Abstract
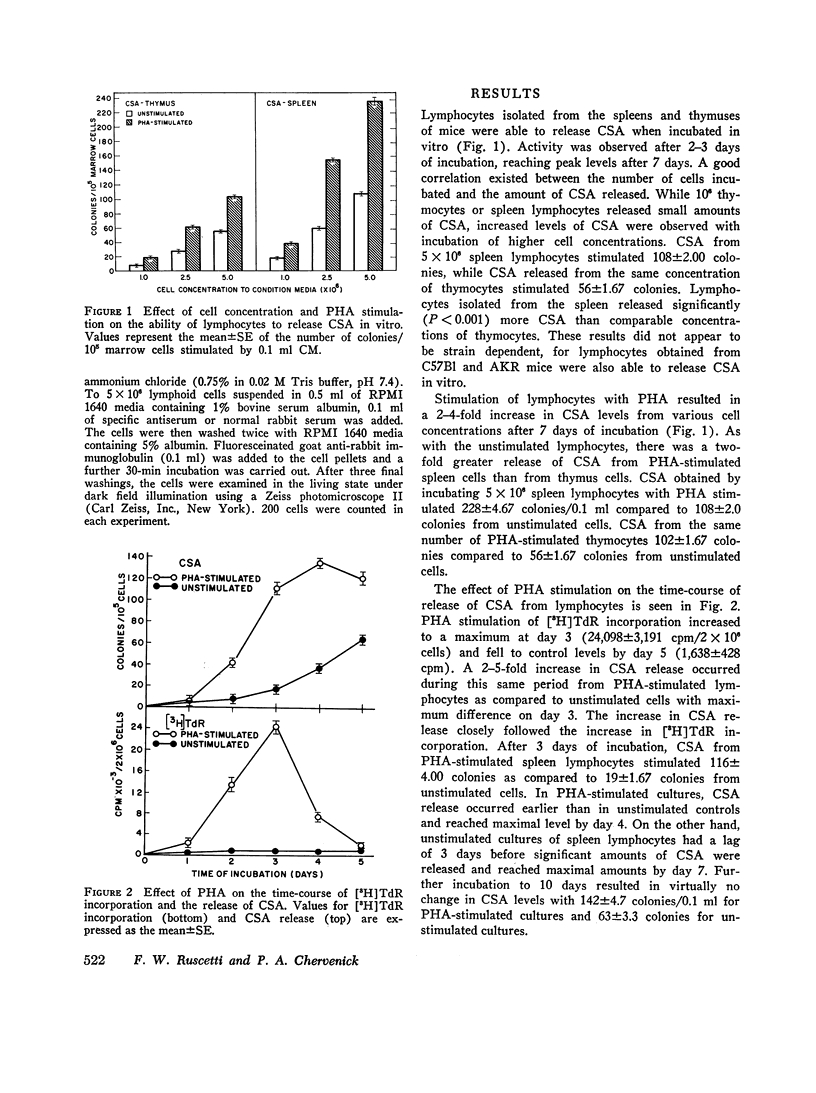
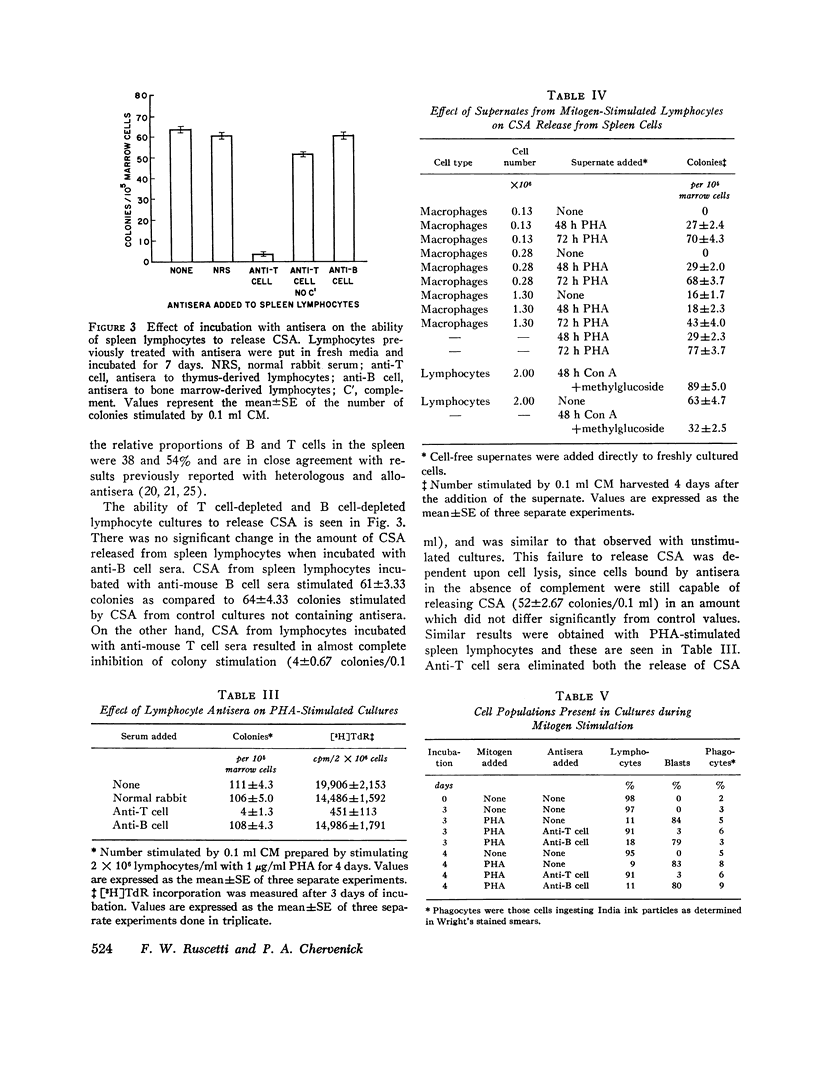
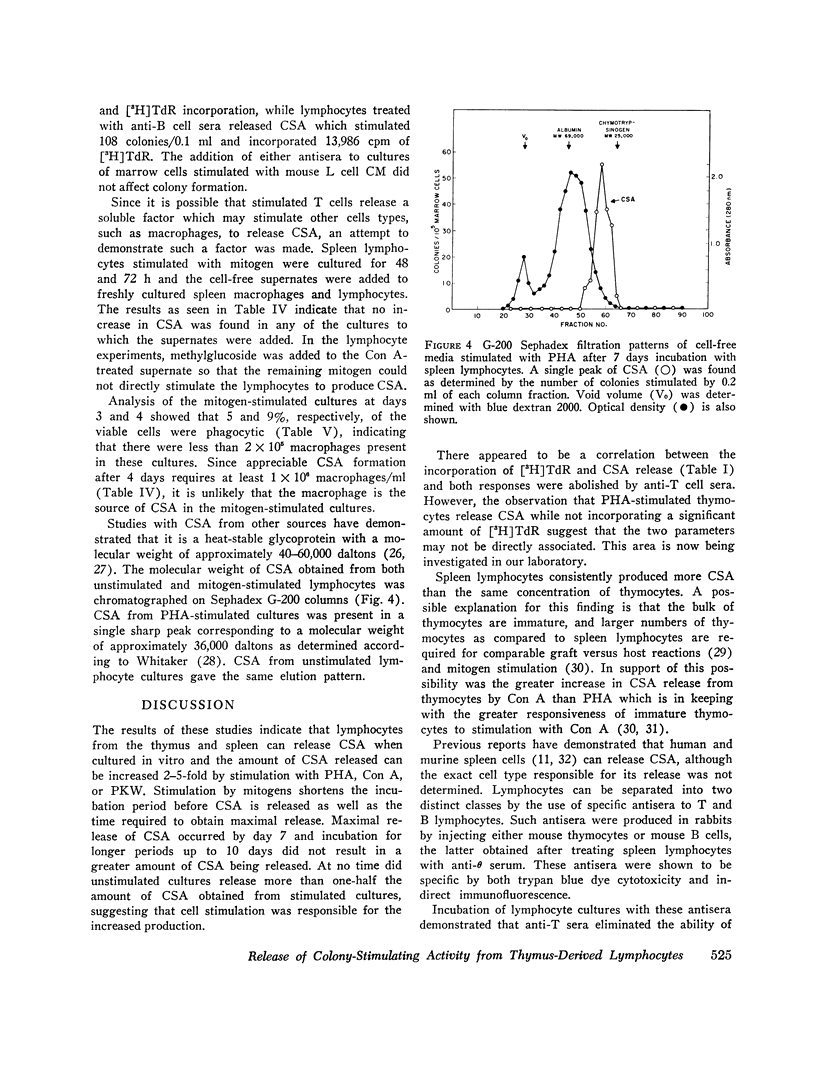
Colony-stimulating activity (CSA) is essential for in vitro differentiation of bone marrow cells into colonies of granulocytes and mononuclear cells. While blood monocytes and macrophages are a major source of CSA, recent studies have indicated that CSA may be produced by lymphocytes responding to immunologic stimulation. Lymphocytes, purified from spleens and thymuses of mice by glass wool columns, were incubated in CMRL-1066 medium with fetal calf serum in vitro. Lymphocytes from the thumus and spleen released CSA when cultured in vitro, with peak levels of CSA observed after 7 days of incubation. Stimulation of cultures with phytohemagglutinin, concanavalin A, or pokeweed mitogen resulted in a 2-5-fold increase in CSA release, with peak levels of CSA released after 4 days of incubation. Thymus-dependent lymphocytes were responsible for the release of CSA from unstimulated and mitogen-stimulated cultures, since the incubation of these cultures with rabbit anti-mouse T cell sera abolished their ability to release CSA. Anti-mouse B cell sera had no effect on the ability of lymphocyte cultures to release CSA. These studies suggest that thymocytes and thymus-derived lymphocytes can release CSA in vitro and may be responsible for the increase in CSA observed in certain immunologic reactions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Blomgren H. Evidence for a small pool of immunocompetent cells in the mouse thymus. Its role in the humoral antibody response against sheep erythrocytes, bovine serum albumin, ovalbumin and the NIP determinant. Cell Immunol. 1970 Oct;1(4):362–371. doi: 10.1016/0008-8749(70)90014-6. [DOI] [PubMed] [Google Scholar]
- Austin P. E., McCulloch E. A., Till J. E. Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. J Cell Physiol. 1971 Apr;77(2):121–134. doi: 10.1002/jcp.1040770202. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Stanley E. R., Sumner M. A. Factors from mouse tissues stimulating colony growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1971 Dec;49(6):595–603. doi: 10.1038/icb.1971.65. