Abstract
Background
Pancreatic-duodenal homeobox 1 (PDX-1) is a transcription factor which regulates embryologic pancreas development and insulin expression in the adult islet, however it is overexpressed in many types of cancer, including pancreatic cancer (PC). The purpose of this study is to investigate the role of PDX-1 in tumorigenesis in human cells.
Methods
In vitro cell proliferation, invasion and transformation were performed in HEK 293, MIA PaCa2 and HPDE cells with transient or stable expressing PDX-1 or GFP PDX-1, with or without co-transfection of PDX-1 shRNA. In vivo tumor formation was carried out in SCID mice with sq injection of HEK 293 and MIA PaCa2 stably transfected cells. Cell cycle was analyzed by Western blot or immunostaining. Microarray of RNA from PANC-1 cells with and without PDX-1 shRNA was performed and analyzed.
Results
Transient and stable expressing PDX-1 significantly increased cell proliferation and invasion in HEK 293, HPDE and MIA PaCa2 cells vs controls (p<0.05), hPDX-1 shRNA reversed these effects. Expression of PDX-1 significantly increased colony formation in HEK 293, HPDE and MIA PaCa2 cells vs controls in vitro(P<0.05). PDX-1 promoted HEK 293 and MIA PaCa2 tumor formation in SCID mice as compared to that of control(P<0.05). PDX-1 overexpression disrupted cell cycles proteins. PDX-1 expression was confirmed by western blot and tracked by viewing of GFP-PDX-1 expression. Microarray data support an oncogenic role of PDX-1 in pancreas cancer cells.
Conclusions
PDX-1 induced increased cell proliferation, invasion, and colony formation in vitro, and resulted in markedly increased HEK 293 and MIA PaCa2 tumor formation in SCID mice. These data suggest that PDX-1 is a potential oncogene that regulates tumorigenesis.
Keywords: PDX-1, transcription factor, oncogene, pancreatic cancer, proliferation, invasion, transformation, microarray
Introduction
Mechanisms involved in oncogenesis include activation of oncogenes and inactivation of tumor suppressor genes1–4, signaling pathways including Wnt, TGFα, TGFβ, EGF5–9, and re-expression of embryonic genes such as sonic Hedgehog (SHH), Notch, and pancreatic and duodenal homeobox-factor 1 (PDX-1). Notch and SHH have been well characterized as oncogenes10, 11, but the role of PDX-1 in tumorigenesis remains unclear.
PDX-1has been demonstrated to play a crucial role in the development and differentiation of the pancreas. In adult pancreas, PDX-1 regulates multiple genes such as insulin, islet amyloid polypeptide, somatostatin, glucokinase and elastase-1 to maintain homeostasis of the endocrine and exocrine pancreas12,13. PDX-1 is required for proliferation and neogenesis of islet cells after chronic hypoglycemic challenge14–16. Re-expression of PDX-1 has been found in adult duct cells in certain conditions such as pancreatectomy and pancreatitis17, 18. PDX-1 expression also was observed in several pancreatic cancer mouse models19–21, supporting the hypothesis that PDX-1 could be participating in the carcinogenesis of pancreatic cancer in mice. Furthermore, persistent expression of PDX-1 induced acinar-to-ductal cell metaplasia in the transgenic mouse pancreas, representing a potential initiating event to malignancy22. Most recently, PDX-1 positive cells have been shown to be an origin of pancreatic cancer, supporting their multipotent role as a stem cell of pancreatic cancer23 .Thus PDX-1 is not only required for pancreatogenesis and maintenance of pancreatic homeostasis, but also plays a key role in mediation of cell differentiation and metaplasia.
Interestingly, PDX-1 was over-expressed in most solid cancers including pancreatic, liver, prostate, ovarian, kidney, gastric, breast, lung and colon24–30. It has been shown that 50–100% of human pancreatic cancers over-express PDX-1 and that PDX-1 overexpression was associated with advanced clinical pathological stages and poor prognosis25, 26, 29, 31, 32. These observations suggest that PDX-1 could be mediating tumorigenesis. In the present study, we have generated in vitro and in vivo data in 4 human cell lines using a number of oncogenesis techniques that strongly support the hypothesis that PDX-1 is a potential oncogene in mediation of tumorigenesis in pancreatic cancer.
MATERIALS AND METHODS
Cell Lines Vectors, and Antibodies
Human embryonic kidney cell line (HEK 293) and human pancreas cancer cell lines, MIA PaCa2 and PANC-1, were obtained from the American Type Culture Collection (ATCC, Bethesda, Md). Human pancreatic ductal epithelial cells (HPDE) cells were maintained in keratinocyte serum-free (KSF) medium supplemented with bovine pituitary extract and epidermal growth factor (Invitrogen). Human PDX-1 cDNA and GFP PDX-1 fusion was cloned into pCMV5 expression vector and pQICXIP (Clontech, Mountain View, CA) retrovirus vector, respectively. PDX-1 shRNA was designed and produced as described28. PDX-1 scrambled shRNA served as control. Mouse goat anti-cyclin E2, mouse anti-Cdk2 and rabbit anti-p21, -p27, -p53, antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, Calif). Rabbit anti-PDX-1 antibody has been previously reported28. Goat anti-rabbit antiserum and sheep anti-mouse antiserum conjugated with horseradish peroxidase were purchased from Amersham (Amersham Life Science Inc, Arlington Heights, Ill). Rabbit anti-goat IgG was obtained from Zymed Laboratories Inc (South San Francisco, Calif).
Transient and Stable Transfection of Cell Lines
Transient transfection of cell lines was performed with 24 μg of plasmid DNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Stable transfections were established in HEK 293 or MIA PaCa2 cells with retrovirus carrying PDX-1 or GFP- PDX-1, which was produced by pQCXIP expressing PDX-1 or GFP-PDX-1 transfection of AmphoPack 293 cell line (Clontech, Mountain View, CA). PDX-1 shRNA or scrambled shRNA was used to co-transfect cells overexpressing PDX-1 or GFP-PDX-1.
Cell Proliferation Assay
Cell proliferation assay was performed on cells with transient or stable PDX-1 or GFP-PDX-1 expression, respectively, and then determined by MTS assay (Promega, Madison, Wis) at 24, 48, and 72 hours after transfection28.
