attempting to utilize AAV for genetic immunization, the group constructed AAV vectors that expressed either herpes simplex virus type 2 glycoprotein B (gB) or glycoprotein D (gD). They demonstrated that, unlike AAV2/2.LacZ, i.m. injection of AAV2/2.gB or gD led to transgene-specific CTL and antibody. Interestingly, a single immunization of AAV2/2.gD was more effective in generating gD-specific antibodies than plasmid DNA or recombinant protein. When restimulated in vitro, the gB-specific T cells demonstrated rapid proliferation in response to antigen and destruction of gB-expressing target cells.
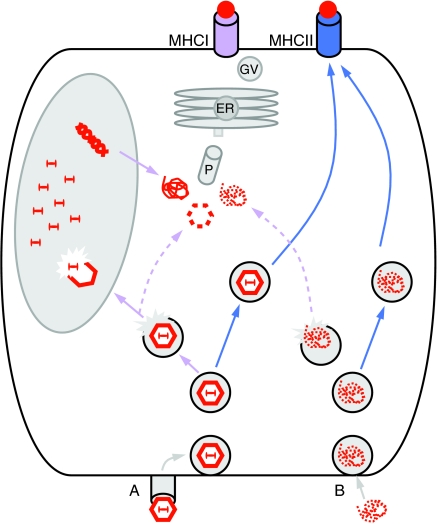
A few years later in 1999, Brockstedt et al. observed that AAV2/2 expressing the secreted protein ovalbumin (Ova) could also elicit Ova-specific cytotoxicity in mice.32 These investigators took the study one-step further by showing that AAV was delivering Ova epitopes into the classical MHC class I pathway of antigen presentation. Briefly, when vector enters the cell, endogenously produced peptides are processed in the cytosol, transported into the endoplasmic reticulum, and subsequently presented on the cell surface in the context of an MHCI molecule in a process called direct presentation (Figure 3). Endogenously expressed transgene products can be presented in MHCI on the surface of all cell types: both on professional APCs, to prime naive CD8+ T cells in the lymph node; or on all peripheral cells, to mark them as targets for CTL-mediated destruction. In this study, AAV2/2.Ova infection resulted in presentation of Ova antigen on MHCI, which was sufficient to activate a CD8+ T cell hybridoma to stimulate IL-2, and also act as a target cell to an Ova-specific MHCI restricted CTL line. Several years later, Wang et al. confirmed this finding by demonstrating that within 10 days postinjection, Ova-specific T helper cells were activated in draining lymph nodes and Ova-expressing muscle fibers were eliminated by a transgene-specific CD8+ T cell response.43
Figure 3.
Processing and presentation of AAV-encoded antigens in the APC. (a) AAV vector transduces the APC by receptor-mediated endocytosis. Endosomal escape and nuclear uncoating lead to production of the endogenous transgene product. In the cytosol, endogenously produced antigen is processed through the proteosome (P), enters the endoplasmic reticulum (ER) via TAP, where it is loaded onto MHCI and shuttled through the Golgi apparatus to the cell surface by “direct presentation.” Capsid fragments can be processed within the endosome for direct presentation onto MHCII; capsid can also escape the endosome and get fed into the ER to be “cross-presented” onto MHCI. (b) Phagocytosis of transduced cells or secreted transgene products enter the APC. Exogenous antigen is processed in the endosome and presented onto MHCII. Conversely, exogenous antigen can escape the endosome and be cross-presented onto MHCI. (Purple solid line, MHCI direct presentation; purple dashed line, MHCI cross-presentation; purple receptor, MHCI; blue line, MHCII presentation; blue receptor, MHCII; GV, golgi vesicle). AAV, adeno-associated virus; APC, antigen-presenting cell; MHC, major histocompatibility complex.
Ovalbumin and herpes simplex virus glycoproteins were not the only transgenes to push AAV vectors beyond the threshold of immune activation. In 2000, Sarukhan et al. demonstrated that i.m. injection of AAV2/2 expressing the strongly immunogenic influenza virus hemagglutinin (HA) protein could also result in a cellular immune response and the elimination of transduced muscle fibers within 4 weeks.44 Interestingly, they observed that the kinetics of CD4+ T cell activation were substantially delayed following AAV2/2.HA as compared to Ad.HA, which could be attributed to mechanistic differences of antigen presentation between the two vectors. Due to AAV's poor transduction of professional APCs, transgene products are not endogenously available for direct presentation onto MHCI. Therefore, in order to prime CD8+ T cells, transgene antigens must be taken up exogenously, released from the endosome and fed into the endoplasmic reticulum for loading onto MHCI in a process known as “cross-presentation” (Figure 3). Adenoviral vector-mediated delivery of HA, on the other hand, could prime transgene-specific CD8+ T cells by both direct transduction of DCs and cross-presentation of the transgene product, which may explain the enhanced kinetics of T cell activation in this case.
These findings indicated that AAV vectors are not always nonimmunogenic and emphasized the impact of the transgene product in activating an immune response. As a result, investigators began to question what other factors might trigger the generation of cellular responses following AAV gene transfer.
Additional Factors Influencing the Generation of Transgene-Specific T cells
Target tissue: route of administration and promoter specificity
When Brockstedt et al. reported Ova-specific CTL responses after a single administration of AAV2/2.Ova by either i.m., intravenous, intraperitoneal, or subcutaneous injection, they also noted that the strength of the T cell response was affected by the route of administration.32 Two weeks postinjection, splenocytes or draining lymph nodes were harvested, restimulated in vitro to expand the transgene-specific CTL population, and then assayed by 51Cr-release of antigen loaded target cells. Cytolytic activity was strongest following subcutaneous or intravenous injection, followed by the intraperitoneal and finally, the i.m. route. This study demonstrated that the route of administration affects the generation of cellular immunity to AAV vectors.
Route of administration can impact both the generation of CTLs and the formation of transgene-specific antibodies. The stability of human factor IX expression achieved following AAV2/2 gene transfer to liver was quite different than that achieved following i.m. injection, where hFIX expression was transient and potent transgene-specific CD4+ T cell and B cell responses were observed.36,39,45,46,47,48 In these studies, transduction of the liver allowed for sustained FIX expression and limited immune activation, whereas delivery of AAV2/2.hFIX to skeletal muscle resulted in a reduction in systemic FIX levels concomitant with robust CD4+ T helper-dependent antibody formation.45,46,47 Here, expression of human FIX was studied in immune-competent mice with intact expression of endogenous FIX. Mice carrying null mutations at the factor IX locus also demonstrated robust CD8+ T cell infiltration in transduced muscle, which was greatly reduced following injection into the liver.48 These findings further illustrate the influence of target tissue on immune responses to AAV vector-encoded transgene products.
