Abstract
Isolated kidney mitochondria prepared from Vitamin D-deficient chicks catalyze the conversion of 25-hydroxyvitamin D3 to 1,25 dihydroxyvitamin D3. It wasfound that changes in the concentrations of Ca-2plus, HPO4-2minus, and Hplus altered synthesis in an interrelated fashion. Increasing the Ca-2plus concentration from 10-6 to 10-5 M caused a four- to fivefold increase in 1 alpha-hydroxylase activity when the medium pH was between 6.5 and 7.0. increasing the [Ca2+] to 10-4 M caused to furhter stimulation. At higher pH values, Ca-2plus had little effect upon 1 alpha-hydroxylase activity. In the absence of calcium [Ca2+] less than or equal to 10-7 M), a change in pH from 6.5 to 7.1 had no effect upon 1 alpha-hydroxylase activity in the presence of 10-5 M calcium, increasing the medium pH had a biphasic effect. An increase in pH from 6.5 to 6.9 caused a 1.5-fold increase in 1 alpha-hydroxylase activity, but a further increase of the pH to 7.1 caused a profound decrease in rate of hydroxylation to approximately 20% of the peak value. Neither 10-5 M LaC13 nor 10 mug/ml of oligomycin altered the effects of Ca2+ upon hydroxylate activity. However, the effect of calcium was blocked by 2.5 times 10-5 M ruthenium red, 0.83 mug/ml of antimycin A, and 500 muM dinitrophenol. The clcium ionophore, A23187, decreased but did not prevent the stimulatory effect of calcium. These data are consistent with the concept that the [Ca2+ in the mitochondrial matrix space is of importance in regulating the 1 alpha-hydroxylase. Phosphate exerted a biphasic effect on 1,25(OH)2D3 production with maximal stimulation (approximately twofold) at 1-3 mM. Calcium enhanced the stimulation by phosphate at all concentrations studied. The presence of potassium modified the interrelated effects of calcium and phosphate in two ways: 10-3 M calcium blocked the stimulation by phosphate; and in the presence of phosphate, 10-3 M calcium resulted in less 1,25(OH)2D3 production by production by isolated mitochondria are qualitatively similar to the effects of these ions on 1,25(OH)2D3 production yb isolated renal tubules.
Full text
PDF

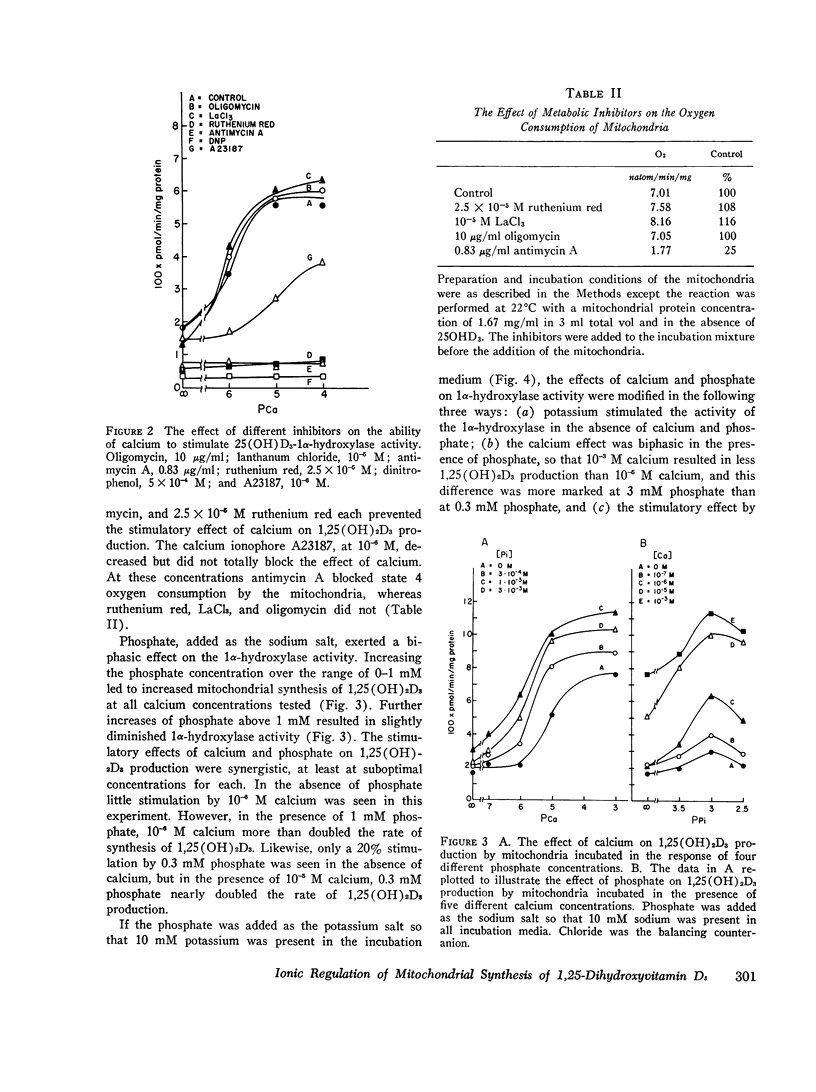
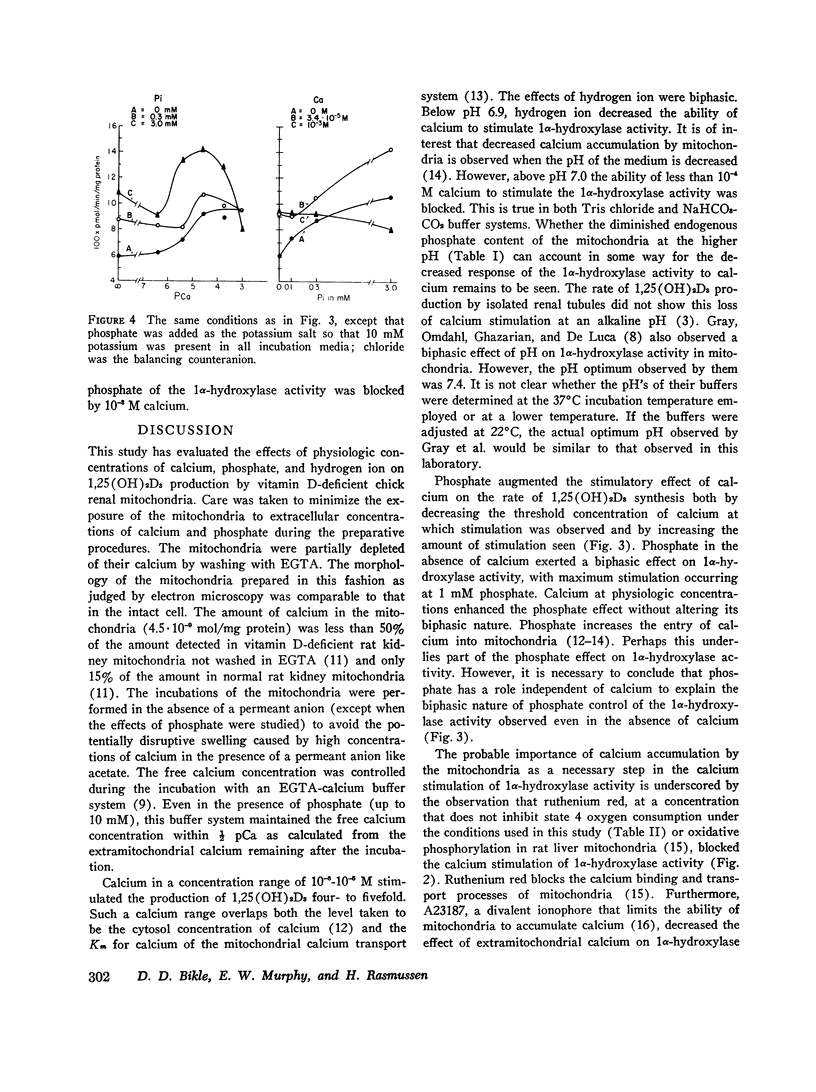


