Summary
Myogenic, or pressure-induced, vasoconstriction is critical for local blood flow autoregulation. Underlying this VSM response are events including membrane depolarization, Ca2+ entry and mobilization, and activation of contractile proteins. BKCa has been implicated in several of these steps including, 1) channel closure causing membrane depolarization, and 2) channel opening causing hyperpolarization to oppose excessive pressure-induced vasoconstriction. As multiple mechanisms regulate BKCa activity, (subunit composition, Em and Ca2+ levels, post-translational modification) tissue level diversity is predicted. Importantly, heterogeneity may contribute to tissue-specific differences in regulation of myogenic vasoconstriction, allowing local hemodynamics to be matched to metabolic requirements. Knowledge of such variability will be important to exploiting the BKCa channel as a therapeutic target and understanding systemic effects of its pharmacological manipulation.
Keywords: arterioles, smooth muscle, myogenic, large conductance, Ca2+ - activated K+ channel, calcium, Ca2+ sparks
K+ channels play key roles in the regulation of diameter of small arteries and arterioles. Principally this results from K+ channel - induced hyperpolarization of smooth muscle cell plasma membranes decreasing the opening of voltage-gated Ca2+ channels. While arterioles are known express a variety of K+ channels (Table 1; see also Jackson[1]) the dominant channels in regulation of vascular tone are the large conductance Ca2+ - activated K+ channel (BKCa) of the vascular smooth muscle (VSM) cells and the small (SK) - and intermediate (IK) – conductance, Ca2+ activated K+ channels which are particularly associated with endothelial cells. As a result of its large single channel conductance (approximately 150 pS under physiological conditions with respect to K+ gradient) and high protein expression levels, BKCa plays a significant role in determining membrane potential (Em). In addition, and of direct relevance to microvascular smooth muscle, BKCa is activated by both Ca2+ and voltage which favors it functioning as a dominant repolarizing current to act as a negative feedback control mechanism for contractile stimuli while being sensitive to a number of metabolic stimuli (pO2, ROS, phosphorylation, fatty acids and their metabolites).
Table 1.
K+ Channels Implicated in Arteriolar Vasomotor Function.
| Channel | Distribution | Activation | Function |
|---|---|---|---|
| BKCa | Predominately smooth muscle |
Ca2+, voltage, protein kinases, steroid hormones, lipids |
Membrane hyperpolarization; smooth muscle relaxation; oppose myogenic tone |
| Kv | Smooth muscle | Voltage, protein kinases |
Membrane hyperpolarization; Appear to play a negative feedback role in limiting myogenic reactivity. Inhibitors of Kv (4-aminopyridine, correolide) cause vasoconstriction of myogenically active small arteries. |
| Kir | Smooth muscle | Extracellular K+, voltage |
Metabolic and endothelial – dependent dilation. Targeted disruption of Kir2.1 leads to impaired dilator responses to 15 mM K+ without altering myogenic tone or cAMP - mediated dilation. |
| Katp | (Endothelium) and smooth muscle |
Availability of ATP/ADP, protein kinases |
Protection against hypoxia/ischemia. Dilation to vasoactive substances including PGI2, adenosine, isoproterenol. Inhibited by vasoconstrictors. |
|
K2p e.g. TREK-1 (TWIK-related K+channels) |
Endothelium and smooth muscle |
Mechanical, osmolality, polyunsaturated fatty acids |
Operate at physiological levels of membrane potential. Basilar and mesenteric arteries from TREK-1−/− mice do not show altered myogenic responsiveness, suggested that under physiological conditions these channels modulate vasconstriction rather than determining levels of myogenic tone. |
| SK | Predominately EC |
Ca2+ via constitutively bound calmodulin |
Membrane hyperpolarization; Endothelial-dependent dilation |
| IK | Predominately EC, but also expressed in proliferating VSM cell |
Ca2+ via Constitutively Bound calmodulin |
Membrane hyperpolarization; Endothelial-dependent dilation |
The role of VSM BKCa in the regulation of arteriolar tone, specifically myogenic tone, is the focus of this review, whereas the role of SK and IK in endothelial-dependent regulation of arteriolar diameter has been the subject of a number of recent publications [2,3]. Diversity in the molecular structure of BKCa, its spatial localization relative to other signaling elements and its ability to be regulated by a number of post-translational mechanisms (including phosphorylation, fatty acids, hormones and redox state) increases the likelihood that this channel may exhibit both tissue and functional heterogeneity. Further, BKCa may not only play a role in control of vascular tone under physiological conditions, but may contribute to the alterations in tone characteristic of pathophysiological states, such as hypertension, stroke, atherosclerosis, diabetes and complications of cardiovascular surgery [4–7].
Molecular and Electrophysiological Characteristics of BKCa
i. Subunit Composition
Based on both functional and structural evidence [8,9], it is now established that the holo-BKCa channel is comprised of four similar α subunits that co-assemble around a central axis to form a single K+-selective conduction pore. Structurally, the BKCa α subunit contains 7 putative transmembrane (TM)-spanning α helical segments (denoted S0–S6), with the ion conduction pathway and selectivity filter formed by the pore-forming loop structure connecting TM segments S5 and S6 (Figure 1). The amino terminus of each α subunit is located extracellularly; an unusual characteristic for ion channels. The selectivity filter itself is comprised of a signature “G-Y-G motif that is evolutionarily conserved amongst K+-selective ion channels. X-ray crystallographic structures of both prokaryotic (i.e. KcsA, KvAP, MthK) [10–13] and eukaryotic (i.e. Kv1.2)[14] K+ channels have demonstrated that the S5-pore loop-S6 tertiary structure is highly conserved, and itself may be considered a distinct module or domain. Moreover, it is now apparent that the S1–S4 segments present in BKCa and other voltage-gated K+ channels (i.e. Shaker and mammalian Kv homologues) function as a voltage sensor domain (VSD), and collectively represent a distinct structural module. In BKCa channels, 4–5 positively and negatively charged residues distributed within the S2-S4 helices appear to represent the major voltage-sensing residues in the VSD of these channels[15]. This situation differs from that of Shaker-type Kv channels, in which the charged residues contributing to voltage sensing (i.e. Arg residues) are largely contained within the S4 segment. BKCa channels also display weaker voltage sensitivity compared to Kv channels (i.e. ~2.5 versus 12–13 effective gating charges per holo-channel, respectively), most likely due to fewer positively charged residues in the BKCa VSD being sensitive to the transmembrane voltage. Electrophysiologically, this weaker voltage sensitivity of BKCa channels is reflected by the shallower slope of plotted conductance-voltage relations describing BKCa versus Kv-channel opening.