In addition to the route of administration, the choice of promoter can also greatly influence the tissue specificity of transgene expression, thus further impacting the generation of immunity or tolerance. Using a murine model of the lysosomal storage disorder, Fabry disease, Ziegler et al. compared intravenous administration of AAV2/2 vectors expressing human α-galactosidase A (α-gal) under the control of either the ubiquitous CMV promoter, or a liver-restricted enhancer/promoter, DC190.49 Expression from the ubiquitous promoter, which allows for some off-target transgene expression in tissues other than the liver, resulted in the formation of transgene-specific antibodies concomitant with a reduction in expression levels over time. In contrast, use of the liver-specific promoter resulted in significantly higher levels of enzyme expression with little to no anti-α-gal antibody formation. Overall, the influence of route of administration and promoter selection on immune outcome clearly illustrates the substantial impact of target tissue on the generation of transgene-specific immunity, where the liver itself appears to be a more tolerogenic organ than skeletal muscle.
Dose
Vector dose has a predictable impact on the amount of transduction and subsequent expression levels in injected animals. Increasing vector dose, however, also increases the amount of foreign antigen available for presentation and T cell priming. Therefore, not surprisingly, numerous studies have shown that vector dose can influence the generation of cellular immunity to AAV-encoded proteins. In 2002, Herzog et al. reported that increasing AAV2/2 vector doses i.m. increased inhibitory anti-canine FIX development in hemophilia B dogs.50,51 Three years later, a study by Wang et al. confirmed that while formation of inhibitory antibodies to human FIX in outbred mice was observed over a wide range of vector doses, increased doses caused stronger immune responses.47 These findings indicate that vector dose has an important affect on the formation of inhibitory antibody responses to FIX, which is most likely caused by influencing the extent of local antigen presentation. From this, we can conclude that higher vector doses (per site of i.m. injection) augment the generation of a transgene-specific immune response following muscle-directed AAV gene transfer.
In contrast, studies have shown an indirect correlation between vector dose and anti-FIX antibody formation in the liver. Unlike i.m. delivery approaches, increasing doses of AAV2/2.hFIX favor the establishment of tolerance following hepatic gene transfer by intraportal injection.52 This finding was also evident in a study by Ziegler et al., who showed a more rapid and complete induction of hepatic tolerance to α-galactosidase A when mice were administered higher doses of recombinant AAV2/8.53 These studies further emphasize the influence of target tissue on the immune response to AAV vector-encoded transgenes, where high levels of transgene expression following liver-directed gene transfer are associated with the induction of tolerance, whereas high-level expression in the muscle leads to transgene-specific immunity. Despite the confounding effects of the target tissue itself, one conclusion remains clear: vector dose has a significant impact on the generation of immunity or the establishment of tolerance to AAV vector-encoded transgene products.
Inflammation in the target organ
In light of the well-described impact of innate immune-mediated inflammation on enhancement of adaptive immune responses, one must also consider the baseline level of inflammation present in the target tissue as potentially confounding the outcome of in vivo gene therapy. In 1997, Snyder et al. began to address this question by using BaCl2 to destroy muscle fibers and induce regeneration and inflammation at the time of vector injection. They observed that while AAV2/2.nLacZ was capable of efficiently and stably transducing postmitotic muscle fibers in the absence of BaCl2, very little gene transfer occurred in the severely damaged, regenerating muscle.54 Instead, gene transfer in this context resulted in an early inflammatory response and the elimination of the majority of transduced myofibers. This is relevant to studies using recombinant AAV vectors for the treatment of muscular dystrophies, where subjects or animal models present a large degree of muscle degeneration, necrosis, and inflammation. In a murine model of muscular dystrophy [gsg (−/−) mice lacking γ-sarcoglycan), Cordier et al. demonstrated that an i.m. injection of AAV2/2.LacZ elicited strong cellular and humoral immunity to the transgene product.55 In wild-type mice, AAV2/2.LacZ generates stable gene expression with no immunogenicity, suggesting that the inflammatory environment in the muscle of gsg (−/−) mice lowered the threshold required for immune activation. Interestingly, the use of a muscle-specific promoter (truncated muscle creatine kinase) was capable of subverting immune activation to permit significant muscle transduction in 3–6-week-old gsg (−/−) mice.55,56 In these mice, signs of fibrosis and necrosis were just beginning to develop, and muscle-specificity may have prevented endogenous transduction of APCs by AAV. Injection of 16–40-week-old gsg (−/−) mice, however, showed very limited transgene-expression in muscle fibers, indicating that the muscle creatine kinase promoter can only drive efficient gene transfer if vector is injected before the development of widespread muscular degeneration and extensive fibrosis.56
The strong degree of inflammation present in the muscle of these mice may create a microenvironment more similar to that seen following adenoviral vector injection. As with Ad vector-mediated gene transfer, this state of increased inflammation appears sufficient to support transgene-specific T cell activation to AAV vectors resulting in the elimination of transduced cells.
