Abstract
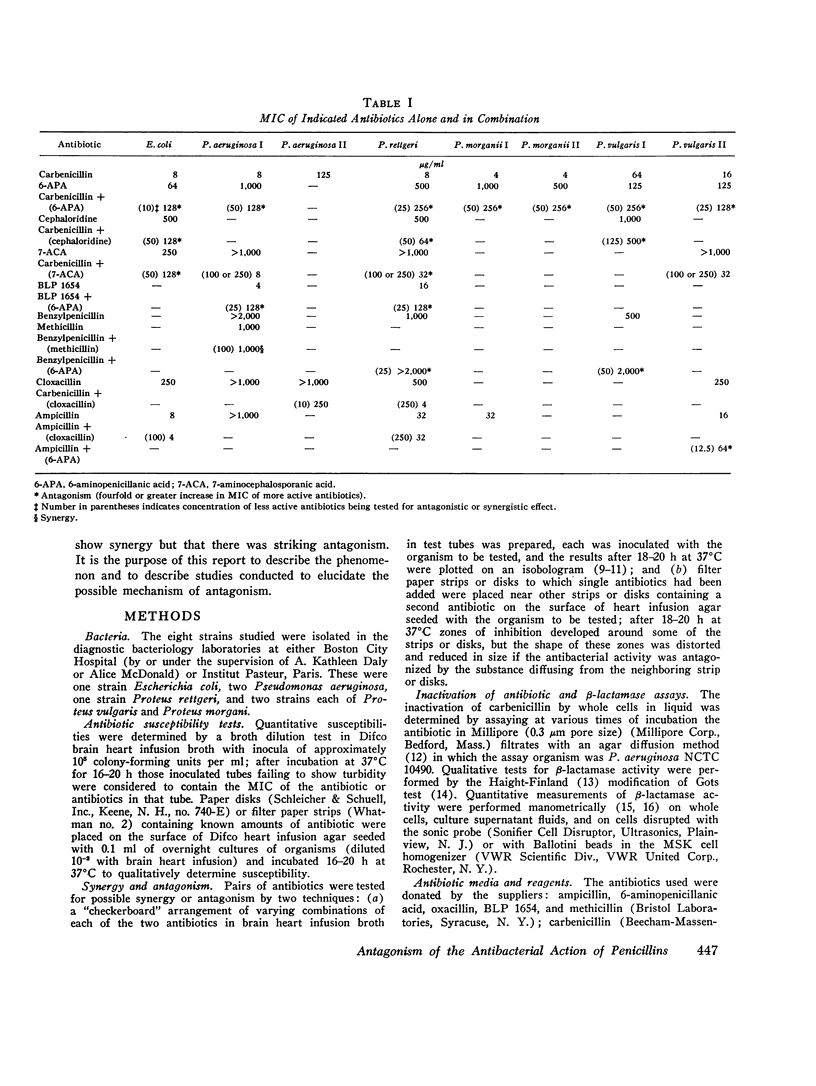
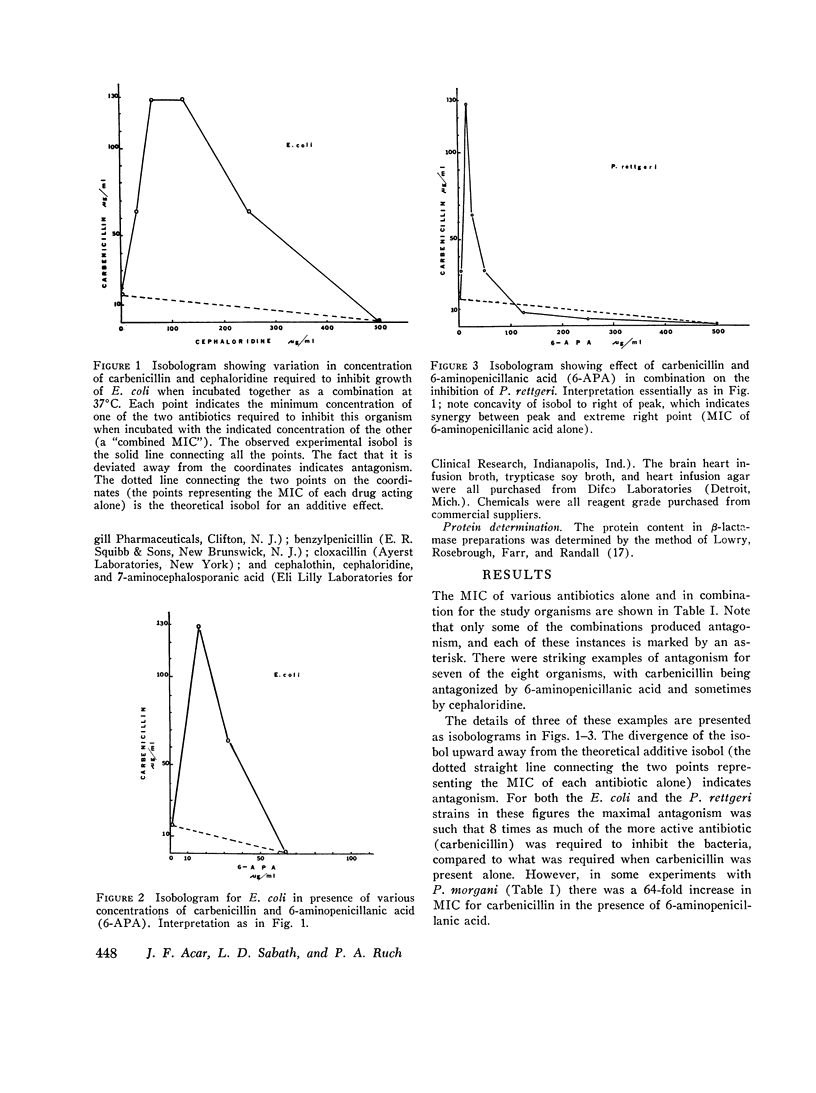
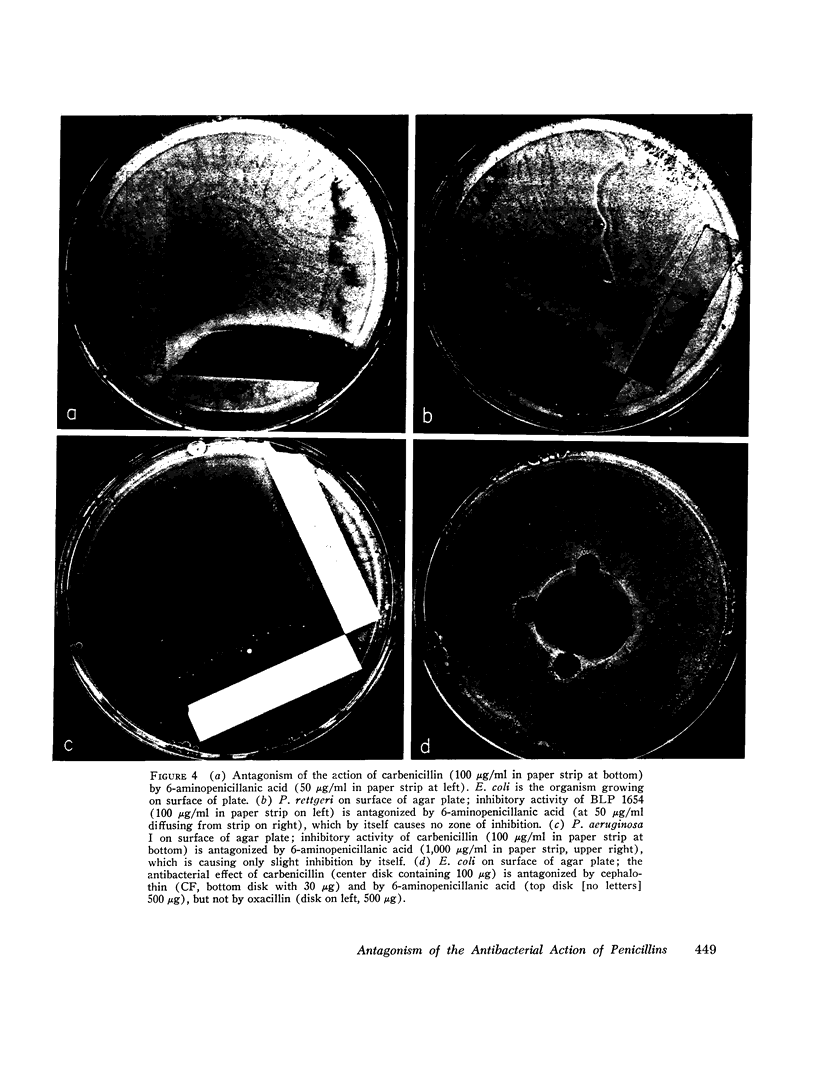
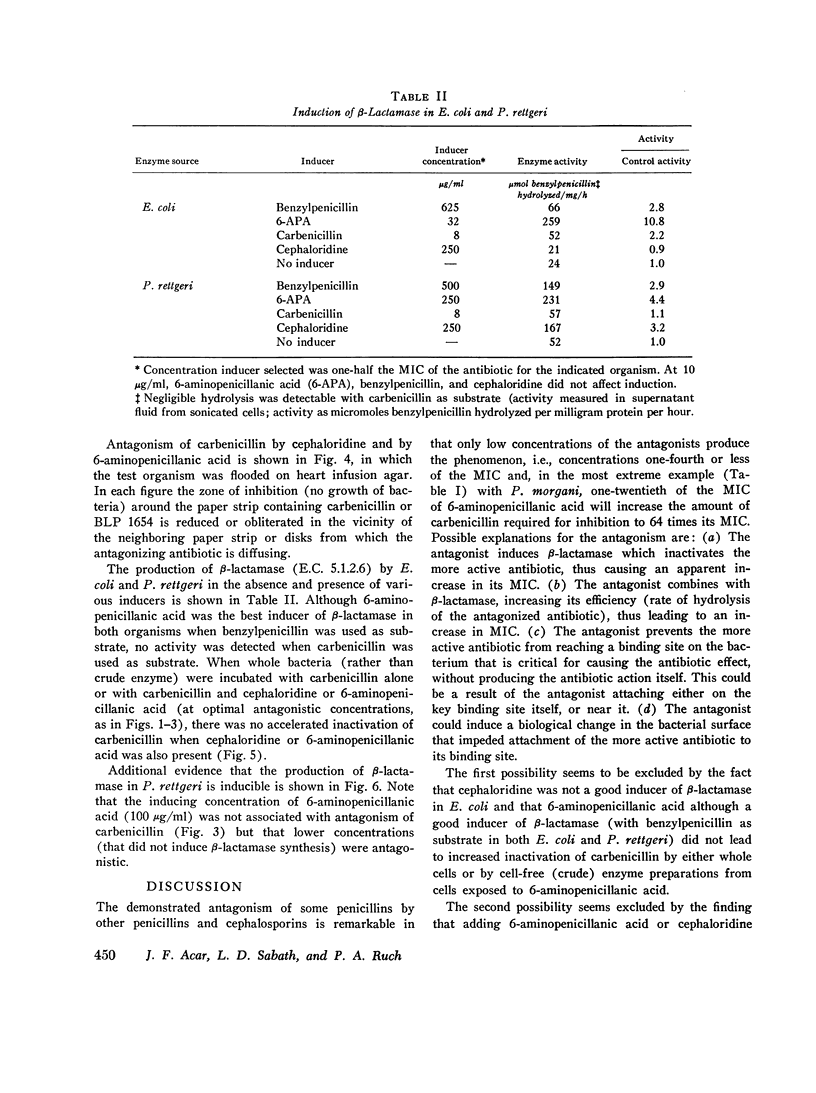
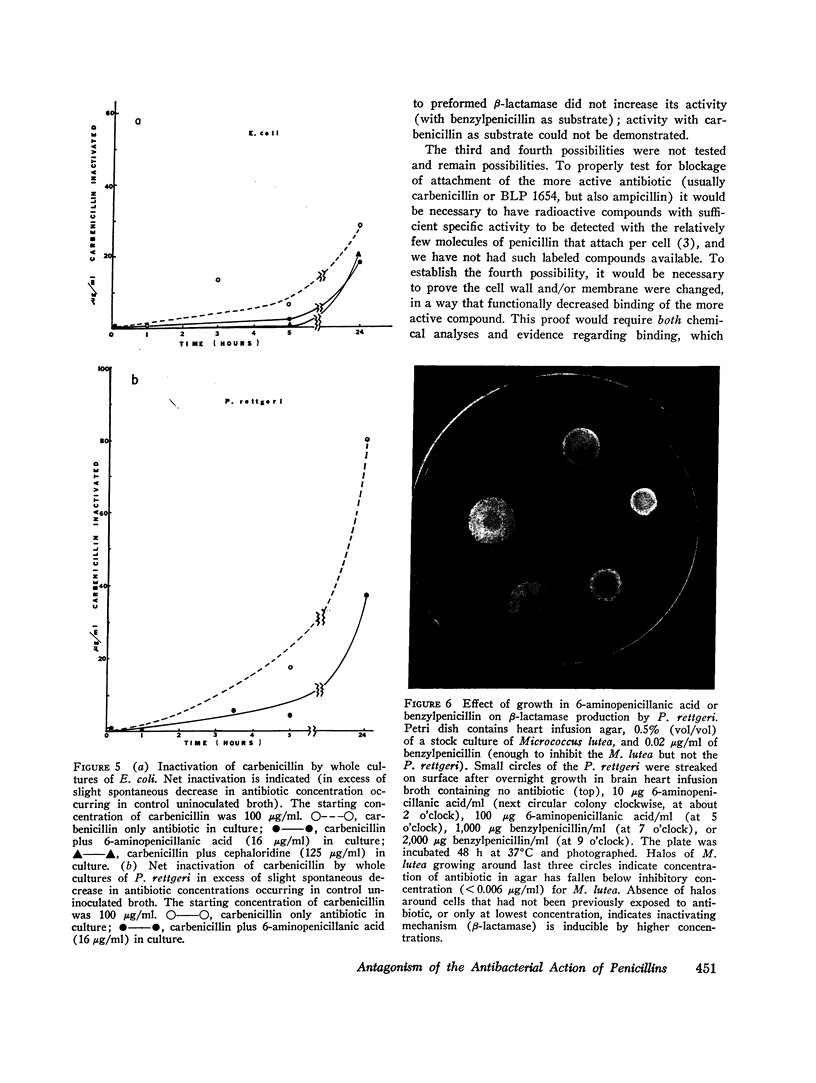
There are many examples of two penicillins acting synergistically, usually by one competitively inhibiting beta-lactamase, thus protecting the other from inactivation. There are few reports on penicillins antagonizing each other. Eight strains of three genera (Proteus, Escherichia, Pseudomonas) isolated at Boston City Hospital or Institut Pasteur, Paris, showed antagonism of carbenicillin or ampicillin by cephaloridine, cloxacillin, or 6-aminopenicillanic acid. Broth dilution tests showed that with seven of the eight strains the minimum inhibiting concentration (MIC) of the more active antibiotic was increased by 800-6,400% by low concentrations (often one-tenth the MIC) of the antagonist, whereas higher concentrations of "antagonist" were not as antagonistic, This suggested that two or more receptor sites of action for penicillins were present; the antagonist thus blocks