In Vitro Invasion Assay
Invasion assays were carried out in a 24-well cell culture insert containing invasion chambers (Chemicon, Temecula, Calif). Invasive cells migrating to the lower surface of the membrane of insert were determined by staining. Invasiveness was quantified by dissolving stained cells in 10% acetic acid and measured of the dye/solution mixture in Multiskan EX plate reader at 560 nm. Experiments were repeated 5 times and representative data are shown.
Anchorage-independent Cell Growth Assay
Stably transfected cells (2500/well) were suspended in 1.0 ml of DMEM with 0.35% agarose (UltraPure TM Invitrogen, Carlsbad CA), and the suspension was placed on top of 1.0 ml of solidified 0.5% base agar (DifcoTM Agar Noble, Becton, Dickinson and Company, Sparks, MD). Triplicate cultures for each cell type were maintained at 37oC in a 5% CO2 atmosphere, and fresh medium was added after 1 week. Colonies were photographed at 21 days under a phase contrast microscope equipped with fluorescence. The numbers and size of colonies were counted and calculated from each experiment, which were reproduced 5 times.
Western Blot Analyses
Western blot analysis of protein levels in transfected cells were performed28. Antibodies against PDX-1, cyclin E, Cdk2, Cdk4, and p21, p27 and p53 were used. Images were captured using the UVP imaging system, and the band was analyzed using ImageJ software.
Tumorigenicity in SCID mice
Cells (3×107) in a 0.1 ml volume of phosphate-buffered saline were inoculated subcutaneously into the right flank of a 20-gm weight male with five mice for each group. Tumor formation was observed 4 weeks later. The formed tumors were dissociated and frozen for further immunostaining studies. Tumors were measured and recorded as the larger (A) and smaller (B) and tumor volume (V; a rotational ellipsoid) was calculated according to the formula: . Mice were scored according to presence or absence of tumor.
Immunohistochemical Staining
Fluorescein isothiocyanate-conjugated anti-rabbit IgG antibodies were purchased from Sigma (St Louis, Mo). Tissue process, section preparation, H&E staining and immunostaining were done as described28. Anti-PDX-1, P53 and PCNA antibodies were used. Images were recorded using a digital camera (Diagnostic Instruments Inc, Sterling Heights, Mich) on a fluorescent microscope (Olympus IX70; Olympus Optical Co Ltd, Japan). Immunostaining for PDX-1 expression was quantified using ImageJ.
Microarray of PANC-1 Cells Before and After Treatment with L-PDX-1 shRNA
PANC-1 cells, which have high endogenous expression of PDX-1, were transfected huPDX-1 shRNA and empty vector. Total mRNA was extracted from treated cells using RNAqueous® Kit (Ambion Inc, Austin Texas). Quality RNA was then subjected to microarray assay using Affymetrix GeneChip arrays (Human Genome U133 Plus 2.0 Array) in the BCM Microarray Core Facility. Each experiment was repeated three times. ComGene expression levels were analyzed and differentially expressed genes were selected using Significance analysis of microarrays (SAMR)33. Genes were normalized using log transformed expression values with GC Robust Multi-array Average (GCRMA)34. Functional gene analysis was carried out using Gene Ontology annotation, followed by (CSPA) using Ingenuity Pathway Analysis (IPA) (Ingenuity Systems, Inc. CA), which takes microarray gene expression data and models it into known signaling networks from the primary literature.
Statistical analysis
Student’s t-test was used to analyze difference in means of the continuous data. Pearson chi-square and Fisher’s exact test were used to analyze difference in ratios of the enumeration data. For microarray, P-values of specific nodes and motif networks were defined by Fisher’s exact test. All numeric data were expressed as mean ±SEM, with P < 0.05 indicating significance.
Results
Generation of stable transfections in HEK 293, HPDE and MIA PaCa2 cell lines
All 3 cell populations stably expressing PDX-1 and GFP-PDX-1 following retroviral infection and puromycine selection. Empty vector transfection served as control. Expression was confirmed by Western blot and immunostaining using anti-PDX-1 antibody against PDX-1 as following described. GFP-PDX-1 fluorescence also was confirmed by microscopy.
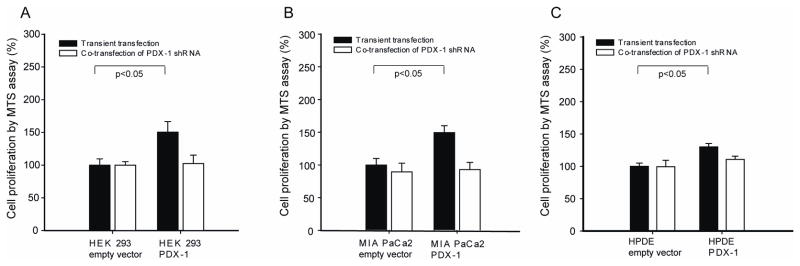
Increased cell proliferation related to transient expression of PDX-1
Transient over-expression of PDX-1 resulted in significant increase of cell proliferation by 150.3%±16.1% in HEK 293, 151.4%±10.5% in MIA PaCa2 and 130.1%±5.1% in HPDE as compared to empty vector transfected cells at 48h, respectively (Fig 1A). Co-transfection of PDX-1 shRNA to knockdown PDX-1 expression blocked cell proliferation (p=0.01, respectively) (Fig 1B).
Fig 1.
Transient expression of PDX-1 increases cell proliferation. HEK 293, MIA PaCa2 and HPDE cells were transfected with human PDX-1 with and without human PDX-1 shRNA. Transient burst expression of PDX-1 results in increased cell proliferation in HEK 293 (A), MIA PACa2 (B) and HPDE (C) cells (P<0.05, respectively). PDX-1 shRNA co-transfection blocks the effect of cell proliferation (white bar).