Host species
As AAV vectors have progressed toward clinical use, numerous animal models have been tested for safety and efficacy. In the majority of cases, investigators have learned that vector performance in mice cannot necessarily predict outcome in nonhuman primates or higher order species. Following direct i.m. injection into random-bred wild-type dogs, for instance, both AAV2/2 and AAV2/6 vectors elicited a robust cellular immune response that was independent of the transgene expressed, the cellular specificity of the promoter, or the muscle type injected.57 Around the same time, another group confirmed these results, showing that despite successful in vitro transduction by AAV2/2.LacZ in primary canine myotubes, gene transfer into skeletal muscles of normal dogs resulted in poor transduction followed by a high degree of cellular infiltration, strong activation of cellular and humoral immunity, and the subsequent loss of transduced cells.58
Furthermore, even in disease models where recombinant AAV vectors show stable expression in canine models, the lack of immunogenicity does not necessarily translate to human subjects enrolled in clinical trials. For instance, in a canine model of severe hemophilia B, liver-directed AAV2/2 vector expressing canine factor IX generated long-term, therapeutic levels of expression.36,37,38 However, a phase 1/2 dose-escalation clinical study to extend this approach to humans with severe hemophilia B resulted in a reduction in hFIX levels over time concomitant with a transient elevation in liver transaminases and a capsid-specific CD8+ T cell response to AAV2/2.33,34
Although preclinical studies are necessary and informative in the design of clinical trials, it is important to note that any lack of immunogenicity observed in animal models is not necessarily predictive of the immune outcome in higher order species. Another recent study directly comparing the performance of identical vectors in both mice and nonhuman primates succinctly illustrates this point. Following injection of AAV2/7.CMV.GFP into mice or cynomolgus macaques, investigators observed similar transduction levels at early time points.35 Over time, green fluorescent protein expression was stable in mice and associated with minimal T cell activation. However, in primates a robust cytotoxic T cell response to green fluorescent protein ensued, resulting in hepatitis and a loss of transgene expression. This indicated that the threshold required to activate immunity to AAV vector-encoded antigens was higher in mice than in nonhuman primates. With the goal of progressing gene therapies toward the clinic, it is essential to understand species-specific differences in immune activation in order to ensure the safety of AAV gene delivery in human subjects.
Capsid serotype
In addition to transgene, dose, route of administration, inflammation, and host, there is yet another factor influencing the activation of immunity: the AAV vector capsid. The AAV capsid can impact transgene T cell responses such that two different serotypes expressing the identical transgene are capable of generating two distinct immune outcomes. A study in 2009 demonstrated that AAV2/8-mediated expression of nuclear targeted β-gal (nLacZ) resulted in stable expression in the absence of T cell responses, whereas novel capsid variant, AAV2/rh32.33, elicited robust nLacZ-specific CD4+ and CD8+ T cells and a loss of β-gal expression in the muscle.59 In this case, the strong CD8+ T cell responses were CD4 helper-dependent; AAV2/8 was incapable of eliciting CD4+ T cell help.
Three recent vaccine studies using recombinant AAV vectors to introduce HIVgag or Env gp160 antigens also showed differences in the level of CD8+ T cell responses primed depending upon whether the transgene antigen was delivered in vectors based on AAV serotypes 1–9.60,61,62 Xin et al. found that AAV2/5 encoding HIV-1 Env gp160 produced the strongest HIV-specific humoral and cell-mediated responses out of serotypes 1–8.60 This correlated with the higher tropism AAV2/5 has for both mouse and human DCs, which is consistent with the theory that poor APC transduction prevents T cell priming to the majority of AAV vectors. Differences in receptor binding and vector uncoating between serotypes undoubtedly influence the transduction and activation of APC populations, leading to variable immune induction.
These studies confirm the ability of vector capsid to impact the generation of T cells toward the encoded transgene-product. It is important to note that the viral capsid itself can also act as a source of antigen for priming T cells. Exogenous antigens, such as the viral capsid, are processed differently than their endogenous counterparts. After entering the endosome, they are loaded onto MHC class II molecules expressed on APCs and presented on the cell surface to prime naive CD4+ T cells (Figure 3). Endosomal escape and cross-presentation onto MHCI also allows for CD8+ T cell priming. Ultimately, differential processing and presentation of capsid versus transgene explains why the generation of cellular immunity to these antigens can be mutually exclusive. While the majority of this review focuses on evidence for the generation of transgene-specific T cells, it is important to note that capsid-specific T cells may also contribute to the clearance of transduced cells following gene transfer.
Becoming Immunologists
With the discovery that AAV was not entirely nonimmunogenic, and the plethora of factors influencing the onset of cellular immunity, the field of gene therapy collectively realized that it was critical to understand the principle factors involved in tipping the balance between tolerance and immunity. In fact, principles of basic host–virus interactions that were characterized in the study of natural infections provided a conceptual framework for studying the immunology of gene therapy. When a foreign antigen is detected, the host's immune system reacts promptly to fight infection and rid the body of the pathogenic threat. During innate immunity, the first wave of the response, pathogen-associated molecular patterns, which distinguish between self- and nonself antigens, are recognized by pattern recognition receptors, such as Toll-like receptors (TLRs), on the surface of professional APCs.63 These cells, including macrophages and DCs, reside in peripheral tissues in an immature state. The interaction of foreign proteins with the APC sends “danger signals” through a signal transduction cascade resulting in the activation of transcription factors, such as nuclear factor-κB, and the production of proinflammatory cytokines, including IL-6, tumor necrosis factor-α, MIP1-α/-β and type I interferons.64 The inflammatory environment stimulates the maturation of APCs, enhancing phagocytic activity and upregulating expression of molecules necessary for antigen processing, presentation, and costimulation, such as MHCII, CD80/86, and CD40.65,66 Endogenously produced antigen is directly presented on MHCI molecules; exogenous antigen is either cross-presented on MHCI or loaded directly onto MHCII (Figure 3).
Adaptive immunity is stimulated later, as APCs migrate to the draining lymph nodes where they present antigens to naive lymphocytes in the context of MHCI or MHCII, priming antigen-specific CD8+ and CD4+ T cells, respectively.66 CD4+ T cells exist in different subsets and provide help and direction to the developing adaptive response.67 The Th2 subclass favors B cell development and antibody production, whereas Th1 CD4+ T cells provide help in the development of cytotoxic CD8+ T lymphocytes (CTLs) (Figure 1). These effector cells undergo rapid proliferation and migration to peripheral sites of infection, where engagement of the CD8+ T cell receptor (TCR) with antigen in the context of MHCI molecules results in recognition and destruction of transduced cells.
Overall, there are three signals required for T cell activation: signal 1, interaction of the T cell receptor with antigen presented in MHC; signal 2, the engagement of costimulatory molecules such as CD80/86 on the APC with CD28 on the T cell; signal 3, additional signals from the APC dictating which T helper subset is primed68,69,70 (Figures 1 and 2). For instance, Th1-inducing DCs provide proinflammatory cytokines and CD40–CD40L interaction to drive a Th1 response. There are also Th2-inducing DCs, which direct a Th2 response, as well as tolerogenic DCs, which favor the development of another subset of CD4s, known as regulatory T cells (Tregs).71,72 Instead of providing help to the developing T or B cell responses, Tregs actively suppress CD8+ effectors. The initial TLR signaling cascade and subsequent inflammation are critical to the unique activation status of APC subsets and their perpetuation of an adaptive response. For this reason, innate immunity is often referred to as signal 0.