the antibacterial action at the more sensitive site but acts additively with the antagonized antibiotic at the less sensitive site. The possibility that the mechanism of antagonism was induction of inactivating enzymes (beta-lactamase, penicillin acylase) was studied in two strains(one Escherichia coli and one Proteus rettgeri), and two antagonists were studied in detail. With E. coli cephaloridine was a poorer inducer of beta-lactamase than were the antagonized antibiotic and 6-aminopenicillanic acid. From these organisms, the good inducers of a beta-lactamase that acted on benzylpenicillin did not induce enzymes that inactivated carbenicillin. Thus, the mechanism of antagonism was not due to beta-lactamase induction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Isolation by covalent affinity chromatography of the penicillin-binding components from membranes of Bacillus subtilis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3751–3755. doi: 10.1073/pnas.69.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. R., Park J. T. Correlation between growth inhibition and the binding of various penicillins and cephalosporins to Staphylococcus aureus. J Bacteriol. 1969 Aug;99(2):459–462. doi: 10.1128/jb.99.2.459-462.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S. THE DETECTION OF PENICILLINASE-PRODUCING PROPERTIES OF MICROORGANISMS. Science. 1945 Sep 21;102(2647):309–309. doi: 10.1126/science.102.2647.309. [DOI] [PubMed] [Google Scholar]
- HAIGHT T. H., FINLAND M. Modified Gots test for penicillinase production. Am J Clin Pathol. 1952 Aug;22(8):806–808. doi: 10.1093/ajcp/22.8_ts.806. [DOI] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M., SMITH J. T., KNOX R. POTENTIATION OF PENICILLIN ACTION BY INHIBITION OF PENICILLINASE. Nature. 1964 Feb 29;201:867–868. doi: 10.1038/201867a0. [DOI] [PubMed] [Google Scholar]
- Heatley N. G. A method for the assay of penicillin. Biochem J. 1944;38(1):61–65. doi: 10.1042/bj0380061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWE S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953 Jun;3(6):285–290. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- POLLOCK M. R. Penicillinase adaptation in Bacillus cereus; an analysis of three phases in the response of logarithmically growing cultures to induction of penicillinase formation by penicillin. Br J Exp Pathol. 1952 Dec;33(6):587–600. [PMC free article] [PubMed] [Google Scholar]
- SABATH L. D., ABRAHAM E. P. SYNERGISTIC ACTION OF PENICILLINS AND CEPHALOSPORINS AGAINST PSEUDOMONAS PYOCYANEA. Nature. 1964 Dec 12;204:1066–1069. doi: 10.1038/2041066a0. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND R., BATCHELOR F. R. SYNERGISTIC ACTIVITY OF PENICILLINS AGAINST PENICILLINASE-PRODUCING GRAM-NEGATIVE BACILLI. Nature. 1964 Feb 29;201:868–869. doi: 10.1038/201868a0. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Elder H. A., McCall C. E., Finland M. Synergistic combinations of penicillins in the treatment of bacteriuria. N Engl J Med. 1967 Aug 3;277(5):232–238. doi: 10.1056/NEJM196708032770503. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Synergy of antibacterial substances by apparently known mechanisms. Antimicrob Agents Chemother (Bethesda) 1967;7:210–217. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]