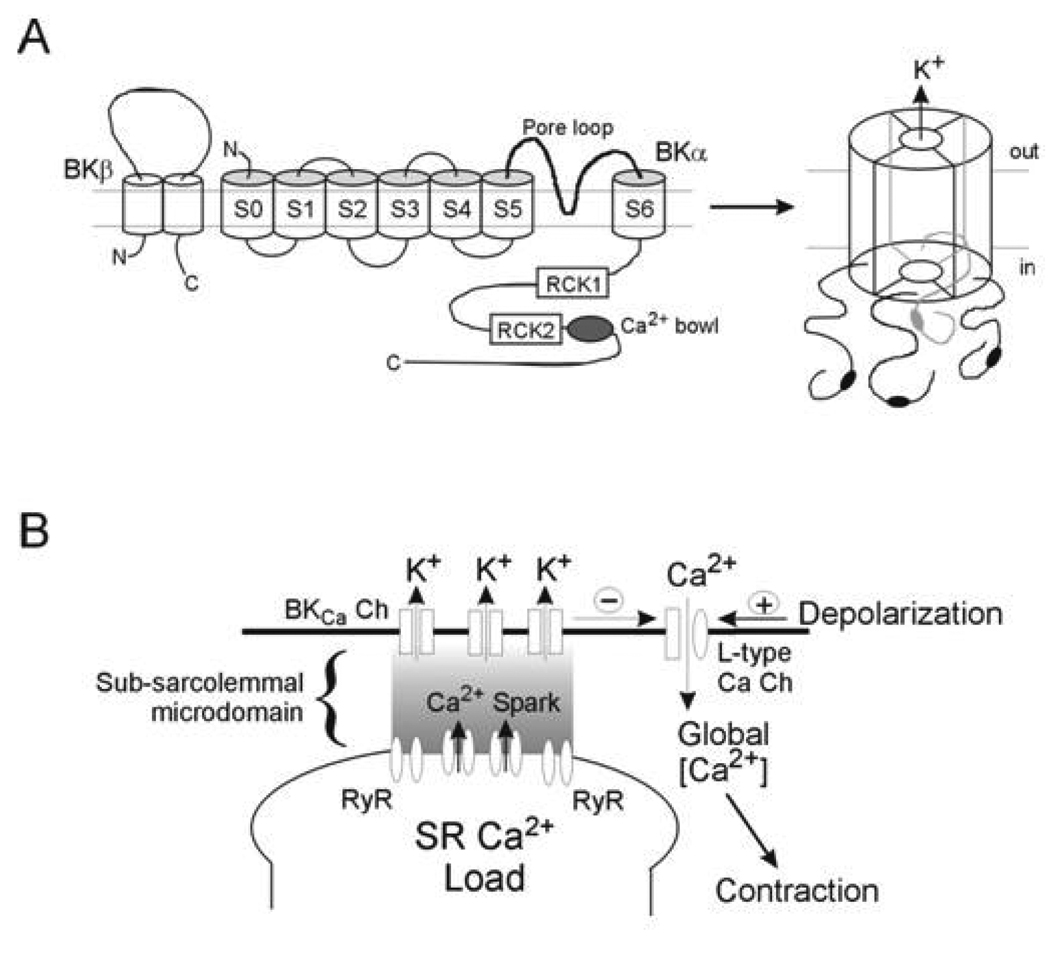
Figure 1.
Schematic diagram illustrating the subunit structure of BKCa (upper panel) and the activation of BKCa through SR-mediated Ca2+ sparks (lower panel). Upper Panel: in VSM cells BKCa exists as a tetramer of α subunits arranged around a central conducting pore. The α subunit has 7 membrane spanning domains and an intracellular C-terminal tail containing a number of regulatory sites (see text for detail). Co-assembled with each α subunit is a β1 subunit, which confers additional Ca2+ and voltage sensitivity on the channel. Lower Panel: close apposition of the SR and plasma membranes creates a restricted space in which Ca2+ can increase to levels required for activation of BKCa. Ca2+ increases occur transiently in the form of Ca2+ sparks due to periodic release from the SR via ryanodine-sensitive mechanisms.
In addition to the transmembrane segments the α subunit has an extensive cytoplasmic carboxy tail (S7–S10) containing a number of regulatory domains. Three distinct Ca2+/divalent metal ion binding sites (RCK 1 and 2) and the ‘Ca2+ bowl’) have been functionally identified by site-directed mutagenesis in this C-terminal region of the α subunit (Figure 1A). The RCK domains in holo-BKCa channels are thought to assemble into “gating rings” beneath the cytosolic mouth of the conduction pore. Direct Ca2+ binding to the high affinity sites within the RCK1 gating ring, in addition to the Ca2+ bowl site, is thought to trigger channel opening via an allosteric coupling mechanism. The α subunit may also exhibit Ca2+ sensing domains within the membrane spanning domains as Ca2+ sensitivity has been shown to persist following deletion of the cytoplasmic sequence [16]. Other studies however, using chimeric channels, have shown the C-terminus to be necessary for Ca2+ sensitivity of the α-subunit [17]. In addition to regulation by Ca2+, the carboxy tail contains phosphorylation sites and RCK1, in particular, has been implicated in the direct gating actions of protons, carbon monoxide and reactive oxygen species[18].
Co-associated with the main channel complex are auxiliary β subunits, which are presumed to be present in a 1:1 ratio with α subunits (Figure 1). This pattern of BKCa subunit co-expression and stoichiometry is thus similar to other members of the large family of voltage-gated K+ (Kv) channel complexes that are typically comprised of α and β subunits [19]. Four distinct genes have been identified for BKCa β subunits (KCNMβ1–4). Thus, four beta subunits (β1–4), have been described with β1 being the principal form found in vascular smooth muscle, with β2 (chromaffin cells, brain, lung), β3 (testis, pancreas, spleen) and β4 (neuronal, lung) generally considered to be restricted to other tissues [20–22]. Structurally, the β subunit exists as 2 transmembrane segments and an extracellular loop with both carboxy and amino termini being intracellular. Further, it is now evident that the BKCa α subunit’s S0 segment is critically important for interaction and functional regulation of channel gating by the auxiliary β subunit [23,24].
Functionally, BKCa β subunits are reported to influence the kinetics and calcium sensitivity of channel gating [25–29], the responses of BKCa activity to pharmacologic modulators [30–34], and the trafficking of channels to the plasma membrane [35–37]. Although differing subunit stoichiometries (i.e. α:β subunit ratio) may exist, a full complement of four β1 subunits are required for maximal effect [38]. Genetic deletion of the β1 subunit in mice leads to hypertension and increased contractility of vascular smooth muscle [39,40], whereas a gain-of-function mutation in the β1 subunit is associated with lower blood pressure in humans [41].
ii. Splice Variants
The BKCa α subunit is translated from a single gene [42] denoted KCNMA1 that contains 27 distinct exons [43], thus the presence of multiple exon boundaries in the pre-messenger RNA provides ample opportunity for structural modification of the final protein product through alternative splicing of the initial gene transcript. Up to 13 alternative splice sites have been described in mammalian BKCa α subunit mRNA. Such variants have been shown to contribute to differences in the regulatory properties of the channel due to variability in responses to steroids, cyclic nucleotides and availability of phosphorylation sites. One such splice variant of the α subunit involves the STRess axis regulated EXon (STREX) which changes the response to cAMP from stimulatory to inhibitory relative to the variant lacking this exon (termed ZERO). Importantly, the expression of different splice variants between tissues could also contribute to differences in voltage sensitivity of the channel [44] with STREX-1 showing a leftward shift in half-maximal voltage for activation (i.e. V1/2 shifted 20 -30 mV more negative [45] to a level of Em consistent with that observed in cannulated arterioles [46,47]. To date, BKCa channels cloned from smooth muscle typically do not contain the STREX sequence, which is consistent with functional data demonstrating that activation of the smooth muscle cAMP/PKA signaling pathway enhances BKCa channel opening, leading to relaxation [48]. Comparisons of the predicted amino acid sequences of smooth muscle BKCa α subunit clones further reveal strong conservation in primary sequence throughout the protein, with the most diversity occurring at the extreme C-terminus. With regard to the β subunit, while considerable variation exists in primary structure between species (reference) there is little information regarding splice variants of the β1 subunit, the predominant beta subunit in vascular smooth muscle. In contrast splice variants of β3 have been reported [49].