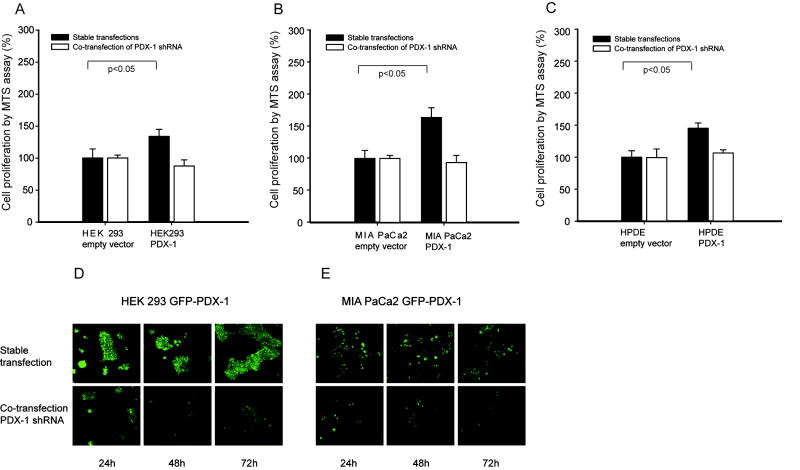
Stable overexpression of PDX-1 is associated with increased cell proliferation
Stably transfected cell lines overexpressing PDX-1 were used to determine long-term influence of PDX-1 on cell proliferation. The cells expressing PDX-1 had 133.7%± 11.2% in HEK 293 (p<0.05) (Fig 2A), 163.9%±15.2 (p<0.05) in MIA PaCa2 (Fig 2B) and 145.1%±8.2% (p<0.05) in HPDE (Fig 2C) increased cell proliferation as compared to control, respectively. PDX-1 shRNA reversed these effects shown Fig 2A, B, C. We noted that the proliferation in stable transfected HPDE was much higher than that in transient transfection of HPDE. To visualize the PDX-1 expression, we developed cells expressing GFP-PDX-1 in HEK 293 and MIA PaCa2 cells. The expression of PDX-1 was seen by GFP expression under microscopy (Fig 2D, 2E). During the 3 days of observation after PDX-1 shRNA transfection, we found a continuous reduction of signal strength and cell number in both HEK 293 (Fig 2D) and MIA PaCa2 cells (Fig 2E).
Fig 2.
HEK 293, MIA PaCa2 and HPDE cells stably expressing PDX-1 with and without human PDX-1 shRNA had increased cell proliferation. PDX-1 overexpression caused significant increase of cell proliferation in all cell lines as shown in A, B and C. PDX-1 shRNA co-transfection was able to reverse the effect induced by PDX-1.
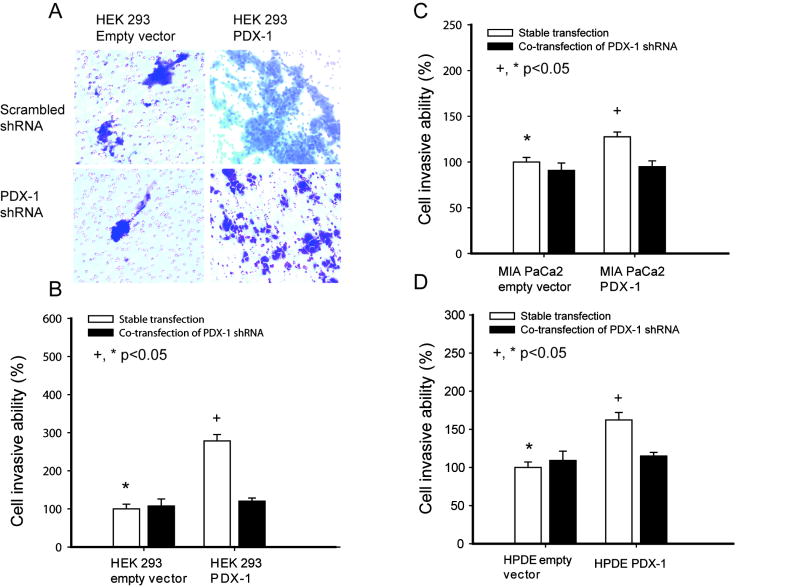
Over-expression of PDX-1 induces cell invasion ability
To determine PDX-1’s effect on cell invasion, we performed invasion studies using stably transfected HEK 293, MIA PaCa2 and HPDE cell lines with or without co-transfection PDX-1 shRNA. Invasive cells were seen by staining of migrated cells (Fig 3A, a representative figure by HEK 293 cells) and invasiveness was quantified by colorimetric assay as shown in Figures 3B, 3C and 3D. PDX-1 overexpression induced an increase of cell invasion by 278.6%±16.5% in HEK 293, 127.6%±5.1% in MIA PaCa2 and 162.4%±9.6% in HPDE cells compared to controls (P<0.05), respectively. Co-transfection of PDX-1 shRNA reversed the effect induced by PDX-1(p=0.01). These data indicate that PDX-1 overexpression increases cell invasion in both benign cells and pancreatic cancer cells, although the increase in MIA PaCa2 was lower than the other cell lines presumably due to the presence of endogenous PDX-1.
Fig 3.
HEK 293, MIA PaCa2 and HPDE cells stably overexpressing PDX-1 were cultured with and without human PDX-1 shRNA, then transferred into invasion inserts for 48h (Fig 3A). Increased quantatative invasiveness iss shown in 3B, 3C and 3D.
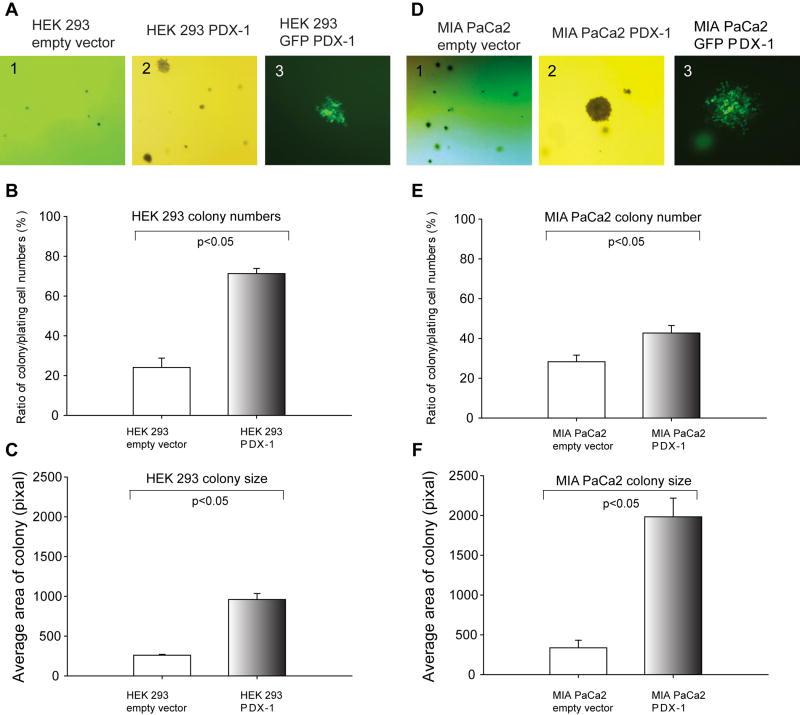
Over-expression of PDX-1 enhances the ability of colonial formation
To test whether PDX-1 induces and promotes cell transformation in human cell lines, we performed anchorage-independent growth assay in soft agar plates. HEK 293 and MIA PaCa2 cells expressing PDX-1, GFP-PDX-1 or empty vectors were seeded in the soft agar plates to grow colonies. During culture, colony formation was studied by observing the number and size of colonies (Fig 4A and 4D), and PDX-1 expression was tracked by viewing GFP signal under microscopy in GFP-PDX-1 expressing colony formation (Fig 4A3 and 4D3). Colony numbers and size are shown Fig 4B, C, E, F. A significant increase in colony formation was found in all cells expressing PDX-1 as compared to controls. PDX-1 overexpression resulted in significantly increased colony numbers (71.3±2.6) in HEK 293 cells (Fig 4B), 42.7±3.8 in MIA PaCa2(Fig 4E) and 121.4±17.7 (not shown) in HPDE cells compared to empty vector controls 24.1±4.7 (p=0.005) in HEK, 28.3±3.3 in MIA PaCa2 (p=0.01) and 0 in HPDE cells, respectively. PDX-1 overexpression also increased colony size by 960.2±75.7 in HEK 293 (Fig 4E) and 1983.1±235.2 in MIA PaCa2 (Fig 4F) as compared to empty vector controls.