Importantly, in the absence of any one signal immune tolerance can result, through either ignorance (lack of signal 1), anergy/deletion (lack of signal 2), or suppression (lack of signal 3). Generally speaking, immunological tolerance describes a state in which the immune system is unable to activate the appropriate cellular or humoral responses following antigen exposure.73 In cases of ignorance, for instance, poor APC transduction may result in insufficient antigen presentation (signal 1), whereby naive T cells are never primed. In the absence of proper costimulation (signal 2), antigen-specific T cells are primed but remain functionally aberrant in a process known as anergy; anergic T cell populations cannot undergo proper proliferation or cytokine secretion in response to antigen. Alternatively, in the absence of the necessary proinflammatory cytokine milieu (signal 3), Tregs may develop, resulting in the active suppression of adaptive immune effectors. It is important to note that signal zero, innate immune-mediated inflammation, is critical in the upregulation of costimulatory molecules on APCs (signal 2) and is therefore necessary to avoid the onset of anergy. Signal 0 may also provide support for signals 1 and 3: by inducing APC maturation, innate immunity enhances antigen uptake and upregulates expression of the antigen processing and presentation machinery that are necessary for signal 1; it also dictates the development of DC subsets, which then supply signal 3 to the naive T cell during priming.
Mechanisms of Avoiding T cell Activation and Inducing Tolerance
Some AAV vectors elicit T cell responses; others avoid them. We have discussed the factors involved in generating cellular immunity. Here, we will discuss the factors involved in avoiding T cell activation by the induction of tolerance. Over the years, numerous studies have investigated the mechanisms of tolerance at work following AAV gene transfer.
Ignorance and the role of innate immunity
As previously discussed, the earliest attempts at defining mechanisms of immune evasion suggested that AAV induces immunologic ignorance through a lack of signal 1. These initial reports concluded that poor transduction of APCs by AAV2/2 resulted in insufficient antigen presentation and a lack of T cell priming to the LacZ transgene.40,41 Over time, however, it became clear that in many cases AAV vectors were capable of eliciting transgene-specific T cell responses, arguing against the ignorance hypothesis, and indicating that tolerance to AAV is often a more complicated story. In 2001, for instance, Sarukhan et al. reported that AAV2/2 expressing a more immunogenic antigen, influenza HA, primed a potent cellular immune response capable of eliminating HA-expressing cells.44 In 2009, Zhu et al. demonstrated that the activation of the CD8+ T cells to AAV2/2.HA was dependent upon TLR9 activation and MyD88 signaling.42 In the absence of TLR9 or MyD88, knockout mice showed diminished CD8+ T cell activation and prolonged HA expression in murine muscle, similar to the state of tolerance observed following AAV2/2.LacZ gene transfer in wild-type mice. These results support the theory that proper innate immune activation (signal 0) is critical to the priming of a functional adaptive response. Taken together, we conclude that poor innate activation and limited APC transduction are two critical mechanisms allowing AAV vectors to avoid immunity.
Compared to adenoviral vectors, AAV typically displays a very minimal inflammatory potential, as it does not commonly engage pattern recognitions receptors, such as TLRs, which are known to initiate innate immune responses.28,74 The use of microarray technology by McCaffrey et al. supported this by showing AAV2's reduced activation of type I interferon-dependent genes when compared to adenoviral vectors.75 In contrast, Zhu et al. reported an increase in type I interferons following AAV2/1, AAV2/2, and AAV2/9, through activation of the TLR9-MyD88 pathway.42 This emphasizes that a closer look should be taken at the inflammatory potential of different AAV serotypes to truly understand their interaction with TLRs and other mediators of the innate response.
Based on the importance of inflammation, investigators have attempted to break AAV vector-mediated tolerance by the addition of TLR ligands to provide supplemental innate immune activation. In an established model of tolerance to AAV2/8.LacZ in the liver, systemic administration of the TLR ligands LPS (TLR4) or CpG (TLR9) could indeed result in the loss of LacZ expression, suggesting that AAV simply lacks the inflammatory signals necessary to render transduced cells targets for CTLs.76 Without supplemental TLR signaling, transduced hepatocytes demonstrated a downregulation of MHCI molecules, preventing necessary antigen presentation on target cell populations. Ultimately, these findings reveal that if the appropriate degree of inflammation and innate activation are reached, AAV vector-induced tolerance can be broken.
Anergy or deletion
Principally, in the priming of CD8+ T cells, two basic signals are paramount: antigen presented on MHC, and the costimulatory molecules CD80/86 and CD40. On one extreme, a low degree of inflammation and APC transduction may prevent antigen presentation altogether, resulting in ignorance. On the other extreme, the response may be strong enough to fuel both signals, resulting in functional T cell priming. It is also possible to fall somewhere in between these two extremes, resulting in antigen presentation without sufficient upregulation of costimulatory factors. In this case, the T cell priming event involves signal 1, but not signal 2, resulting in either anergy or deletion. A state of anergy is evidenced by the presence of a T cell population that is antigen-specific, but nonfunctional. Unlike normal effectors, anergic T cells are unable to proliferate or secrete cytokines, particularly IL-2, in response to antigenic stimulation.
The importance of costimulatory molecules in priming a functional T cell response to AAV was noted early on. In 2000, Zhang et al. reported that T cell immunity to AAV was dependent upon CD40L signaling.41 In this study, AAV2/2.LacZ-injected mice received an adoptive transfer of ex vivo AAV2/2.LacZ-infected iDCs to support the generation of cellular immunity; wild-type C57BL/6 mice, but not CD40L deficient (CD40L−/−) mice, were capable of eliciting a transgene-specific CTL response following adoptive transfer. Furthermore, in 2001, Sarukhan et al. demonstrated that the transgene-specific T cell response generated to AAV2/2.HA could be ablated by using an anti-CD40L monoclonal antibody to block the APC:T cell interaction in these mice.44 While normal mice developed robust cellular immunity to the HA transgene, leading to the elimination of transduced muscle fibers within 4 weeks, “tolerized” mice showed stable expression of HA after 28 days with no evidence of cellular infiltration in muscle sections. These studies confirm that improper costimulation is sufficient to circumvent immune responses to AAV, supporting the anergy/deletion hypothesis.