iii. Regulation of BKCa by Ca2+ and Voltage
BKCa channels belong to the family of voltage-gated K+ channels as shown by both primary sequence analysis and their biophysical behavior [50–52], however, the presence of intrinsic binding sites for intracellular divalent metals (i.e. Ca2+ and Mg2+) affords an independent and synergistic mechanism of activation via stimulus-evoked elevations in cytosolic free Ca2+ concentration. Detailed biophysical characterization of BKCa channel gating by Aldrich and colleagues, along with Stefani and co-workers, have demonstrated that BKCa channel opening may be induced by voltage alone, in the absence of calcium, or solely by cytosolic free calcium, under conditions in which the voltage sensor domains remain inactive [52,53]. At resting levels of cytosolic Ca2+ (i.e. 100 nM), very positive voltages are required to achieve significant BKCa channel opening (i.e. half-maximal voltage of activation is >100 mV), due to the inherently weak voltage sensitivity of these channels. With increasing cytosolic free [Ca2+], however, this voltage-dependent activation is shifted left-ward along the voltage axis to more negative membrane potentials [50,54], so that more channel activity is observed within the physiologic range of membrane potentials. Calcium thus appears to enhance the voltage-dependent activation of BKCa channels, such that channel opening will be greater at any given membrane potential with increasing levels of cytosolic Ca2+. This mechanism is particularly important in smooth muscle cells of myogencially active resistance arteries, which do not typically use action potentials to produce graded changes in tone and display depolarized membrane potentials in the range of −40 to −30 mV. In this situation, stimulated rises in cytosolic free Ca2+ will be the major determinant of cellular BKCa activity, as channel open probability over this negative voltage range will only be ~0.1% (at an intracellular Ca2+ level of 100 nM). Thus, in vascular smooth muscle, BKCa channels appear to operate primarily as ligand-gated channels, with membrane potential in the physiologic range producing only a modest level of background activity upon which Ca2+ elevations evoke further increases. Functionally, the smooth muscle-associated BKβ1 subunit significantly increases the activity of the holo-channel, primarily by enhancing the apparent sensitivity of channel activation by Ca2+ [25,27,55,56]. Smooth muscle BKCa channels assembled from α + β1 subunits will thus have greater activity at a given [Ca2+] compared to channels containing only α subunit, as well as channels containing less than a 1:1 ratio of β1 to α subunits [38].
Spatial Considerations in BKCa Activity
The local environment in which BKCa resides in part determines its function within a given tissue. For example, efficient activation by locally produced Ca2+ transients, or sparks (via ryanodine-sensitive release channels) requires a close spatial arrangement between SR and plasma membranes (Figure 1B). In smooth muscle the distance between the plasma membrane and superficial SR has been reported to be less than 20 nm [57]. Facilitating such a spatial arrangement is the superficial SR found in smooth muscle and possibly plasma membrane invaginations or caveolae. The α-subunit of BKCa is known to contain two caveolin binding sites and BKCa has been demonstrated to associate with caveolin 1 in cultured endothelium [58] and myometrium [59]. In cerebral arteries, caveolae have been shown to be functionally important in coupling Ca2+ sparks to spontaneous transient outward currents (STOCs) (Lohn et al., 2000; Drab et al., 2001) presumably, which are presumably mediated through the opening of a population/cluster of BKCa channels [60]. Thus, genetic ablation of caveolin-1 was shown to lead to both a loss of caveolae and a decrease in STOC frequency [61] while cholesterol depletion with β-methyl cyclodextrin decreased spark frequency, amplitude and spatial dimensions by ablating caveolae and possibly disrupting the local coupling between plasmalemmal VGCC and RyR [62]. A consequence of this architecture is that subpopulations of BKCa may exist [63] to perform specific functions independently of the more global BKCa complement.
Additional localized regulation of BKCa activity may result from the colocalization of other signaling molecules (for example, c-Src; G-protein coupled receptors) and cytoskeletal elements (for example, actin) [64]. Similarly, targeting of BKCa to specific membrane domains has also been implicated in the functional coupling of this population of BKCa to other ion channels, including cation channels comprised of TRP proteins and voltage-gated Ca2+ channels [65,66].
iv. Post-Translational Modification
Regulation of BKCa conductance is also exerted via direct phosphorylation by a number of protein kinases (including PKA, PKG, PKC, Src) and BKCa is reported to have a large number of potential phosphorylation sites [67]. Importantly, a number of vasodilator and vasoconstrictor stimuli utilize phosphorylation-mediated mechanisms to regulate ion channel activities. In general, VSM cell BKCa is activated by PKA [48,68] and PKG [48,69] while being inhibited by PKC (Schubert et al., 1999) and the tyrosine kinase cSrc [70,71], although the latter effect is controversial [72,73]. It is apparent, however, that tissue specific differences exist in responses to various protein kinases, as pulmonary artery smooth muscle BKCa is activated by particular isoforms of PKC [74]. Cross-talk between cyclic nucleotide-dependent systems may also occur as cAMP-dependent activation of BKCa has been reported to occur via cGMP-dependent protein kinase G [75]. Similarly, the activation of BKCa in pulmonary artery smooth muscle by PKC is reported to occur via a cGMP-dependent mechanism [74]. Collectively, these kinase-based mechanisms allow for modulation of BKCa open probability by a number of vasoactive substances. Biophysically, vasodilators acting through cAMP or cGMP tend to increase channel open probability by shifting voltage-activation curves towards more negative membrane potentials [48]. In VSM of skeletal muscle arterioles, such pathways could provide a mechanism whereby a relatively quiescent channel could be activated during metabolic activity to oppose the normally high resting vascular resistance [47,76].
In addition to phosphorylation, BKCa is potentially regulated by a number of other post-translational modifications. For example, palmitoylation of a cysteine-rich domain within the STREX insert can potentially affect membrane targeting/anchoring, trafficking and signaling [77]. Interestingly, when the palmitoylated STREX variant is phosphosphorylated by PKA, the C-terminus of the α-subunit alters its interaction with the plasma membrane and channel activity is inhibited [77]. These observations highlight the complexity of BKCa regulation and the potential for interaction between such mechanisms.
BKCa is also modulated by a number of small molecules including steroids, fatty acids, PIP2 and reactive oxygen species [18,34,78]. In the case of steroid hormones, modulation of BKCa activity has been shown to occur through genomic [79] and/or non-genomic [33,34] effects, with the latter being mediated via cGMP in the case of estrogen-induced relaxation of coronary arteries [80]. In cerebral arteries it is likely that the increase in cGMP occurs secondary to increased NO production [81] although NO has been suggested to exert direct effects on BKCa [82]. Estrogen - like molecules, including 17β-estradiol and tamoxifen exert a direct non-genomic effect to cause enhanced opening of BKCa through an action on the β1 subunit of BKCa [33,34]. Genomically-mediated effects include control of expression of spice variants. During pregnancy, the hormonal environment dictates alternate splicing patterns of the BKCa α subunit expression, with the STREX variant diminishing and PKA signaling switching from inhibitory to excitatory [79]. Similarly, splice variant expression (i.e. STREX) is regulated by stress hormones of the hypothalamic – adrenal axis [83].