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Bone marrow colonies: stimulation in vitro by supernatant from incubated human blood cells. Science. 1970 Aug 14;169(3946):691–692. doi: 10.1126/science.169.3946.691. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., LoBuglio A. F. Human blood monocytes: stimulators of granulocyte and mononuclear colony formation in vitro. Science. 1972 Oct 13;178(4057):164–166. doi: 10.1126/science.178.4057.164. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Production of colony-stimulating activity by human lymphocytes. Nature. 1974 Apr 19;248(5450):703–704. doi: 10.1038/248703a0. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. I. Receptor specificity of cells participating in a cellular immune response. Cell Immunol. 1970 Oct;1(4):404–418. doi: 10.1016/0008-8749(70)90017-1. [DOI] [PubMed] [Google Scholar]
- Davis R. C., Cooperband S. R., Mannick J. A. Preparation and in vitro assay of effective and ineffective antilymphocyte sera. Surgery. 1969 Jul;66(1):58–64. [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Identification of the colony-stimulating cell in human peripheral blood. J Clin Invest. 1972 Nov;51(11):2981–2983. doi: 10.1172/JCI107124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. Production of colony-stimulating factor by human macrophages. Lancet. 1972 Dec 30;2(7792):1397–1399. doi: 10.1016/s0140-6736(72)92966-2. [DOI] [PubMed] [Google Scholar]
- Lamelin J. P., Lisowska-Bernstein B., Matter A., Ryser J. E., Vassalli P. Mouse thymus-independent and thymus-derived lymphoid cells. I. Immunofluorescent and functional studies. J Exp Med. 1972 Nov 1;136(5):984–1007. doi: 10.1084/jem.136.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband E., Boyse E. A. Immunocompetent cells among mouse thymocytes: a minor population. Science. 1971 Jun 18;172(3989):1258–1260. doi: 10.1126/science.172.3989.1258. [DOI] [PubMed] [Google Scholar]
- McNeil T. A. Release of bone marrow colony stimulating activity during immunological reactions in vitro. Nat New Biol. 1973 Aug 8;244(136):175–176. [PubMed] [Google Scholar]