Two recent reports have provided evidence for functionally aberrant T cell populations. In both cases, the use of AAV carrying an HIV-1 gag antigen for vaccination purposes demonstrated that gag-specific CD8+ T cells could be primed, but they failed to expand upon secondary antigen exposure.61,62 Transgene-specific CD8+ T cell responses to AAV2/7.HIVgag demonstrated proliferative impairment. Coimmunization with TLR adjuvants was unable to restore the proliferative capacity of these T cells, which also expressed markers of exhaustion, including CTLA-4, granzyme B, and PD-1.61 In the second study, using AAV2/8.HIVgag, a high frequency of gag-specific CD8+ T cells were initially primed, however as the response matured and contracted, it failed to elicit the production of IL-2 and the generation of central memory cells. As a result, upon re-exposure to antigen in an adenoviral vector-mediated boost, AAV2/8 primed CD8+ T cells were unable to undergo proper expansion, reaching levels far lower than that seen in the initial prime.62 In addition, at the peak of the primary response, a disproportionately lower percentage of gag-specific CD8+ T cells was detected by intracellular cytokine staining versus MHCI tetramer staining, indicating the presence of substantial numbers of nonresponsive gag-specific CD8+ T cells. Taken together, CD8+ T cells appear to be primed in high frequency, but their functional ability to proliferate and secrete cytokines is impaired, providing further support for the anergy hypothesis.
Furthermore, in an Ova-specific T cell receptor transgenic mouse model, liver-directed AAV2/2.Ova gene transfer resulted in CD4+ T cell tolerance suggestive of anergy and deletion.77 Following injection, Ova expression remained stable, and upon in vitro stimulation of lymphocytes with Ova antigen, proliferation and secretion of IL-2 were substantially reduced. Proliferation could be restored in the presence of exogenous IL-2, which is a trademark of anergic cells. Mice receiving AAV2/2.Ova also demonstrated an increased number of apoptotic cells within the TCR+ population, reducing the overall number of CD4+ TCR+ cells and indicating a mechanism of deletion at work.
In 2009, Velazquez et al. reported a confounding observation where AAV vector-mediated gene transfer to the muscle resulted in stable transgene expression despite the priming of a functional (nonanergic) transgene-specific CD8+ T cell response detectable in spleen and liver.78 Investigators described a mechanism in which antigen-specific CD8+ effectors were primed, but after migration to the muscle, they underwent functional impairment followed by programmed cell death. Based on the finding that CD8+ T cells primed in the absence of CD4+ T cell help are highly susceptible to activation-induced cell death upon antigen-restimulation,79 the authors speculated that inadequate costimulation during the initial prime could predispose CD8+ effectors to enhanced death signaling upon encounter of transduced target cells. It is also possible, however, that programmed death is induced by Tregs working to actively suppress muscle-infiltrating CD8+ effectors in this system.
Suppression
In addition to ignorance, anergy or deletion, an alternative theory of tolerance is that of active suppression, where a special class of Tregs is primed with the ability to eliminate activated CD8+ effectors. In the first description of hepatic tolerance to a human FIX transgene following AAV gene transfer, Mingozzi et al. provided mechanistic evidence for active suppression together with Fas-FasL-mediated deletion of reactive T cells.52 Initially, high vector doses of AAV2/2.hFIX resulted in stable expression in the liver in the absence of anti-hFIX antibody formation. After challenge with hFIX in complete Freund's adjuvant, previously treated mice continued to express hFIX with no evidence of antibody formation, confirming the induction of tolerance. To establish the role of suppression, investigators showed that adoptive transfer of either pooled splenocytes or purified CD4+ T cells from AAV2/2 treated, hFIX-tolerant mice was capable of significantly reducing the formation of hFIX-specific antibodies in recipient mice following challenge. This confirmed that a class of CD4+ T cells was mediating suppression to FIX in the liver. The strength of this suppression was attested to several years later, when Dobrzynski et al. showed that hepatic tolerance to AAV2/2.hFIX could even prevent FIX-specific CTL responses following challenge with a highly immunogenic adenoviral vector.80
Since that time, the role of suppression in liver-directed gene transfer has been confirmed by several groups expressing therapeutic transgenes for the purposes of correction in animal models of disease. Ziegler et al. observed the induction of hepatic tolerance to human α-galactosidase A (α-gal) following intravenous administration of AAV2/2 or AAV2/8 in a murine model of Fabry disease.49,53 Adoptive transfer of CD4+ T cells isolated from tolerized mice was able to suppress the formation of anti-α-gal antibodies in naive recipients, confirming that tolerance in this system was also mediated by a CD4+ suppressor. In 2007, Sun et al. demonstrated a similar mechanism of hepatic tolerance to human acid α-glucosidase (GAA) in the murine model of a second lysosomal storage disorder, Pompe disease.81 Together, these studies demonstrated that the phenomenon of AAV vector-mediated suppression first observed in a murine model of hemophilia B was also associated with other clinically relevant disease models.
To date, numerous types of suppressor cells and mechanisms of suppression have been described. The predominant subclass, Tregs, are characterized by expression of high CD25 and the transcription factor Foxp3.82 In addition to CD4+CD25+Foxp3+ Tregs, there are also type 1 regulatory (Tr1) cells, which do not express Foxp3 and function via production of high levels of IL-10, as well as Foxp3-expressing T helper 3 cells that mediate suppression through the secretion of transforming growth factor-β.83,84 Due to the diversity in suppressor cell phenotype and function, further studies were required to fully characterize the subset of CD4+ T cells mediating suppression to AAV vector-encoded transgenes.