BKCa and the Arteriolar Myogenic Response
Introduction to the Myogenic Response
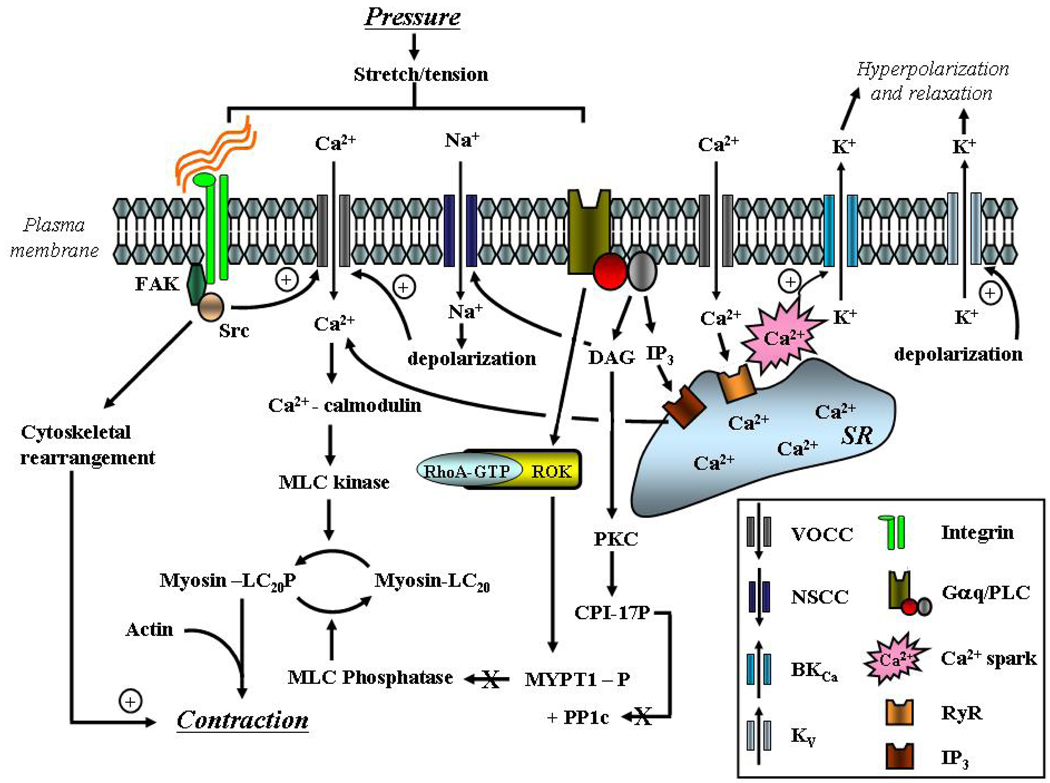
The term ‘myogenic response’ refers to the ability of arterioles to respond to changes in intraluminal pressure. Specifically vasoconstriction follows an increase in pressure while vasodilation occurs in response to a pressure decrease. The physiological significance of myogenic responsiveness relates to its role in the local autoregulation of microvascular blood flow and provision of a level of basal vascular tone (resistance) upon which vasodilators and vasoconstrictors can act. A further role relates to the control of capillary pressure, affording protection against pressure-induced capillary leakage and the formation of edema. This mechanically-induced vasomotor response has been shown to be a function inherent to vascular smooth muscle and is independent of the endothelium or neural input. While the exact signaling mechanisms underlying myogenic vasoconstriction are not completely defined, a plausible pathway involves activation of non-selective cation and/or stretch-activated channels, membrane depolarization, opening of voltage-gated Ca2+ channels, Ca2+ influx and activation of the contractile proteins (Figure 2). These events are likely supported by a number of other mechanisms, including the rearrangement of cytoskletal elements, Ca2+ release from the sarcoplasmic reticulum and increased Ca2+ sensitivity of the contractile elements (Figure 2). As changes in Em and global intracellular Ca2+ appear central to myogenic constriction, considerable interest has been shown in the potential influence of dynamic BKCa activity. Further detail regarding myogenic signaling mechanisms can be found in recent reviews [84–87].
Figure 2.
Schematic diagram illustrating signaling mechanisms underlying arteriolar myogenic vasoconstriction. Emphasis is placed on ion channels, membrane depolarization, mobilization of Ca2+ and activation of the contractile proteins. In addition, negative feedback activation of BKCa by SR-mediated Ca2+ sparks is shown (from Hill et al.[124]).
Mechanisms Linking BKCa to Myogenic Vasoconstriction
i. Modulation of BKCa by stretch (direct and indirect)
Several studies have examined the direct stretch sensitivity of BKCa in smooth muscle. The potential relevance of such a mechanism to the myogenic response is that mechanical stretch, during an alteration in intraluminal pressure, would alter BKCa channel activity thereby impacting Em and Ca2+ entry via voltage-gated Ca2+ channels. Kirber et al (1992) and Dopico et al (1994) in studies (pulmonary and mesenteric arteries, respectively) at both the whole cell and excised patch levels reported that stretch exerts a direct stimulatory effect on BKCa channel opening. The data from excised membrane patches were used to exclude a role for SR-mediated Ca2+ release. These data would not, however, be consistent with mechanical activation of BKCa being involved in myogenic contraction, per se, as stretch would induce hyperpolarization leading to relaxation. In studies of coronary arteriolar smooth muscle cells, [88] showed that the stretch-induced activation of BKCa channels was largely overridden by the concurrent activation of an inward cation current. Thus, collectively, these studies support direct stretch activation of BKCa as acting as a brake on stretch-induced depolarization to limit the magnitude of depolarization and contraction.
A number of alternative mechanisms indirectly linking BKCa to a stretch stimulus have been proposed. An increase in intraluminal pressure has been shown to lead to the production of the arachidonic acid metabolite 20-HETE, which causes membrane depolarization by closure of BKCa [89]. Interestingly, arachidonic acid and other metabolites of this fatty acid have been shown to cause an increase in opening of BKCa [90]. A possible explanation for these differences may relate to specific actions such as 20-HETE-induced activation of PKC [91], as this kinase has been reported to inhibit BKCa. Alternatively, differences may relate to the physico-chemical properties of lipids which have been shown to dictate both inhibitory and stimulatory actions on BKCa [92].
A further mechanism which may link BKCa to the pressure/stretch-induced mechanical stimulus involves an action on cell surface integrins mediated through extracellular matrix proteins. Integrins have been implicated in both myogenic responsiveness and activation of BKCa. In particular, α5β1 integrin activation with fibronectin was shown to enhance BKCa opening in arteriolar smooth muscle cells [93] through a mechanism dependent upon cSrc [73]. Blockade of α5β1 integrin with specific antibodies, however, inhibits myogenic constriction in intact arterioles [94].
ii. Ca2+ entry
Direct activation of BKCa in arteriolar smooth muscle by stretch-induced Ca2+ entry is unlikely. Ca2+ levels in the vicinity of the channel are required to reach 5 – 10 µM to markedly affect channel opening, whereas smooth muscle global cytosolic Ca2+ concentrations remain in the range 100 – 300 nM in cannulated arterioles over a physiological range of intraluminal pressures. It is further unlikely that this level of Ca2+, in combination with the expected pressure-induced depolarization, would significantly alter BKCa opening.
Clustering of voltage-gated Ca2+ channels in a membrane domain in which BKCa channels are co-localized could provide a mechanism for focal increases in Ca2+, conceivably of significant magnitude to stimulate BKCa opening. Consistent with this possibility Ca2+ sparklets (resulting from Ca2+ entry through clustered VGCCs) have been identified in arterial smooth muscle cells [95,96], while other studies have suggested a functional co-localization of BKCa and VGCC in caveolae [97]. Data are not currently available, however, linking these events to pressure-induced activation of BKCa in intact arterioles.
Ca2+ entry may also indirectly facilitate BKCa activation through increased filling of SR stores and increasing the sensitivity of RyR to subsequent CICR. In cerebral artery smooth muscle cells, Ca2+ sparks and Ca2+ waves are increased in frequency by depolarization (30 mM KCl) and inhibited by blockade of voltage-gated Ca2+ channels [98]. Similarly, increasing intraluminal pressure from 10 to 60 mmHg in isolated cerebral arteries (a stimulus expected to cause both depolarization and Ca2+ entry via VGCC) caused an approximate 2.5 fold increase in the frequency of Ca2+ sparks and waves. Whether a similar situation applies in all vascular beds is uncertain. In cremaster muscle, elevating intraluminal pressure increases the frequency of Ca2+ waves, and this relationship persists in the presence of nifedipine, but is blocked by putative inhibitors of non-selective cation channels [99]. Conceivably this may point to some of the effects of increasing Ca2+ entry being independent of the precise entry mechanism.
iii. Ca2+ sparks and SR-mediated events
While myogenic constriction lowers pressure distal to the point of constriction there is a tendency for upstream pressure to increase. The result of this situation is that myogenic constriction can act as a positive feedback system with the potential for uncontrolled vasoconstriction. Under physiological conditions, this is not observed, however, as myogenic constriction exhibits upper and lower pressure limits while the increased pressure may also activate hyperpolarizing mechanisms that oppose the pressure-induced depolarization and contraction. A candidate hyperpolarizing pathway involves the large conductance, Ca2+-activated, K+ channel (BKCa).
The idea that BKCa could be activated as a repolarizing event thus opposing the action of vasoconstrictor stimuli evolved from the work of Benham and colleagues [60,100]. Nelson and colleagues [101,102] subsequently implicated BKCa as a negative feedback mechanism limiting pressure-induced or myogenic constriction. Activation of BKCa was hypothesized to occur in response to focal Ca2+ release (Ca2+ sparks) from the SR. Specifically, a spark was proposed to allow local Ca2+ concentrations below the plasma membrane to reach levels approaching 10 µM for brief periods. Such high local Ca2+ concentrations would be sufficient to activate a cluster of BKCa channels (giving rise to spontaneous transient outward currents) while not contributing markedly to global cytosolic Ca2+ levels and contraction. In fact Ca2+ sparks are viewed as activating hyperpolarizing current which leads to closure of voltage-gated Ca2+ channels and vasodilation.