Fig 4.
PDX-1 overexpression in HEK 293 (A, G) and MIA PaCa2 (D, H) cells causes cell transformation in anchorage-independent growth assay. Colonies were photographed at 21 days under a phase contrast fluorescent microscope. The number and size of colonies were counted and calculated from each experiment at B, E and C, F, and the experiment was reproduced 5 times.
Over-expression of PDX-1 promote tumor growth in SCID mice
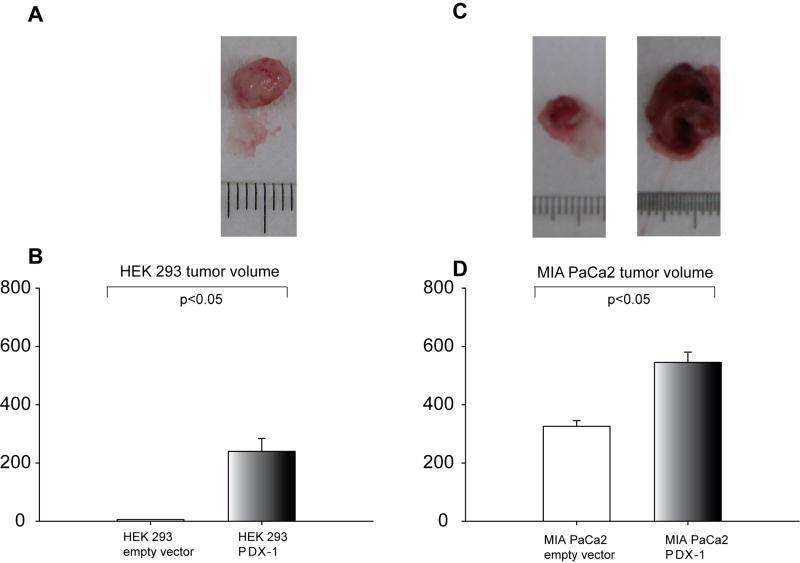
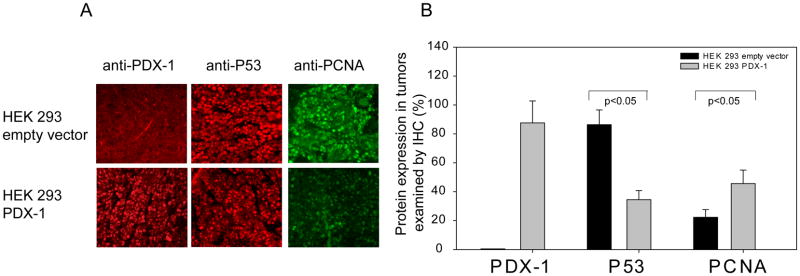
To study the role of PDX-1 overexpression in vivo, we implanted HEK 293 or MIA PaCa2 cells, expressing PDX-1, GFP-PDX-1, or empty vector in SCID mice by sq injection at a dose of 3×107 cells/mouse. The mice were sacrificed at day 30 after cell inoculation and tumor information was collected. 5/5 mice developed tumors from PDX-1 overexpressing HEK 293 cells, whereas only 1/5 mouse developed a very small tumor from HEK 293 empty vector cells. The volume of PDX-1 overexpressing HEK tumors (Fig 5A, B) was 239.9±84.4mm3 vs 5.8mm3 for the empty vector cells(P<0.05 between all groups). As expected, both PDX-1 overexpressing and empty vector MIA PaCa2 cells generated tumors in mice, however, the volume of PDX-1 overexpressing MIA PaCa2 tumor was 545.3±35.2mm3 in PDX-1 and 325.8±19.3mm3 in empty vector control cells(P<0.05) (Fig 5C and D). Tumor volume in HEK 293 empty vector control cells was markedly smaller than that of the MIA PaC2 control cells reflecting that HEK 293 can serve as benign cells. PDX-1 expression in tumors was confirmed by immunostaining as shown in Fig 6A top panel. Changes of P53 and PCNA were detected in HEK 293 tumor samples in the presence or absence of PDX-1. As shown in Fig 6A middle and bottom panel, PDX-1 overexpression down-regulated P53 (34.5%±6.3%) expression and up-regulated PCNA expression (45.6%±9.4%) as compared to controls at 86.4%±10.2% (p=0.005) and 22.4%±5.3% (p=0.01), respectively.
Fig 5.
PDX-1 overexpression promotes tumor formation and growth in SCID mice. HEK 293 or MIA PaCa2 cells overexpressing PDX-1 vs empty vector were implanted in SCID. Gross tumor volume at 30 days was was evaluated compared using x2 test (A) and tumor size was measured and compared using Student’s t test(B). Tumor size was significantly induced by PDX-1 overexpression in HEK 293 cells (A, B) and MIA PaCa2(C, D).
Fig 6.
PDX-1 regulates cell cycle proteins. Immunostaining study was performed on tumor sections using anti-PDX-1, P53 and PCNA antibodies. Red staining in upper panel and middle panel of A indicates PDX-1 and P53 expression, respectively, and green staining at bottom of panel A represented PCNA expression. Quantity was defined as positive cells in total cells as analyzed by image J and shown in B. Magnification×200.