In 2007, Cao et al. provided the first definitive evidence that tolerance induction following hepatic gene transfer was specifically induced by a population of regulatory CD4+CD25+Foxp3+ T cells.85 In an Ova transgenic mouse model in which mice are Treg-deficient, injection of AAV2/2.Ova resulted in a CD4+CD25+ cell population capable of expressing Foxp3, GITR, and CTLA-4. When cocultured in the presence of Ova antigen, Tregs suppressed IL-2 secretion by CD4+CD25— T cells, confirming their regulatory function in vitro. Furthermore, in the standard model of tolerance to AAV2/2.hFIX after hepatic delivery, adoptive transfer and in vivo depletion experiments confirmed that CD4+CD25+ cells were responsible for suppression of hFIX antibody formation.85 Using depletion experiments, Sun et al. confirmed the role of Tregs in a murine model of Pompe disease.86 Furthermore, administration of AAV2/2.hFIX to the liver of nonhuman primates under transient immunosuppression provided the first evidence of CD4+CD25+Foxp3+ Treg-mediated tolerance in a large animal model.87
Tolerance induction to FIX in the liver was not only observed with AAV2/2, but also AAV2/8.53,88 Compared with AAV2/2, an equal dose of AAV2/8 was capable of generating a significantly higher frequency of transgene-specific regulatory CD4+CD25+Foxp3+ T cells, permitting stable expression of the secreted transgene product in three different strains of wild-type mice.88 Martino et al. went on to demonstrate that hepatic gene delivery of AAV2/2 expressing cytoplasmic β-gal also induced a Treg population that actively suppressed β-gal-specific CD8+ T cells.89 This confirmed that the role of Tregs in tolerance induction following hepatic gene transfer is not limited to a systemic transgene product and antibody responses, but can also be elicited following the delivery of a cytoplasmic antigen to suppress a cytotoxic T cell response.
In 2009, Breous et al. further explored the mechanisms of Treg-mediated suppression in C57BL/6 mice using AAV2/8 expressing the human α-1 antitrypsin (hAAT) reporter gene.90 They discovered that Tregs in the liver secrete the immunosuppressive cytokine IL-10 in response to antigen. In addition, Kupffer cells also adopt a tolerogenic phenotype in this model, where Kupffer cell depletion abrogates IL-10 production by hepatic Tregs, indicating an interaction between Tregs and Kupffer cells to create a local cytokine microenvironment that suppresses the CTL response. The following year, this group went on to caution that the mechanisms of suppression observed in C57BL/6 mice differed from that mediated in an alternate strain. In contrast, BALB/c mice displayed impaired hepatic tolerance which could be broken by strong immunogenic challenge using an adenoviral vector, indicating the importance of host- and strain-specific differences in defining the subtleties of mechanism.91
Although AAV gene transfer to liver is associated with the induction of transgene tolerance mediated by active suppression, there is no evidence that i.m. delivery is associated with Treg expansion.43,52,85,92 In fact, direct i.m. injection of high vector doses is often associated with CD4+ T helper-dependent antibody responses limiting sustained transgene expression.51 Recently, a protocol of regional intravascular delivery of AAV2/2 using afferent transvenular retrograde extravasation and transient immunosuppression was used in place of direct i.m. injection in order to achieve sustained expression of the canine FIX transgene in the muscle of hemophilia B dogs.93 In this case, stable transgene expression and minimal antibody formation were associated with high levels of IL-10 secretion in response to cFIX antigen, as well as expansion of a population of antigen-specific CD4+Foxp3+IL-10+ T cells.94 These findings suggested that the mechanisms of tolerance described following hepatic gene transfer may not be limited to the liver; protocols involving transient immunosuppression, lower vector doses, and/or modified vector delivery approaches may also lead to Treg-mediated suppression following muscle-directed gene transfer.
Conclusion
Over the years, the field of AAV gene therapy has addressed numerous issues relevant to ensuring the efficacy and safety of our vectors. Initial preclinical studies showing stable, high-level gene transfer in the muscle revealed the promise of recombinant AAV vector-mediated gene delivery over other viral and nonviral vector systems being explored. Evidence of T cell and antibody responses to different transgene products, delivered by various doses or routes of administration, fueled investigation into the number of factors influencing the generation of cellular immunity to these vectors. Finally, in cases of transgene-specific tolerance, researchers delved into translational immunology in an attempt to determine the mechanisms of tolerance at work. A growing pool of evidence is present to investigate the roles of either ignorance, anergy/deletion, or suppression in AAV vector-mediated gene delivery under varying conditions.
We believe that it is important to view AAV immunogenicity as a delicate balance between tolerance and immunity, to which numerous facets of the AAV-host cell interaction contribute. Depending upon the conditions, AAV is capable of priming immunity or inducing tolerance—both passively, through ignorance or anergy/deletion, as well as through active forms of suppression. However, upstream of these immune outcomes, there is one common denominator: innate immunity. AAV vectors are unique in their ability to deliver genes with little to no danger signals, avoiding the activation of an innate response. Combined with poor transduction of APCs, the end result is the priming of a continuum of dysfunctional T cells, which can only overcome the threshold for immune activation with the support of supplemental triggers.
From the very beginning, AAV's poor transduction of APCs reduces the amount of endogenous antigen available for presentation to naive T cells. If this antigen load does not reach a critical level, ignorance would be inevitable. Additionally, as a poor activator of innate immunity, local inflammation is limited, preventing the recruitment and activation of APC populations and proper upregulation of molecules critical to antigen processing and presentation. Limited MHCII upregulation may prevent priming of CD4+ T helper cells, which have been shown to play a critical role in CD8+ T cell activation, effecting either initial CD8 priming, or the establishment of functional CD8 memory. Furthermore, if the inflammatory response to the AAV capsid does not reach a certain threshold, APCs do not mature to express proper costimulatory molecules, such as CD80/86 or CD40, preventing the support of signal 2 and leading to anergy or deletion.
The absence of a sufficient inflammatory response to AAV prevents proper upregulation of antigen presentation machinery not only in the APC population, but also in target cells. While, poor upregulation of these molecules in DCs prevents functional T cell priming, poor expression on non-APC populations makes AAV-transduced cells poor targets for CTL-mediated clearance. Evidence in the literature has shown that AAV2/8's poor inflammatory response limits upregulation of MHCI on hepatocytes, preventing the elimination of transduced cells following hepatic gene transfer.76 As such, in the absence of supplemental immune triggers, the standard host response to AAV vectors can be viewed as one involving minimal danger signals or inflammation, with insufficient upregulation of the molecules required for T cell priming or target cell recognition.