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikle D. D., Rasmussen H. The ionic control of 1,25-dihydroxyvitamin D3 production in isolated chick renal tubules. J Clin Invest. 1975 Feb;55(2):292–298. doi: 10.1172/JCI107932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Rasmussen H. The metabolism of 25-hydroxycholecalciferol by isolated renal tubules in vitro as studied by a new chromatographic technique. Biochim Biophys Acta. 1974 Oct 8;362(3):425–438. doi: 10.1016/0304-4165(74)90138-x. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Boyle I. T., Gray R. W., DeLuca H. F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston K. W., Evans I. M., Galante L., MacIntyre I., Moss D. W. Regulation of vitamin D metabolism: factors influencing the rate of formation of 1,25-dihydroxycholecalciferol by kidney homogenates. Biochem J. 1973 Jul;134(3):817–820. [PMC free article] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat New Biol. 1973 Feb 7;241(110):163–166. doi: 10.1038/newbio241163a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972 Dec 10;247(23):7528–7532. [PubMed] [Google Scholar]
- Kimmich G. A., Rasmussen H. Regulation of pyruvate carboxylase activity by calcium in intact rat liver mitochondria. J Biol Chem. 1969 Jan 10;244(1):190–199. [PubMed] [Google Scholar]
- Lehninger A. L., Carafoli E. The interaction of La 3+ with mitochondria in relation to respiration-coupled Ca 2+ transport. Arch Biochem Biophys. 1971 Apr;143(2):506–515. doi: 10.1016/0003-9861(71)90235-9. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela L. Interactions of La3+ and local anesthetic drugs with mitochondrial Ca++ and Mn++ uptake. Arch Biochem Biophys. 1968 Feb;123(2):286–293. doi: 10.1016/0003-9861(68)90136-7. [DOI] [PubMed] [Google Scholar]
- REILLEY C. N. Methods of detecting and controlling metal ion levels. Fed Proc. 1961 Sep;2:22–32. [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Spencer T., Bygrave F. L. The role of mitochondria in modifying the cellular ionic environment: studies of the kinetic accumulation of calcium by rat liver mitochondria. J Bioenerg. 1973 Apr;4(3):347–362. doi: 10.1007/BF01648977. [DOI] [PubMed] [Google Scholar]
- Suda T., Horiuchi N., Sasaki S., Ogata E., Ezawa I. Direct control by calcium of 25-hydroxycholecalciferol-1-hydroxylase activity in chick kidney mitochondria. Biochem Biophys Res Commun. 1973 Sep 18;54(2):512–518. doi: 10.1016/0006-291x(73)91451-4. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973 Feb;154(2):566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- VASINGTON F. D. Calcium ion uptake by fragments of rat liver mitochondria and its dependence on electron transport. J Biol Chem. 1963 May;238:1841–1847. [PubMed] [Google Scholar]