Stable expression of PDX-1 lead to disruption of cell cycle
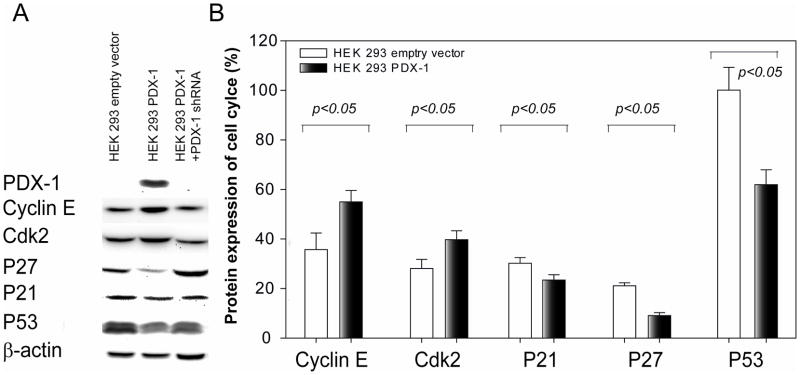
To study potential mechanisms by which PDX-1 regulates cell proliferation, expression of a series of cell cycle related proteins was evaluated by Western blot analysis in HEK 293 cells stably expressing PDX-1 with and without. PDX-1 shRNA. As shown in Figure 7A, stable overexpression of PDX-1 in HEK 293 cells resulted in down-regulation of expression of P21, P27 and P53 and up-regulation of expression of cyclin E and Cdk2 as compared to control cells (P<0.05, excluding P27). Protein levels in PDX-1 stably transfected cells versus control vector treated cells were quantified as p21 (23.4±2.1 vs 30.2±2.3), P27 (9.1±1.2 vs 21.1±1.2) P53 (61.9±6.0 vs 100.1±9.2), Cyclin E (55.0±4.6 vs 35.7±6.7), Cdk2 (39.7±3.7 vs 28.1±2.8) shown in Fig 7B. Co-transfection with hPDX-1 shRNA reversed the effect induced by PDX-1 overexpression.
Fig 7.
PDX-1 overexpression results in cell cycle disruption. Western blot was performed on PDX-1 stably trasnfected HEK 293 cells with or without PDX-1 shRNA. Protein levels of PDX-1, cyclin E, cdk2 P21, p27 and P53 were determined by bands profiles (A) and quantified by relative dentistry of aimed band vs internal control (B).
Knockdown PDX-1 expression in PANC-1 cells is associated with alterations of signaling pathways of cell proliferation, invasion and apoptosis
PANC-1 cells were used for this study as they have high endogenous PDX-1 expression. Microarray analysis showed that the highest scoring biological networks were decreased Cancer (p = 1×10−23 − 0.0002), decreased Cell cycle (p= 2.02×10−28 − 0.0003), and increased Cell death (p = 2.02×10−37 − 0.0002 ). These networks contained at least 190 molecules from the gene list. For these three networks, expression of sp-1, HDAC, p53, CDKN1A and CDKN1B, CDKN2A, v-rel, E2Fs, were critical to direct and indirect pathways for significant genes. Figure 8 represents over-represented motifs and proliferative pathways identified by Ingenuity Systems and DAVID/EASE. Red molecules represent over-expression, whereas green molecules represent down regulation. Direct interactions are indicated as (green) promoting or (red) inhibiting interacting arrows. The data suggest that knockdown of PDX-1 in PANC-1 cells stimulates apoptosis through blockade of NFkB signaling, as well as arrest of cell cycle by downregulation of cyclins. Interestingly, decreased gene expression related to angiogenesis (VEGF) and invasion were also demonstrated (HBEGF, PAI-1, uPA), along with over-expression of DNA methylating proteins/ histone de-acetylases. These data support the hypothesis that PDX-1 stimulates cell proliferation, invasion and angiogenesis and inhibits cell apoptosis.
Fig 8.
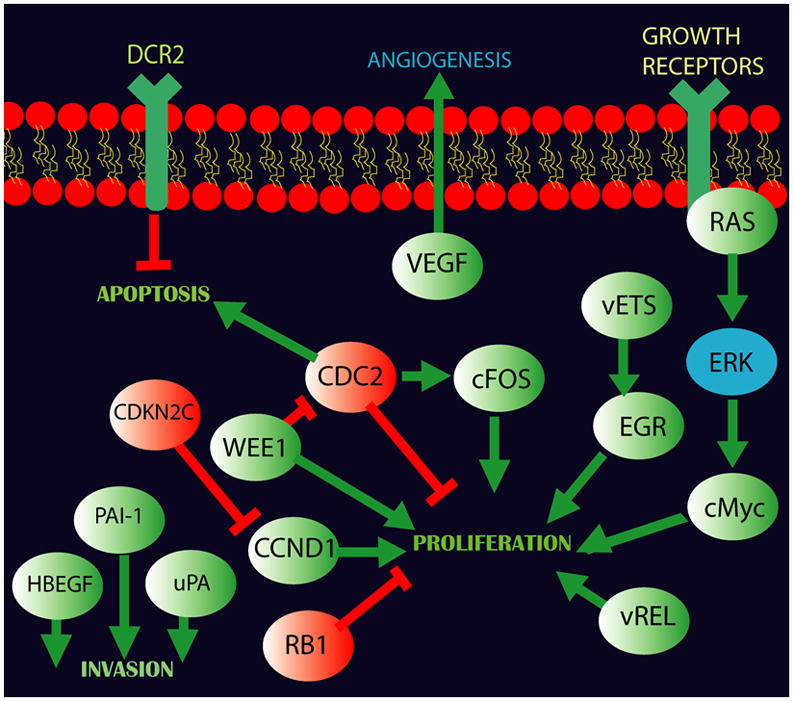
PDX-1 knockdown of PANC-1 cells with high endogenous PDX-1 expression affected gene expression in signaling pathways of cell proliferation, invasion, apoptosis and angiogenesis using Affymetrix GeneChip arrays (Human Genome U133 Plus 2.0 Array). Molecules in red represent gene over-expression, whereas molecules in green represent down regulation. Direct interactions are indicated as (green) promoting or (red) inhibiting interacting arrows. PDX-1 knockdown stimulates apoptosis and cell cycle arrest by downregulation of cyclins. Decreased gene expression related to angiogenesis (VEGF) and invasion (HBEGF, PAI-1, uPA) was also seen in the analysis.