- Moore M. A., Williams N. Physical separation of colony stimulating cells from in vitro colony forming cells in hemopoietic tissue. J Cell Physiol. 1972 Oct;80(2):195–206. doi: 10.1002/jcp.1040800206. [DOI] [PubMed] [Google Scholar]
- Paran M., Sachs L., Barak Y., Resnitzky P. In vitro induction of granulocyte differentiation in hematopoietic cells from leukemic and non-leukemic patients. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1542–1549. doi: 10.1073/pnas.67.3.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. W., Metcalf D. Production of colony-stimulating factor in mitogen-stimulated lymphocyte cultures. J Immunol. 1974 Feb;112(2):502–510. [PubMed] [Google Scholar]
- Perper R. J., Zee T. W., Mickelson M. M. Purification of lymphocytes and platelets by gradient centrifugation. J Lab Clin Med. 1968 Nov;72(5):842–848. [PubMed] [Google Scholar]
- Pick E., Turk J. L. The biological activities of soluble lymphocyte products. Clin Exp Immunol. 1972 Jan;10(1):1–23. [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Price G. B., McCulloch E. A., Till J. E. A new human low molecular weight granulocyte colony stimulating activity. Blood. 1973 Sep;42(3):341–348. [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Sheridan J. W., Metcalf D. A low molecular weight factor in lung-conditioned medium stimulating granulocyte and monocyte colony formation in vitro. J Cell Physiol. 1973 Feb;81(1):11–23. doi: 10.1002/jcp.1040810103. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D. The molecular weight of colony-stimulating factor (CSF). Proc Soc Exp Biol Med. 1971 Jul;137(3):1029–1031. doi: 10.3181/00379727-137-35721. [DOI] [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. V. Lymphocytes lacking detectable surface theta or immunoglobulin determinants. J Exp Med. 1973 Jul 1;138(1):71–88. doi: 10.1084/jem.138.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIERFELDER S. A METHOD FOR THE ISOLATION OF HUMAN LYMPHOCYTES. Vox Sang. 1964 Jul-Aug;9:447–454. doi: 10.1111/j.1423-0410.1964.tb03312.x. [DOI] [PubMed] [Google Scholar]