The idea that Ca2+ sparks oppose myogenic constriction through opening of BKCa would appear to be at odds with the hypothesis that an increase in intraluminal pressure causes the production of the arachidonic acid metabolite, 20-HETE, resulting in closure of BKCa with subsequent depolarization and contraction [89,103]. Interestingly, evidence for both Ca2+ sparks and 20-HETE production has been provided in rat cerebral blood vessels [98,102,103]. The precise relationship between these apparently opposing pathways, however, remains unclear, although it is questionable that both mechanisms could be simultaneously present, even if arguments of spatio-temporal separation are proposed.
Heterogeneity in BKCa Function
i. Variation at the Level of the Channel
As outlined above, a number of characteristics of the BKCa channel are consistent with the possibility of vascular heterogeneity. Differences in subunit stoichiometry, isoform expression or the expression of splice variants could result in channels of varying Ca2+ sensitivity and responsiveness to post translational modifications including phosphorylation.
On the basis of differences in the relationships between intraluminal pressure, level of smooth muscle Em, and observed myogenic tone in small cerebral arteries and arterioles from skeletal muscle of the rat, it has been hypothesized that these two tissues may exhibit differing contributions of BKCa. At the tissue level, it was further suggested that these differences allowed arterioles in resting skeletal muscle to maintain a relatively high level of vascular resistance [47]. This idea was supported by the observation that cremaster muscle arterioles showed relatively small changes in Em and diameter in response to IBTX and that the extent of the constriction (i.e. effectiveness of IBTX) was apparently independent of the level of intraluminal pressure. Jackson and Blair [76] similarly reported a comparative lack of effect of IBTX on the in vivo resting diameters of cremaster muscle second and third order arterioles (diameter approximately 20 – 40 µm) from hamster while Frisbee et al[104] found that IBTX did not alter myogenic responsiveness in isolated gracilis muscle from normotensive Dahl rat. Similarly, both in vivo and in vitro studies of renal [105] and coronary arteries [106] have shown little effect of BKCa inhibitors (IBTX and penitrem A) on resting vascular resistance. Collectively, these observations appear to provide evidence against BKCa being recruited as a general negative feedback mechanism as pressure and hence myogenic tone increase.
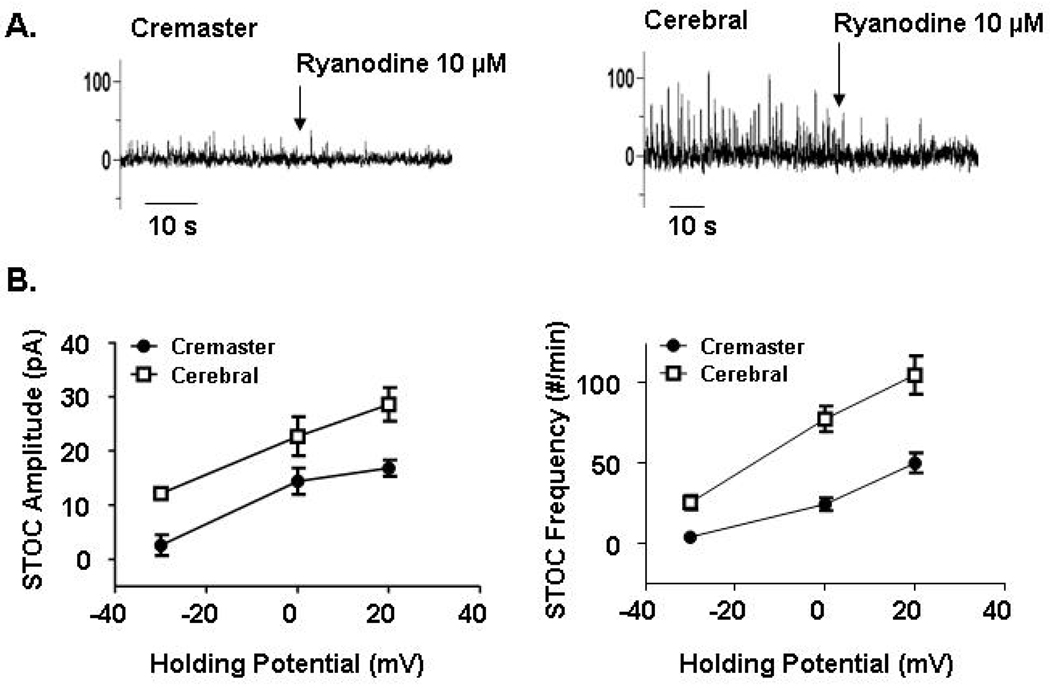
To examine the apparent differences in BKCa between two different vascular beds, comparative electrophysiological studies were performed using patch clamp on freshly isolated VSM cells. Relative to VSM cells from cerebral arteries, VSM from skeletal muscle arterioles displayed a lower density of IBTX-sensitive K+ current while Kv-associated current appeared similar. This difference was particularly evident at high pipette Ca2+ levels (5 µM), consistent with the observed difference in whole-cell K+ current being mediated by differences in BKCa. Further supporting a decreased contribution of BKCa was the observation that STOCs were less frequent and of smaller amplitude in VSM cells from skeletal muscle arterioles compared to cells from cerebral arteries despite identical recording conditions (Figure 3). Pharmacological studies suggested a difference at the level of the β1 regulatory subunit, as skeletal muscle VSM showed diminished sensitivity to acute estrogen exposure compared to cerebral VSM while both vasculatures showed comparable increases in K+ current in response to NS-1619. Estrogen had previously been shown to exert an enhancing effect on BKCa opening via the β1 subunit, while NS-1619 acts through the α subunit. A relative decrease in expression of BKCa was shown both at the mRNA and protein levels in skeletal muscle VSM. Further to this, the ratio of β1:α subunit protein expression in cremaster VSM was shown by Western blotting to be decreased. The importance of this relates to the fact that a simple reduction in protein might not necessarily explain the differences in BKCa characteristics, due to its known large conductance while the differences in subunit composition are consistent with the observed heterogeneity involving Ca2+ and Em sensitivities. Interestingly, in vitro treatment of cerebral artery VSM with siRNA directed at the β1 subunit causes BKCa function to more closely resemble that of native skeletal muscle muscle VSM [107].
Figure 3.
Characteristics of STOCs in VSM cells enzymatically isolated from cremaster muscle arterioles and small cerebral arteries. The upper panels show example STOC tracings and illustrate that despite differences in apparent voltage dependency, ryanodine eliminates the outward currents in both cell preparations. Recordings were made at a pipette [Ca2+] of 100 nM. The lower panel shows that at any given holding potential STOCs are both less frequent and of smaller amplitude in cremaster VSM cells compared to those from cerebral arteries. Data are re-plotted from Yan et al and are shown as mean SEM for n = 6 – 8 cells.