Discussion
It is well known that PDX-1 is required for embryologic development of the pancreas and for maintenance of hormone expression in the adult islet35–40. PDX-1 has also been found to regulate proliferation of islet cells in mice15 as well as proliferation and invasion of pancreatic cancer cells in vitro14–16. Re-expression of PDX-1 in adult duct cells, under certain conditions such as pancreatectomy and pancreatitis, suggests that PDX-1 may be associated with the regenerative responses that accompany these conditions. It has been demonstrated to be a key regulator of the induction of cell differentiation from non islet cells to insulin-secreting cells41, especially acinar to ductal cell metaplasia, a common cellular change that may progress to malignancy22. These studies, together with the finding that PDX-1 is overexpressed in most solid cancers, which is associated with advanced clinical pathological stages and poor prognosis of patients with pancreas cancer, suggests that PDX-1 could be playing a role in oncogenesis 25, 26, 29, 31, 32. To test the hypothesis that PDX-1 is an oncogene, we utilized techniques similar to those performed on Kras, which is a well known pancreas cancer oncogene that induces malignant cellular transformation when over-expressed as an activating point mutation at codon 12( KrasG12D)1, 2, 42–44. Our cumulative data show that over-expression of PDX-1, in addition to affecting cell proliferation and invasion in both benign and malignant human cell lines, induced significant cell transformation by colony formation and promoted tumor growth in vivo, strongly supporting the role of PDX-1 as a potential oncogene. An opposite view has been mentioned in one study in which PDX-1 was considered as a tumor suppressor gene in human gastric cancer 45. Further studies are needed to clarify this issue, since most studies showed high level of PDX-1 in gastric cancer specimens46–50, as opposite to this study showing low expression of PDX-1 in a single human gastric cancer cell line.
To strengthen the evidence of PDX-1’s role in tumorigenesis, we also utilized visualized PDX-1 expression and PDX-1 siRNA knockdown techniques for in vitro studies in multiple human cell lines. HEK 293 cells are routinely used as a “normal” utility cell to test the function of oncogenic or tumor suppressor genes51. HPDE cells, which originate from human pancreatic ductal epithelial, have been widely used as tool to investigate the oncogenic property in pancreatic cancer studies 52, 53. MIA PaCa2 cells and PANC-1 cells are human pancreatic cancer cell lines with low and high endogenous expression of PDX-1, respectively. Consistent results were obtained from different human cell lines, demonstrating reliable approaches used in the study, emphasizing the crucial role of PDX-1 in mediating tumorigenesis. Secondly, visualized PDX-1 expression offered kinetic observation of cellular responses to PDX-1 expression. It was particularly useful in monitoring PDX-1 expression in colony formation of PDX-1-transformed cells. Lastly, RNAi technique was used to validate the role of PDX-1 in regulating cell proliferation and invasion. By knockdown of PDX-1 expression, the effectiveness of PDX-1 induction was accordingly reversed, providing confirmative evidence of PDX-1’s role in cell proliferation and invasion. In combination of three approaches, the study provides reliable evidence to demonstrate that PDX-1 mediates tumorigenesis and further support our previous view that PDX-1 is therapeutic target for pancreatic cancer28. Using a xenograft human cell-SCID mouse model, this study demonstrates in vivo evidence that PDX-1 overexpression induces tumor formation and growth. HEK 293 cells harboring PDX-1 expression grew large tumors in all implanted mice, whereas HEK 293 control cells developed only a very small tumor in one of five mice. The results are consistence with other studies using HEK 293 cells to study the function of other oncogenes51, 54. PDX-1 also promoted MIA PaCa2 cell tumor growth in SCID mice, indicating PDX-1 also has a cumulative effect on tumorigenesis, as MIA PaCa2 control cells have low endogenous PDX-1 expression. These data further support the hypothesis that PDX-1 is an oncogene.
In terms of the mechanism by which PDX-1 is involved in pancreas cancer tumorigenesis, we have shown that overexpression mouse PDX-1 in human pancreas cancer cell lines, as well as PDX-1 shRNA knock down of PDX-1, results in disruption of cell cycle proteins28. Previous PDX-1 studies have shown dependence on several signal transduction pathways such as those involving Stat322, MAPK55, and phosphatidylinositol 3-kinase/Akt/mTOR signaling pathways56. There is great deal of overlap between these transduction cascades and those described in SHH regulation of proliferation in pancreatic cancer57. The data in this study demonstrate PDX-1 up-regulates expression of Cdk2, cyclin E, and down-regulation of p27, p21 and P53 expression in human PDX-1 overexpressed HEK 293 cells both in vivo and in vitro; these data are consistent with our previous observations in other cell lines, addressing the role of PDX-1 on G1 to S transition of cell cycle, emphasizing the critical role of PDX-1 in the mediation of cell proliferation.
Further mechanistic evidence was obtained from microarray analysis of genes involving in signaling pathways following PDX-1 knockdown in human PANC-1 cells, which have high endogenous expression of PDX-1. The computerized analysis of the microarray data also helped to identify additional potential molecular targets involved in the PDX-1 pathway, which provides potential insight into the molecular mechanism of PDX-1 in tumorigenesis. These data are consistent with our findings that PDX-1 regulates proliferation and invasion of PC cells and that suppression of PDX-1 expression via PDX-1 shRNA activates apoptotic pathways, as well as suppression of proliferation and invasion pathways28. However, further studies are needed to determine precisely how PDX-1 regulates transformation.
In conclusion, the data in the present study demonstrate that PDX-1 overexpression resulted in: i) increased cell proliferation and invasion, as well as transformation of non malignant human cells; ii) promotion of tumor formation and growth of human cells implanted in SCID mice; and iii) disruption of the cell cycle in non malignant cells. These data, along with the demonstration that PDX-1 is over-expressed in more than 80 pancreatic cancer specimens, support the hypothesis that PDX-1 is an oncogene mediating tumorigenesis in pancreatic cancer.
Acknowledgments
We would like to thank our colleagues working in collaboration with Dr. Brunicardi’s laboratory for their support and suggestions. We also thank Katie Elsbury for her continuous assistance and editorial support.
Sources: The work was supported by National Institutes of Health (NIH) grants, NIDDK R01-DK46441 and NCI R01 – CA095731, the Vivian L. Smith Foundation, the MD Anderson Foundation, the Elkins Pancreas Center at Baylor College of Medicine, and the generosity of Mr. and Mrs. Lloyd Kirchner.