However, cases of AAV inducing robust, polyfunctional CD4+ and CD8+ T cell responses capable of clearing transduced target cells clearly indicate that under certain circumstances AAV sufficiently transduces APCs and activates innate immunity, providing ample signal 1 and signal 2 during T cell priming, and sufficient upregulation of MHCI:Ag complexes on target cells. We believe there is a threshold required for the activation of functional CD8+ effectors, where multiple factors contribute to either helping AAV overcome this threshold or keeping it below the critical level, resulting in tolerance. The degree of innate immunity dictated by the AAV vector capsid, transgene, host species, and the level of inflammation in the target organ are the driving forces determining whether or not this threshold is met. Higher order species and tissues with a greater degree of regeneration and inflammation are known to more readily generate immune activation to AAV vectors. Certain capsid variants, such as AAV2/rh32.33, readily promote robust T cell responses even in wild-type mice, whereas AAV2/8 vectors expressing the identical transgene are unable to do so. AAV2/rh32.33 has been shown to provide robust CD4+ T cell help, of which AAV2/8 is incapable of priming. Moreover, unpublished work in our lab has provided evidence that AAV2/rh32.33's enhanced immunogenicity over AAV2/8 also correlates with enhanced transduction of APCs in vitro, as well as significantly greater upregulation of CD80/86, CD40, and MHCII on CD11c+ DCs in the draining lymph nodes following i.m. injection (L.E. Mays and J.M. Wilson, unpublished results). This supports our view that sufficient APC transduction and innate immune activation contribute to enhanced CD4+ T cell help, and ultimately CD8+ T cell priming.
If AAV vector administration does not initiate sufficient innate immune activation, a mechanism of passive tolerance results, through either ignorance, anergy or deletion. However, even if AAV reaches the threshold sufficient for T cell priming, a mechanism of dominant tolerance via active suppression may still ensue. The vector's innate effect on the pro- or anti-inflammatory cytokine milieu may impact the immunogenic versus tolerogenic nature of APCs, which dictate the priming of either effector T cells or suppressors, respectively. Thus, even if the innate response is strong enough to support naive T cell priming, the tissue microenvironment is responsible for swaying the T cell priming event toward immunity versus tolerance. In the case of AAV vector-mediated hepatic gene transfer, the AAV capsid promotes a cytokine milieu which favors the generation of CD4+CD25+Foxp3+ Treg suppressors over the priming of functional CD8+ effectors. In contrast, priming of Tregs has not been observed following direct i.m. injection of AAV vectors, only via a protocol of afferent transvenular retrograde extravasation under transient immunosuppression, thus highlighting the more immunogenic nature of this target tissue in comparison to liver.
Overall, the ability of the AAV vector to induce innate immunity—transducing APCs, activating APC maturation and eliciting a proinflammatory environment—largely dictates the successful acquisition of signals 1, 2, and 3, and the generation of a functional CD8+ T cell response to vector-encoded proteins. Future studies will certainly continue in the further elucidation of immunologic mechanisms, with the ultimate goal of establishing a thorough understanding of the threshold between establishing tolerance and generating immunity. In order to avoid destructive T cell responses to AAV vector-encoded transgene products, efforts should be taken to minimize the factors that may lead to innate immune activation. This highlights the critical role of selecting the appropriate capsid, transgene, dose, route of administration, and host species. For gene therapy applications aimed toward a clinical setting, selection of host species, transgene-product, and the degree of inflammation in the target tissue may be predetermined by the disease model itself. This emphasizes the importance of screening for the AAV serotype or capsid variant which can minimize innate immune activation in multiple preclinical models. The ability to predict and circumvent deleterious cellular responses is essential to the safe and efficacious translation of AAV vectors into the clinic.
REFERENCES
- Verma IM., and, Somia N. Gene therapy – promises, problems and prospects. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- Katsumi A, Emi N, Abe A, Hasegawa Y, Ito M., and, Saito H. Humoral and cellular immunity to an encoded protein induced by direct DNA injection. Hum Gene Ther. 1994;5:1335–1339. doi: 10.1089/hum.1994.5.11-1335. [DOI] [PubMed] [Google Scholar]
- Pickering JG, Jekanowski J, Weir L, Takeshita S, Losordo DW., and, Isner JM. Liposome-mediated gene transfer into human vascular smooth muscle cells. Circulation. 1994;89:13–21. doi: 10.1161/01.cir.89.1.13. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gönczöl E., and, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilwell JL., and, Samulski RJ. Adeno-associated virus vectors for therapeutic gene transfer. BioTechniques. 2003;34:148–50, 152, 154 passim. doi: 10.2144/03341dd01. [DOI] [PubMed] [Google Scholar]
- Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Li J., and, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- Atchison RW, Casto BC., and, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Melnick JL, Mayor HD, Smith KO., and, Rapp F. Association of 20-Millimicron Particles with Adenoviruses. J Bacteriol. 1965;90:271–274. doi: 10.1128/jb.90.1.271-274.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Mizukami H, Young NS., and, Brown KE. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- Chiorini JA, Yang L, Liu Y, Safer B., and, Kotin RM. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorini JA, Kim F, Yang L., and, Kotin RM. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge EA, Halbert CL., and, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wang L, Takeuchi T., and, Kanda T. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology. 2004;330:375–383. doi: 10.1016/j.virol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Nakai H, Herzog RW, Hagstrom JN, Walter J, Kung SH, Yang EY, et al. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood. 1998;91:4600–4607. [PubMed] [Google Scholar]
- Xiao W, Berta SC, Lu MM, Moscioni AD, Tazelaar J., and, Wilson JM. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H, et al. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Bosch A, Perret E, Desmaris N., and, Heard JM. Long-term and significant correction of brain lesions in adult mucopolysaccharidosis type VII mice using recombinant AAV vectors. Mol Ther. 2000;1:63–70. doi: 10.1006/mthe.1999.0005. [DOI] [PubMed] [Google Scholar]
- Grant CA, Ponnazhagan S, Wang XS, Srivastava A., and, Li T. Evaluation of recombinant adeno-associated virus as a gene transfer vector for the retina. Curr Eye Res. 1997;16:949–956. doi: 10.1076/ceyr.16.9.949.5046. [DOI] [PubMed] [Google Scholar]
- Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, Briot D, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7:774–781. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS., and, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning WC, Zhou S, Bland MP, Escobedo JA., and, Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Standaert TA, Wilson CB., and, Miller AD. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning WC, Paliard X, Zhou S, Pat Bland M, Lee AY, Hong K, et al. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockstedt DG, Podsakoff GM, Fong L, Kurtzman G, Mueller-Ruchholtz W., and, Engleman EG. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Gao G, Wang Q, Calcedo R, Mays L, Bell P, Wang L, et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther. 2009;20:930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- Wang L, Nichols TC, Read MS, Bellinger DA., and, Verma IM. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- Jooss K, Yang Y, Fisher KJ., and, Wilson JM. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao G., and, Wilson J. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: role of immature dendritic cells. J Virol. 2000;74:8003–8010. doi: 10.1128/jvi.74.17.8003-8010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang X., and, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dobrzynski E, Schlachterman A, Cao O., and, Herzog RW. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood. 2005;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarukhan A, Camugli S, Gjata B, von Boehmer H, Danos O., and, Jooss K. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J Virol. 2001;75:269–277. doi: 10.1128/JVI.75.1.269-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, et al. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- Ge Y, Powell S, Van Roey M., and, McArthur JG. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood. 2001;97:3733–3737. doi: 10.1182/blood.v97.12.3733. [DOI] [PubMed] [Google Scholar]
- Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F., and, Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- Cao O, Hoffman BE, Moghimi B, Nayak S, Cooper M, Zhou S, et al. Impact of the underlying mutation and the route of vector administration on immune responses to factor IX in gene therapy for hemophilia B. Mol Ther. 2009;17:1733–1742. doi: 10.1038/mt.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Cherry M, Barbon CM, Li C, Bercury SD, Armentano D, et al. Correction of the biochemical and functional deficits in fabry mice following AAV8-mediated hepatic expression of alpha-galactosidase A. Mol Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Spratt SK, Lagarde C, Bohl D, Kaspar B, Sloan B, et al. Efficient and stable adeno-associated virus-mediated transduction in the skeletal muscle of adult immunocompetent mice. Hum Gene Ther. 1997;8:1891–1900. doi: 10.1089/hum.1997.8.16-1891. [DOI] [PubMed] [Google Scholar]
- Cordier L, Gao GP, Hack AA, McNally EM, Wilson JM, Chirmule N, et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12:205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- Cordier L, Hack AA, Scott MO, Barton-Davis ER, Gao G, Wilson JM, et al. Rescue of skeletal muscles of gamma-sarcoglycan-deficient mice with adeno-associated virus-mediated gene transfer. Mol Ther. 2000;1:119–129. doi: 10.1006/mthe.1999.0019. [DOI] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- Mays LE, Vandenberghe LH, Xiao R, Bell P, Nam HJ, Agbandje-McKenna M, et al. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol. 2009;182:6051–6060. doi: 10.4049/jimmunol.0803965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin KQ, Mizukami H, Urabe M, Toda Y, Shinoda K, Yoshida A, et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J Virol. 2006;80:11899–11910. doi: 10.1128/JVI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SW, Hensley SE, Tatsis N, Lasaro MO., and, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Zhi Y, Mays L., and, Wilson JM. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J Virol. 2007;81:11840–11849. doi: 10.1128/JVI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K., and, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Gallucci S., and, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr, and, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 1998;10:349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- Banchereau J., and, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Zhu J., and, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM., and, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- Cui W, Joshi NS, Jiang A., and, Kaech SM. Effects of Signal 3 during CD8 T cell priming: Bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R. Immunogenic versus tolerogenic dendritic cells: a matter of maturation. Int Rev Immunol. 2010;29:111–118. doi: 10.3109/08830181003602515. [DOI] [PubMed] [Google Scholar]
- Lombardi V., and, Akbari O. Dendritic cell modulation as a new interventional approach for the treatment of asthma. Drug News Perspect. 2009;22:445–451. [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M., and, Frauwirth KA. Peripheral tolerance in CD8+ T cells. Cytokine. 2009;46:147–159. doi: 10.1016/j.cyto.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Hensley SE., and, Amalfitano A. Toll-like receptors impact on safety and efficacy of gene transfer vectors. Mol Ther. 2007;15:1417–1422. doi: 10.1038/sj.mt.6300217. [DOI] [PubMed] [Google Scholar]
- McCaffrey AP, Fawcett P, Nakai H, McCaffrey RL, Ehrhardt A, Pham TT, et al. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol Ther. 2008;16:931–941. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- Somanathan S, Breous E, Bell P., and, Wilson JM. AAV vectors avoid inflammatory signals necessary to render transduced hepatocyte targets for destructive T cells. Mol Ther. 2010;18:977–982. doi: 10.1038/mt.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski E, Mingozzi F, Liu YL, Bendo E, Cao O, Wang L, et al. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104:969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- Velazquez VM, Bowen DG., and, Walker CM. Silencing of T lymphocytes by antigen-driven programmed death in recombinant adeno-associated virus vector-mediated gene therapy. Blood. 2009;113:538–545. doi: 10.1182/blood-2008-01-131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- Dobrzynski E, Fitzgerald JC, Cao O, Mingozzi F, Wang L., and, Herzog RW. Prevention of cytotoxic T lymphocyte responses to factor IX-expressing hepatocytes by gene transfer-induced regulatory T cells. Proc Natl Acad Sci USA. 2006;103:4592–4597. doi: 10.1073/pnas.0508685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Bird A, Young SP, Kishnani PS, Chen YT., and, Koeberl DD. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am J Hum Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays LE., and, Chen YH. Maintaining immunological tolerance with Foxp3. Cell Res. 2007;17:904–918. doi: 10.1038/cr.2007.84. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Gregori S, Bacchetta R., and, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Kulis MD, Young SP, Hobeika AC, Li S, Bird A, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol Ther. 2010;18:353–360. doi: 10.1038/mt.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O., and, Herzog RW. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transfer. Hum Gene Ther. 2009;20:767–776. doi: 10.1089/hum.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino AT, Nayak S, Hoffman BE, Cooper M, Liao G, Markusic DM, et al. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS ONE. 2009;4:e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Somanathan S, Vandenberghe LH., and, Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breous E, Somanathan S., and, Wilson JM. BALB/c mice show impaired hepatic tolerogenic response following AAV gene transfer to the liver. Mol Ther. 2010;18:766–774. doi: 10.1038/mt.2009.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda VR, Stedman HH, Nichols TC, Haskins ME, Nicholson M, Herzog RW, et al. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood. 2005;105:3458–3464. doi: 10.1182/blood-2004-07-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurigot V, Mingozzi F, Buchlis G, Hui DJ, Chen Y, Basner-Tschakarjan E, et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther. 2010;18:1318–1329. doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]