To provide additional insight into differences in BKCa function in skeletal muscle and cerebral artery VSM cells STOCs were recorded at various holding potentials. STOCs have previously been suggested to arise from the opening of clusters of BKCa [108] and reflect the simultaneous opening of a variable number of individual channels [108–110]. At any holding potential cerebral VSM cells showed an increased frequency of STOCs and further the outward currents were greater in amplitude compared to those recorded in skeletal muscle VSM cells [111] (Figure 3). From the comparative data, it can be seen that skeletal muscle VSM cells require approximately 30 mV additional depolarization (compared to cerebral VSM cells) to generate a comparable STOC frequency. Although STOCs could be evoked in cerebral VSM cells at levels of Em approximating the physiological range, cells from skeletal muscle muscle required considerably more positive holding potentials (Figure 3).
ii. Variation in Activation of the Channel
As outlined above, Ca2+-mediated activation of BKCa is thought to occur as a result of focal Ca2+ release in the form of ‘sparks’ from the SR. Ca2+ sparks comparable to those in cerebral VSM cells do not, however, appear to be found in similarly prepared cells from skeletal muscle arterioles[111]. However, as STOCs are inhibited in skeletal muscle VSM cells by both IBTX and ryanodine, it is evident that, at least under the conditions of whole cell patch clamp, a BKCa activation mechanism involving SR Ca2+ release does exist (Figure 3). The question is, why are comparable Ca2+ spark events not identified in the two cell preparations? The difference does not appear to relate to methods used for isolating the cells nor differences in resting Em as determined using a perforated patch recording strategy under current clamp conditions. Differences therefore reflect variation in how the VSM cell preparations respond to the patch clamp conditions or the occurrence of Ca2+ release events in skeletal muscle VSM cells that were below the limits of detection for the approach used. Regardless, as both cell types were investigated using the same experimental strategy, this suggests differences in the exact cellular mechanism by which the SR is coupled to activation of BKCa.
The possibility that Ca2+ release events are yet to be detected in some VSM cell types, including those of skeletal muscle muscle arterioles, is highly likely. It is clear that there is considerable variation in spatiotemporal aspects of Ca2+ signaling [112]. Further, even in cerebral VSM cells, approximately 50% of BKCa current was not associated with typical Ca2+ sparks [113]. Interestingly, the currents not associated with sparks tended to be of smaller amplitude, perhaps more consistent with the situation in cremaster VSM, where sparks were not detected and STOCs were of smaller amplitude (at a given Em) compared to cerebral VSM cells yet the observed STOCs were inhibited by depletion of SR Ca2+ with ryanodine (Figure 3).
An alternate explanation is that BKCa may be activated by distinct mechanisms in differing vascular beds. Variation in phosphorylation–mediated mechanisms perhaps may result from tissue – specific expression of α subunit splice variant. Similarly, regional variation in the production of vasoactive factors that activate BKCa may contribute to apparent functional heterogeneity of the channel. In this regard Hercule et al [114] have demonstrated that the vasodilator 17,18-epoxyeicosatetrenoic acid stimulates BKCa by an action on the α subunit. Further, these authors showed that this action occurred despite the presence of low Ca2+ concentrations (100 nM) or genetic deletion of either RyR3 or BKCa β1, indicating that neither local nor global Ca2+ increases were required.
iii. Other Possible Factors Contributing to BKCa Heterogeneity Between Vascular Beds
In addition to differences in the contribution of the β1 subunit to BKCa function between vascular beds it is conceivable that heterogeneity arises from the existence of tissue-specific expression of splice variants. Such expression patterns may subsequently confer differences in response to vasoactive stimuli, which post-translationally regulate BKCa (for example, via phosphorylation) as well as determining cellular localization and trafficking. To date, however, relatively little data specific to arteriolar smooth muscle are available. Consistent with this possibility, Poulsen et al. [115] have recently shown C-terminal splice variants in rat cerebral blood vessels. Further, these authors suggest that while the β1 subunit is the predominant form in the vessel wall of cerebral arteries the β2 and β3 isoforms can also be detected. These results await confirmation in purified VSM cell preparations and extension to other vascular beds.
It is currently also unknown whether the same cellular architecture exists in all vascular smooth muscle cells or if variation affecting the exact localization of BKCa occurs between vascular beds. As the extent of peripheral SR has been reported to vary between blood vessels [116–118] it may be expected that the relationship between the SR, Ca2+ sparks and BKCa-mediated STOCs also show regional variation. Consistent with this, the coupling efficiency between Ca2+ sparks and STOCs has been reported to vary between smooth muscle tissues [119].
Similarly, differences in coupling between the SR and BKCa between vascular beds could also relate to variation in the expression, or cellular distribution, of ryanodine receptors. In regard to this, little is known as to whether there are quantitative differences in the various ryanodine receptor isoforms (RyR 1, 2 and 3) between cerebral and cremaster muscle VSM cells.
Although not discussed in detail in this review, variation in the role of BKCa in the vasculature may exist between species and as a result of differing genetic backgrounds within strains of the same species. In relation to the latter point, Stadnickna and colleagues have reported that differences in the expression and function of BKCa between normotensive Dahl salt-sensitive and Brown Norway rats underlies differences in the cardiovascular responsiveness to the anesthetic agent, propofol [120]. Specifically, differences in chromosome 13 resulted in an increased expression of BKCa (α-subunit) and enhanced hyperpolarization of mesenteric artery smooth muscle cells in the Dahl rats. Similarly, age-dependent changes in BKCa expression and function may contribute to differences in the physiological role of the channel within the cardiovascular system [121–123].
Conclusion
Apparent differences in the structure and function of BKCa in VSM cells of skeletal muscle compared to cerebral vasculature raise challenges to the classical view of Ca2+ sparks as the primary mechanism for channel activation and dilation in all arteries. The complex array of mechanisms that regulate BKCa in vascular smooth muscle appear likely to contribute to this regional heterogeneity in channel function and may allow the channel function to be adapted to local requirements. Current challenges important to understanding the physiological significance of such variation include identifying vascular bed-specific differences in channel subunit composition, BKCa regulation by post-translational mechanisms and its exact relationship to SR-mediated Ca2+ release. In regard to the involvement of BKCa in the regulation of pressure-induced membrane depolarization and contraction, such heterogeneity may be utilized to allow for variation in the regional control of vascular resistance to match local hemodynamics with metabolic requirements. Further, knowledge of the molecular and cellular basis for tissue heterogeneity will be important to both exploiting BKCa as a potential therapeutic target and understanding the systemic effects of pharmacologically manipulating this K+ channel.
Acknowledgements
MAH and MJD are supported by grants from the National Institutes of Health (NHLBI) and APB by grants from the Canadian Heart and Stroke Foundation and Alberta Heritage Foundation for Medical Research. SRE was supported by a pre doctoral training award from NIH. Sincere thanks are extended to Dr Chris Triggle for constructive comments and suggestions.