Footnotes
There is no conflict of interest in this study
References
- 1.Deramaudt T, Rustgi AK. Mutant KRAS in the initiation of pancreatic cancer. Biochim Biophys Acta. 2005 Nov 25;1756(2):97–101. doi: 10.1016/j.bbcan.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005 May;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Tian X, Chakrabarti A, Amirkhanov NV, et al. External imaging of CCND1, MYC, and KRAS oncogene mRNAs with tumor-targeted radionuclide-PNA-peptide chimeras. Ann N Y Acad Sci. 2005 Nov;1059:106–144. doi: 10.1196/annals.1339.038. [DOI] [PubMed] [Google Scholar]
- 4.Naka T, Kobayashi M, Ashida K, Toyota N, Kaneko T, Kaibara N. Aberrant p16INK4 expression related to clinical stage and prognosis in patients with pancreatic cancer. Int J Oncol. 1998 May;12(5):1111–1116. doi: 10.3892/ijo.12.5.1111. [DOI] [PubMed] [Google Scholar]
- 5.Pasca di Magliano M, Biankin AV, Heiser PW, et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS One. 2007;2(11):e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heiser PW, Cano DA, Landsman L, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008 Oct;135(4):1288–1300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truty MJ, Urrutia R. Basics of TGF-beta and pancreatic cancer. Pancreatology. 2007;7(5–6):423–435. doi: 10.1159/000108959. [DOI] [PubMed] [Google Scholar]
- 8.Katoh M. Transcriptional mechanisms of WNT5A based on NF-kappaB, Hedgehog, TGFbeta, and Notch signaling cascades. Int J Mol Med. 2009 Jun;23(6):763–769. doi: 10.3892/ijmm_00000190. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto Y, Maitra A, Ghosh B, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003 Jun;3(6):565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 10.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003 Oct;3(10):756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 11.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003 Oct 23;425(6960):851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashizawa S, Brunicardi FC, Wang XP. PDX-1 and the pancreas. Pancreas. 2004 Mar;28(2):109–120. doi: 10.1097/00006676-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Swift GH, Liu Y, Rose SD, et al. An endocrine-exocrine switch in the activity of the pancreatic homeodomain protein PDX1 through formation of a trimeric complex with PBX1b and MRG1 (MEIS2) Mol Cell Biol. 1998;18:5109–5120. doi: 10.1128/mcb.18.9.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beattie GM, Itkin-Ansari P, Cirulli V, et al. Sustained proliferation of PDX-1+ cells derived from human islets. Diabetes. 1999 May;48(5):1013–1019. doi: 10.2337/diabetes.48.5.1013. [DOI] [PubMed] [Google Scholar]
- 15.Feanny MA, Fagan SP, Ballian N, et al. PDX-1 expression is associated with islet proliferation in vitro and in vivo. J Surg Res. 2008 Jan;144(1):8–16. doi: 10.1016/j.jss.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Dutta S, Gannon M, Peers B, Wright C, Bonner-Weir S, Montminy M. PDX:PBX complexes are required for normal proliferation of pancreatic cells during development. Proc Natl Acad Sci U S A. 2001 Jan 30;98(3):1065–1070. doi: 10.1073/pnas.031561298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi M, Yamaguchi T, Otsuki M. Induction of PDX-1-positive cells in the main duct during regeneration after acute necrotizing pancreatitis in rats. J Pathol. 2002 Aug;197(5):638–646. doi: 10.1002/path.1134. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Wang C, Wan C, Xiong J, Xu Y, Zhou F. PDX-1 expression in pancreatic ductal cells after partial pancreatectomy in adult rats. J Huazhong Univ Sci Technolog Med Sci. 2004;24(5):464–466. doi: 10.1007/BF02831109. [DOI] [PubMed] [Google Scholar]
- 19.Du YC, Klimstra DS, Varmus H. Activation of PyMT in beta cells induces irreversible hyperplasia, but oncogene-dependent acinar cell carcinomas when activated in pancreatic progenitors. PLoS One. 2009;4(9):e6932. doi: 10.1371/journal.pone.0006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanger BZ, Stiles B, Lauwers GY, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005 Sep;8(3):185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Lewis BC, Klimstra DS, Varmus HE. The c-myc and PyMT oncogenes induce different tumor types in a somatic mouse model for pancreatic cancer. Genes Dev. 2003 Dec 15;17(24):3127–3138. doi: 10.1101/gad.1140403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyatsuka TKH, Shiraiwa T, Matsuoka TA, Yamamoto K, et al. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20(11):1435–1440. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gidekel Friedlander SY, Chu GC, Snyder EL, et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009 Nov 6;16(5):379–389. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballian N, Liu SH, Brunicardi FC. Transcription factor PDX-1 in human colorectal adenocarcinoma: a potential tumor marker? World J Gastroenterol. 2008 Oct 14;14(38):5823–5826. doi: 10.3748/wjg.14.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonmarker S, Glaessgen A, Culp WD, et al. Expression of PDX-1 in prostate cancer, prostatic intraepithelial neoplasia and benign prostatic tissue. APMIS. 2008 Jun;116(6):491–498. doi: 10.1111/j.1600-0463.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi M, Doi R, Toyoda E, et al. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery. 2003 Aug;134(2):260–266. doi: 10.1067/msy.2003.231. [DOI] [PubMed] [Google Scholar]
- 27.Leys CM, Nomura S, Rudzinski E, et al. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006 Sep;37(9):1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Ballian N, Belaguli NS, et al. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas. 2008 Aug;37(2):210–220. doi: 10.1097/MPA.0b013e31816a4a33. [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Gou SM, Wang CY, Wu HS, Xiong JX, Zhou F. Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J Gastroenterol. 2007 May 14;13(18):2615–2618. doi: 10.3748/wjg.v13.i18.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XP, Li ZJ, Magnusson J, Brunicardi FC. Tissue MicroArray analyses of pancreatic duodenal homeobox-1 (PDX-1) in human cancers. World J Surgery. 2005 doi: 10.1007/s00268-004-7823-4. in press. [DOI] [PubMed] [Google Scholar]