Abbreviations
- BKCa
Large conductance, Ca2+ - activated, K+ channel
- CICR
Ca2+ - induced Ca2+ release
- EC
Endothelial cell
- Em
Membrane potential
- IBTX
Iberiotoxin
- IK
Intermediate conductance, Ca2+ - activated, K+ channel
- IP3
Inositol trisphosphate
- MLC
Myosin light chain
- PIP2
Phosphatidylinositol 4,5 – bisphosphate
- PLC
Phospholipase C
- RCK
Regulator of conductance for K+
- ROS
Reactive oxygen species
- RyR
Ryanodine receptor
- SK
Small conductance, Ca2+ - activated, K+ channel
- SR
Sarcoplasmic reticulum
- STOC
Spontaneous transient outward current
- STREX
STRess axis regulated EXon
- VGCC
Voltage-gated Ca2+ channel
- VSD
Voltage sensor domain
- VSM
Vascular smooth muscle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- 5.McGahon MK, et al. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng J, Liu Y, Khabbaz KR, Sodha NR, Osipov RM, Hagberg R, Alper SL, Sellke FW. Large conductance calcium-activated potassium channels contribute to the reduced myogenic tone of peripheral microvasculature after cardiopulmonary bypass. J Surg Res. 2009;157:123–128. doi: 10.1016/j.jss.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- 8.Shen KZ, Lagrutta A, Davies NW, Standen NB, Adelman JP, North RA. Tetraethylammonium block of Slowpoke calcium-activated potassium channels expressed in Xenopus oocytes: evidence for tetrameric channel formation. Pflugers Arch. 1994;426:440–445. doi: 10.1007/BF00388308. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Sigworth FJ. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 2009;461:292–295. doi: 10.1038/nature08291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 14.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 15.Ma Z, Lou XJ, Horrigan FT. Role of charged residues in the S1-S4 voltage sensor of BK channels. J Gen Physiol. 2006;127:309–328. doi: 10.1085/jgp.200509421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piskorowski R, Aldrich RW. Calcium activation of BK(Ca) potassium channels lacking the calcium bowl and RCK domains. Nature. 2002;420:499–502. doi: 10.1038/nature01199. [DOI] [PubMed] [Google Scholar]
- 17.Xia XM, Zhang X, Lingle CJ. Ligand-dependent activation of Slo family channels is defined by interchangeable cytosolic domains. J Neurosci. 2004;24:5585–5591. doi: 10.1523/JNEUROSCI.1296-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 2009;24:26–35. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42:167–195. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- 20.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 21.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 22.Wang B, Rothberg BS, Brenner R. Mechanism of beta4 subunit modulation of BK channels. J Gen Physiol. 2006;127:449–465. doi: 10.1085/jgp.200509436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vassilev PM. A simple hanging-drop patch-clamp technique for studying single channel activities in excised membrane patches. Pflugers Arch. 1990;415:497–500. doi: 10.1007/BF00373631. [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Zakharov SI, Yang L, Wu RS, Deng SX, Landry DW, Karlin A, Marx SO. Locations of the beta1 transmembrane helices in the BK potassium channel. Proc Natl Acad Sci U S A. 2008;105:10727–10732. doi: 10.1073/pnas.0805212105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 26.Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang CP, Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci. 1996;16:4543–4550. doi: 10.1523/JNEUROSCI.16-15-04543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao L, Cox DH. Gating and ionic currents reveal how the BKCa channel's Ca2+ sensitivity is enhanced by its beta1 subunit. J Gen Physiol. 2005;126:393–412. doi: 10.1085/jgp.200509346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 29.Sweet TB, Cox DH. Measurements of the BKCa channel's high-affinity Ca2+ binding constants: effects of membrane voltage. J Gen Physiol. 2008;132:491–505. doi: 10.1085/jgp.200810094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan G, Yi H, Chen M, Sun L, Li W, Wu Y, Ding J. Structural basis for toxin resistance of beta4-associated calcium-activated potassium (BK) channels. J Biol Chem. 2008;283:24177–24184. doi: 10.1074/jbc.M800179200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valverde MA, et al. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 33.Dick GM, Rossow CF, Smirnov S, Horowitz B, Sanders KM. Tamoxifen activates smooth muscle BK channels through the regulatory beta 1 subunit. J Biol Chem. 2001;276:34594–34599. doi: 10.1074/jbc.M104689200. [DOI] [PubMed] [Google Scholar]
- 34.Dick GM, Sanders KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory beta1 subunit: a study of beta1 knockout mice. J Biol Chem. 2001;276:44835–44840. doi: 10.1074/jbc.M106851200. [DOI] [PubMed] [Google Scholar]
- 35.Kim EY, Zou S, Ridgway LD, Dryer SE. Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. J Neurophysiol. 2007;97:3508–3516. doi: 10.1152/jn.00009.2007. [DOI] [PubMed] [Google Scholar]
- 36.Toro B, Cox N, Wilson RJ, Garrido-Sanabria E, Stefani E, Toro L, Zarei MM. KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience. 2006;142:661–669. doi: 10.1016/j.neuroscience.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 37.Zarei MM, Song M, Wilson RJ, Cox N, Colom LV, Knaus HG, Stefani E, Toro L. Endocytic trafficking signals in KCNMB2 regulate surface expression of a large conductance voltage and Ca(2+)-activated K+ channel. Neuroscience. 2007;147:80–89. doi: 10.1016/j.neuroscience.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Wang YW, Ding JP, Xia XM, Lingle CJ. Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci. 2002;22:1550–1561. doi: 10.1523/JNEUROSCI.22-05-01550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brenner R, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 40.Pluger S, et al. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–E60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest. 2004;113:1032–1039. doi: 10.1172/JCI20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 43.Fodor AA, Aldrich RW. Convergent evolution of alternative splices at domain boundaries of the BK channel. Annu Rev Physiol. 2009;71:19–36. doi: 10.1146/annurev.physiol.010908.163124. [DOI] [PubMed] [Google Scholar]
- 44.Saleem F, Rowe IC, Shipston MJ. Characterization of BK channel splice variants using membrane potential dyes. Br J Pharmacol. 2009;156:143–152. doi: 10.1111/j.1476-5381.2008.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saito M, Nelson C, Salkoff L, Lingle CJ. A cysteine-rich domain defined by a novel exon in a slo variant in rat adrenal chromaffin cells and PC12 cells. J Biol Chem. 1997;272:11710–11717. doi: 10.1074/jbc.272.18.11710. [DOI] [PubMed] [Google Scholar]
- 46.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca(2+) signaling. Am J Physiol Heart Circ Physiol. 2005;289:H1326–H1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- 48.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 49.Uebele VN, et al. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 50.Cui J, Cox DH, Aldrich RW. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J Gen Physiol. 1997;109:647–673. doi: 10.1085/jgp.109.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefani E, Ottolia M, Noceti F, Olcese R, Wallner M, Latorre R, Toro L. Voltage-controlled gating in a large conductance Ca2+-sensitive K+channel (hslo) Proc Natl Acad Sci U S A. 1997;94:5427–5431. doi: 10.1073/pnas.94.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horrigan FT, Aldrich RW. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca(2+) J Gen Physiol. 1999;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horrigan FT, Cui J, Aldrich RW. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca(2+) J Gen Physiol. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui J, Yang H, Lee US. Molecular mechanisms of BK channel activation. Cell Mol Life Sci. 2009;66:852–875. doi: 10.1007/s00018-008-8609-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox DH, Aldrich RW. Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity. J Gen Physiol. 2000;116:411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nimigean CM, Magleby KL. The beta subunit increases the Ca2+ sensitivity of large conductance Ca2+-activated potassium channels by retaining the gating in the bursting states. J Gen Physiol. 1999;113:425–440. doi: 10.1085/jgp.113.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Somlyo AP, Somlyo AV, Shuman H, Sloane B, Scarpa A. Electron probe analysis of the sarcoplasmic reticulum and mitochondria in muscle. Microsc Acta. 1978 Suppl:79–91. [PubMed] [Google Scholar]
- 58.Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee HC. Caveolae targeting and regulation of large conductance Ca(2+)-activated K+ channels in vascular endothelial cells. J Biol Chem. 2005;280:11656–11664. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- 59.Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol. 2005;289:C49–C57. doi: 10.1152/ajpcell.00399.2004. [DOI] [PubMed] [Google Scholar]
- 60.Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drab M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 62.Lohn M, Furstenau M, Sagach V, Elger M, Schulze W, Luft FC, Haller H, Gollasch M. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res. 2000;87:1034–1039. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- 63.Brainard AM, Korovkina VP, England SK. Disruption of the maxi-K-caveolin-1 interaction alters current expression in human myometrial cells. Reprod Biol Endocrinol. 2009;7:131. doi: 10.1186/1477-7827-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol. 2006;570:65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 66.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 67.Yan J, et al. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics. 2008;7:2188–2198. doi: 10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perez G, Toro L. Differential modulation of large-conductance KCa channels by PKA in pregnant and nonpregnant myometrium. Am J Physiol. 1994;266:C1459–C1463. doi: 10.1152/ajpcell.1994.266.5.C1459. [DOI] [PubMed] [Google Scholar]
- 69.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- 70.Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci U S A. 2002;99:14560–14565. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toro L, Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E. MaxiK, c-Src and vasoconstriction. J Muscle Res Cell Motil. 2004;25:616–617. [PubMed] [Google Scholar]
- 72.Ling S, Woronuk G, Sy L, Lev S, Braun AP. Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J Biol Chem. 2000;275:30683–30689. doi: 10.1074/jbc.M004292200. [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, et al. {alpha}5{beta} 1 integrin engagement increases BK channel current and Ca2+ sensitivity through c-Src mediated channel phosphorylation. J Biol Chem. 2009 doi: 10.1074/jbc.M109.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1275–L1281. doi: 10.1152/ajplung.00259.2003. [DOI] [PubMed] [Google Scholar]
- 75.Barman SA, Zhu S, Han G, White RE. cAMP activates BKCa channels in pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol. 2003;284:L1004–L1011. doi: 10.1152/ajplung.00295.2002. [DOI] [PubMed] [Google Scholar]
- 76.Jackson WF, Blair KL. Characterization and function of Ca(2+)-activated K+ channels in arteriolar muscle cells. Am J Physiol. 1998;274:H27–H34. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- 77.Tian L, et al. Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc Natl Acad Sci U S A. 2008;105:21006–21011. doi: 10.1073/pnas.0806700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol. 2008;132:13–28. doi: 10.1085/jgp.200709913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett. 2005;579:4856–4860. doi: 10.1016/j.febslet.2005.07.069. [DOI] [PubMed] [Google Scholar]
- 80.White RE, Darkow DJ, Lang JL. Estrogen relaxes coronary arteries by opening BKCa channels through a cGMP-dependent mechanism. Circ Res. 1995;77:936–942. doi: 10.1161/01.res.77.5.936. [DOI] [PubMed] [Google Scholar]
- 81.Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am J Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- 82.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 83.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 84.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, Honore E. Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 85.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 86.Davis MJ, Hill MA, Kuo L. Local regulation of microvascular perfusion. In: Tuma RF, Ley WNDK, editors. Handbook of Physiology, Microcirculation. San Diego: Academic Press; 2008. pp. 161–184. [Google Scholar]
- 87.Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 2008;77:8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 88.Wu X, Davis MJ. Characterization of stretch-activated cation current in coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1751–H1761. doi: 10.1152/ajpheart.2001.280.4.H1751. [DOI] [PubMed] [Google Scholar]
- 89.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 90.Kirber MT, Ordway RW, Clapp LH, Walsh JV, Jr, Singer JJ. Both membrane stretch and fatty acids directly activate large conductance Ca(2+)-activated K+channels in vascular smooth muscle cells. FEBS Lett. 1992;297:24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- 91.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 92.Clarke AL, Petrou S, Walsh JV, Jr, Singer JJ. Modulation of BK(Ca) channel activity by fatty acids: structural requirements and mechanism of action. Am J Physiol Cell Physiol. 2002;283:C1441–C1453. doi: 10.1152/ajpcell.00035.2002. [DOI] [PubMed] [Google Scholar]
- 93.Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, Braun AP, Davis MJ. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by alpha5beta1 integrin activation in arteriolar smooth muscle. J Physiol. 2008;586:1699–1713. doi: 10.1113/jphysiol.2007.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. alphavbeta3-and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H399. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- 95.Amberg GC, Navedo MF, Nieves-Cintron M, Molkentin JD, Santana LF. Calcium sparklets regulate local and global calcium in murine arterial smooth muscle. J Physiol. 2007;579:187–201. doi: 10.1113/jphysiol.2006.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng X, Jaggar JH. Genetic ablation of caveolin-1 modifies Ca2+ spark coupling in murine arterial smooth muscle cells. Am J Physiol. 2006 doi: 10.1152/ajpheart.01226.2005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jaggar JH. Intravascular pressure regulates local and global Ca(2+) signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 99.Ella SR, Davis MJ, Meininger GA, Yang Y, Dora KA, Hill MA. Mechanisms underlying smooth muscle Ca2+ waves in cremaster muscle arterioles (Abstract) Faseb J. 2009 [Google Scholar]
- 100.Benham CD, Bolton TB, Lang RJ, Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 102.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 103.Gebremedhin D, et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 104.Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. 20-HETE modulates myogenic response of skeletal muscle resistance arteries from hypertensive Dahl-SS rats. Am J Physiol Heart Circ Physiol. 2001;280:H1066–H1074. doi: 10.1152/ajpheart.2001.280.3.H1066. [DOI] [PubMed] [Google Scholar]
- 105.Magnusson L, Sorensen CM, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Renovascular BK(Ca) channels are not activated in vivo under resting conditions and during agonist stimulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R345–R353. doi: 10.1152/ajpregu.00337.2006. [DOI] [PubMed] [Google Scholar]
- 106.Borbouse L, et al. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–H1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang Y, Ella SR, Braun AP, Korhuis RJ, Davis MJ, Hill MA. Manipulation of arteriolar BKCa using subunit directed siRNA (Abstract) FASEB J. 2010 [Google Scholar]
- 108.Zhuge R, Fogarty KE, Tuft RA, Walsh JV., Jr Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca(2+) concentration on the order of 10 microM during a Ca(2+) spark. J Gen Physiol. 2002;120:15–27. doi: 10.1085/jgp.20028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/s0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- 110.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–C1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 111.Yang Y, et al. Relative lack of ♌ 1-subunit-mediated regulation of BKCa in cremaster arteriolar smooth muscle. J Physiol. 2009;587:3025–3044. doi: 10.1113/jphysiol.2009.169920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Niggli E, Shirokova N. A guide to sparkology: the taxonomy of elementary cellular Ca2+signaling events. Cell Calcium. 2007;42:379–387. doi: 10.1016/j.ceca.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 113.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hercule HC, et al. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp Physiol. 2007;92:1067–1076. doi: 10.1113/expphysiol.2007.038166. [DOI] [PubMed] [Google Scholar]
- 115.Poulsen AN, Wulf H, Hay-Schmidt A, Jansen-Olesen I, Olesen J, Klaerke DA. Differential expression of BK channel isoforms and beta-subunits in rat neuro-vascular tissues. Biochim Biophys Acta. 2009;1788:380–389. doi: 10.1016/j.bbamem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Nixon GF, Mignery GA, Somlyo AV. Immunogold localization of inositol 1,4,5-trisphosphate receptors and characterization of ultrastructural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. J Muscle Res Cell Motil. 1994;15:682–700. doi: 10.1007/BF00121075. [DOI] [PubMed] [Google Scholar]
- 117.Lesh RE, Nixon GF, Fleischer S, Airey JA, Somlyo AP, Somlyo AV. Localization of ryanodine receptors in smooth muscle. Circ Res. 1998;82:175–185. doi: 10.1161/01.res.82.2.175. [DOI] [PubMed] [Google Scholar]
- 118.Sweeney M, Jones CJ, Greenwood SL, Baker PN, Taggart MJ. Ultrastructural features of smooth muscle and endothelial cells of isolated isobaric human placental and maternal arteries. Placenta. 2006;27:635–647. doi: 10.1016/j.placenta.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 119.Zhuge R, Fogarty KE, Baker SP, McCarron JG, Tuft RA, Lifshitz LM, Walsh JV., Jr Ca(2+) spark sites in smooth muscle cells are numerous and differ in number of ryanodine receptors, large-conductance K(+) channels, and coupling ratio between them. Am J Physiol Cell Physiol. 2004;287:C1577–C1588. doi: 10.1152/ajpcell.00153.2004. [DOI] [PubMed] [Google Scholar]
- 120.Stadnicka A, Contney SJ, Moreno C, Weihrauch D, Bosnjak ZJ, Roman RJ, Stekiel TA. Mechanism of differential cardiovascular response to propofol in Dahl salt-sensitive, Brown Norway, and chromosome 13-substituted consomic rat strains: role of large conductance Ca2+ and voltage-activated potassium channels. J Pharmacol Exp Ther. 2009;330:727–735. doi: 10.1124/jpet.109.154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res. 2001;88:210–216. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- 122.Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004;559:849–862. doi: 10.1113/jphysiol.2004.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin MT, Hessinger DA, Pearce WJ, Longo LD. Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes. Am J Physiol Heart Circ Physiol. 2006;291:H732–H740. doi: 10.1152/ajpheart.01357.2005. [DOI] [PubMed] [Google Scholar]
- 124.Hill MA, Meininger GA, Davis MJ, Laher I. Therapeutic potential of pharmacologically targeting arteriolar myogenic tone. Trends Pharmacol Sci. 2009;30:363–374. doi: 10.1016/j.tips.2009.04.008. [DOI] [PubMed] [Google Scholar]