- 31.Wang XP, Li ZJ, Magnusson J, Brunicardi FC. Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J Surg. 2005 Mar;29(3):334–338. doi: 10.1007/s00268-004-7823-4. [DOI] [PubMed] [Google Scholar]
- 32.Quint K, Stintzing S, Alinger B, et al. The expression pattern of PDX-1, SHH, Patched and Gli-1 is associated with pathological and clinical features in human pancreatic cancer. Pancreatology. 2009;9(1–2):116–126. doi: 10.1159/000178882. [DOI] [PubMed] [Google Scholar]
- 33.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhijin Wu RAI, Gentleman Robert, Martinez-Murillo Francisco, Spencer Forrest. A Model-Based Background Adjustment for Oligonucleotide Expression Arrays. Journal of the American Statistical Association. 2004 December 1;99(468) [Google Scholar]
- 35.Yokoi N, Serikawa T, Walther R. Pdx-1, a homeodomain transcription factor required for pancreas development, maps to rat chromosome 12. Exp Anim. 1997;46:323–324. doi: 10.1538/expanim.46.323. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson KS, McGlynn L, Hodge M, et al. Isolation, characterization, and differentiation of thy1.1-sorted pancreatic adult progenitor cell populations. Stem Cells Dev. 2009 Dec;18(10):1389–1398. doi: 10.1089/scd.2008.0301. [DOI] [PubMed] [Google Scholar]
- 37.Peshavaria M, Cissell MA, Henderson E, Petersen HV, Stein R. The PDX-1 activation domain provides specific functions necessary for transcriptional stimulation in pancreatic beta-cells. Mol Endocrinol. 2000 Dec;14(12):1907–1917. doi: 10.1210/mend.14.12.0563. [DOI] [PubMed] [Google Scholar]
- 38.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996 Mar;122(3):983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 39.Melloul D. Transcription factors in islet development and physiology: role of PDX-1 in beta-cell function. Ann N Y Acad Sci. 2004 Apr;1014:28–37. doi: 10.1196/annals.1294.003. [DOI] [PubMed] [Google Scholar]
- 40.McKinnon CM, Docherty K. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function. Diabetologia. 2001 Oct;44(10):1203–1214. doi: 10.1007/s001250100628. [DOI] [PubMed] [Google Scholar]
- 41.Meivar-Levy I, Sapir T, Gefen-Halevi S, et al. Pancreatic and duodenal homeobox gene 1 induces hepatic dedifferentiation by suppressing the expression of CCAAT/enhancer-binding protein beta. Hepatology. 2007 Sep;46(3):898–905. doi: 10.1002/hep.21766. [DOI] [PubMed] [Google Scholar]
- 42.Tuveson DA, Zhu L, Gopinathan A, et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006 Jan 1;66(1):242–247. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 43.Kojima K, Vickers SM, Adsay NV, et al. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res. 2007 Sep 1;67(17):8121–8130. doi: 10.1158/0008-5472.CAN-06-4167. [DOI] [PubMed] [Google Scholar]
- 44.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007 Mar;11(3):229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 45.Ma J, Chen M, Wang J, et al. Pancreatic duodenal homeobox-1 (PDX1) functions as a tumor suppressor in gastric cancer. Carcinogenesis. 2008 Jul;29(7):1327–1333. doi: 10.1093/carcin/bgn112. [DOI] [PubMed] [Google Scholar]
- 46.Buettner M, Dimmler A, Magener A, et al. Gastric PDX-1 expression in pancreatic metaplasia and endocrine cell hyperplasia in atrophic corpus gastritis. Mod Pathol. 2004 Jan;17(1):56–61. doi: 10.1038/modpathol.3800015. [DOI] [PubMed] [Google Scholar]
- 47.Leys CMNS, Rudzinski E, Kaminishi M, Montgomery E, Washington MK, Goldenring JR. Expression of PDX-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37(9):1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Sakai HEY, Li XL, Akiyama Y, Miyake S, Takizawa T, Konishi N, Tatematsu M, Koike M, Yuasa Y. PDX-1 homeobox protein expression in pseudopyloric glands and gastric carcinomas. Gut. 2004;53(3):323–330. doi: 10.1136/gut.2003.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava A, Hornick JL. Immunohistochemical staining for CDX-2, PDX-1, NESP-55, and TTF-1 can help distinguish gastrointestinal carcinoid tumors from pancreatic endocrine and pulmonary carcinoid tumors. Am J Surg Pathol. 2009 Apr;33(4):626–632. doi: 10.1097/PAS.0b013e31818d7d8b. [DOI] [PubMed] [Google Scholar]
- 50.Tingstedt JE, Edlund H, Madsen OD, Larsson LI. Gastric amylin expression. Cellular identity and lack of requirement for the homeobox protein PDX-1. A study in normal and PDX-1-deficient animals with a cautionary note on antiserum evaluation. J Histochem Cytochem. 1999 Aug;47(8):973–980. doi: 10.1177/002215549904700801. [DOI] [PubMed] [Google Scholar]
- 51.Malik MT, Kakar SS. Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2) Mol Cancer. 2006;5:61. doi: 10.1186/1476-4598-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouyang H, Mou L, Luk C, et al. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000 Nov;157(5):1623–1631. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell PM, Groehler AL, Lee KM, Ouellette MM, Khazak V, Der CJ. K-Ras promotes growth transformation and invasion of immortalized human pancreatic cells by Raf and phosphatidylinositol 3-kinase signaling. Cancer Res. 2007 Mar 1;67(5):2098–2106. doi: 10.1158/0008-5472.CAN-06-3752. [DOI] [PubMed] [Google Scholar]
- 54.Chao C, Goluszko E, Lee YT, et al. Constitutively active CCK2 receptor splice variant increases Src-dependent HIF-1 alpha expression and tumor growth. Oncogene. 2007 Feb 15;26(7):1013–1019. doi: 10.1038/sj.onc.1209862. [DOI] [PubMed] [Google Scholar]
- 55.Rafiq I, da Silva Xavier G, Hooper S, Rutter GA. Glucose-stimulated preproinsulin gene expression and nuclear trans-location of pancreatic duodenum homeobox-1 require activation of phosphatidylinositol 3-kinase but not p38 MAPK/SAPK2. J Biol Chem. 2000 May 26;275(21):15977–15984. doi: 10.1074/jbc.275.21.15977. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe H, Saito H, Nishimura H, Ueda J, Evers BM. Activation of phosphatidylinositol-3 kinase regulates pancreatic duodenal homeobox-1 in duct cells during pancreatic regeneration. Pancreas. 2008 Mar;36(2):153–159. doi: 10.1097/MPA.0b013e318157753e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morton JP, Lewis BC. Shh signaling and pancreatic cancer: implications for therapy? Cell Cycle. 2007 Jul 1;6(13):1553–1557. doi: 10.4161/cc.6.13.4467. [DOI] [PubMed] [Google Scholar]