Abstract
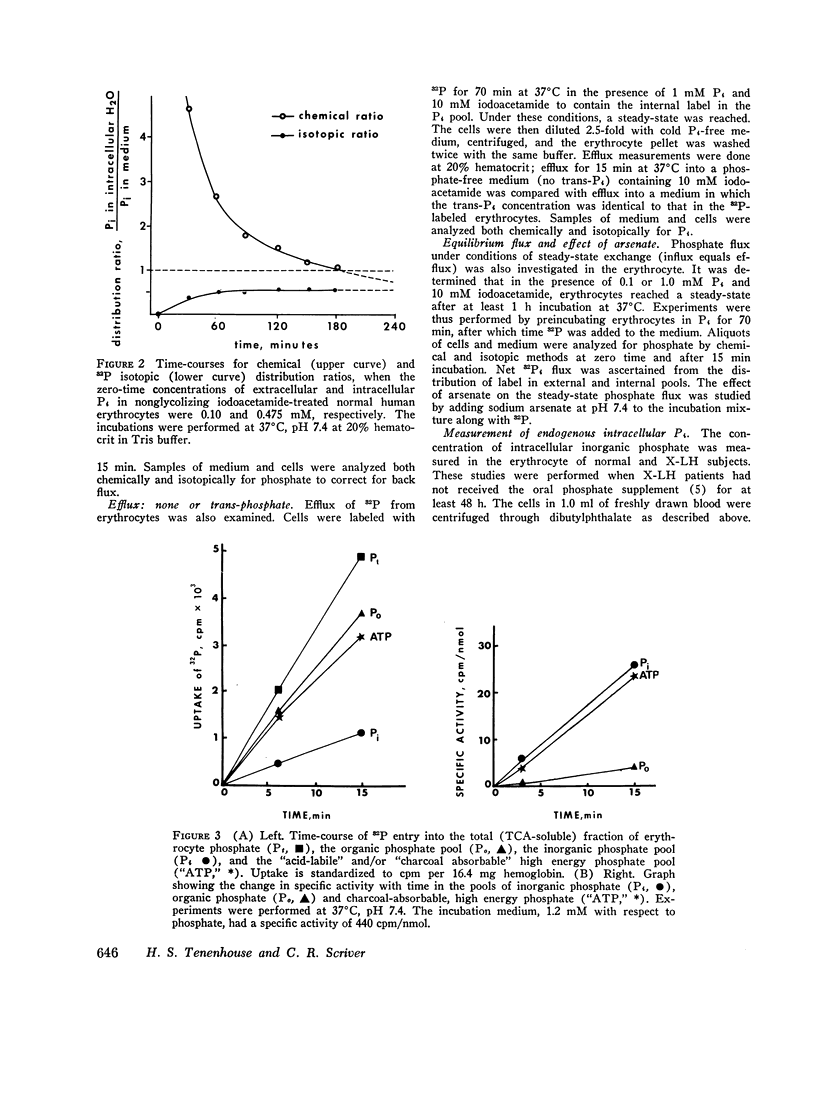
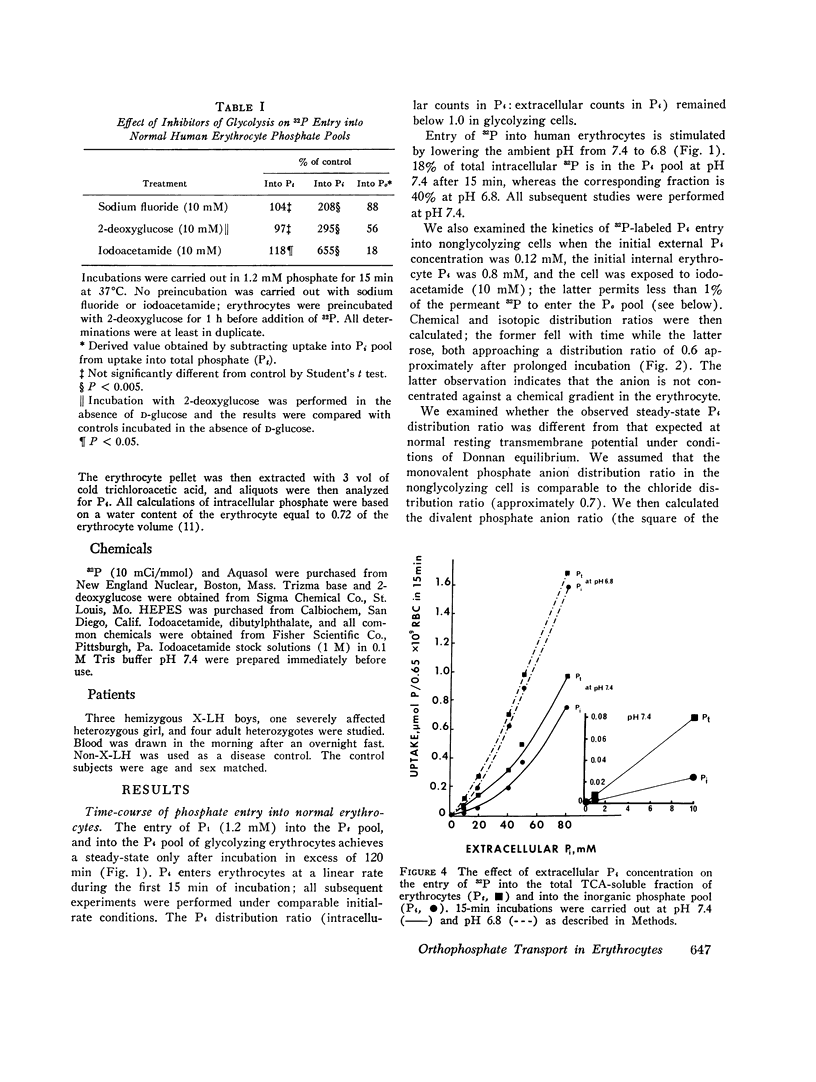
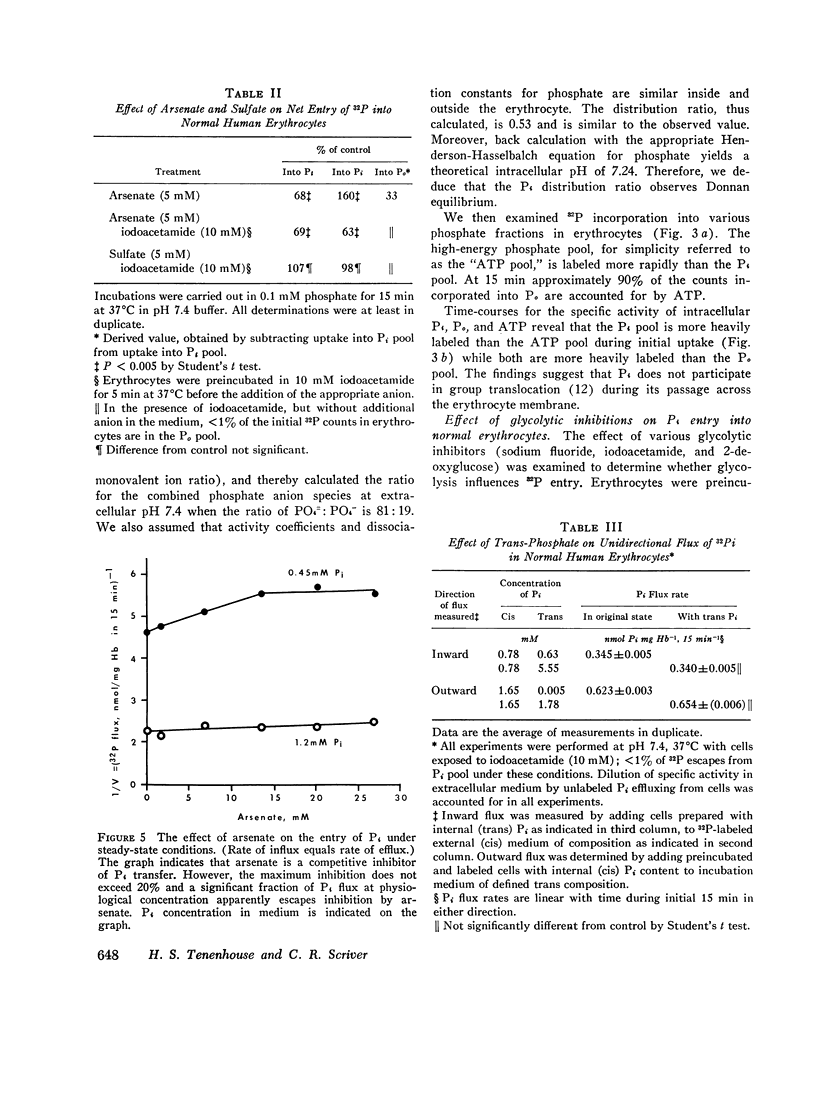
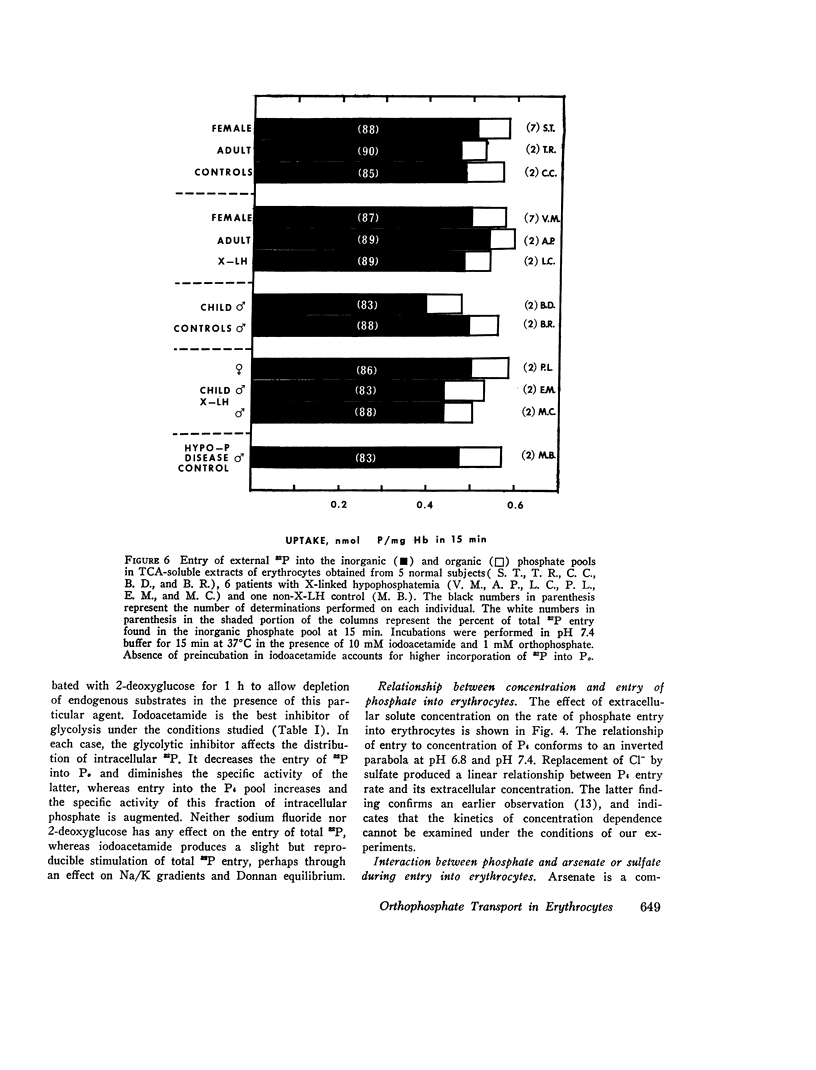
We have examined the mechanism of TCA-soluble orthophosphate (Pi) transfer across the membrane of mature human erythrocytes in normal subjects and in patients with X-linked hypophosphatemia (X-LH). The studies were carried out largely at pH 7.4 and 37 degrees C, in partial stimulation of conditions in vivo. (a) At physiological concentrations (1-2 mM) Pi enters the intact normal erythrocyte down its chemical gradient and under no conditions could we identify a steady-state trans-membrane gradient for Pi greater than 0.6. Calculations of the phosphate anion distribution ratio using the Nernst equation yield theoretical values that closely approximate observed values. (b) Glycolytic inhibitors have little effect on total entry of 32Pi inti erythrocytes but they do affect the intracellular distribution of Pi. In the presence of iodoacetamide, label accumulates almost exclusively in the orthophosphate pool and less than 1% enters the organic phosphate pool. (c) Specific activity measurements in unblocked cells indicate that Pi anion equilibrates first with its intracellular Pi pool. These initial findings imply that neither group translocation, nor energy coupling, influence Pi permeation into the human erythrocytes. (d) The relationship between 32P entry and extracellular Pi concentration is parabolic in the presence of chloride, and linear in the presence of sulfate. The kinetics of concentration dependent entrance cannot be examined and saturability of Pi entry cannot be identified under these conditions. (e) The competitive inhibitor arsenate partially inhibits the initial rate and steady-state flux of orthophosphate in erythrocytes treated with iodoacetamide to inhibit glycolysis. However, a significant portion of Pi transport escapes arsenate inhibition. (f) Activation energies for Pi entry, in nonglycolizing erythrocytes are much higher than those required by simple diffusion in an aqueous system. (g) Neither the inward or outward movement of Pi is modulated by trans-phosphate. These latter findings suggest that transport of phosphate across the human erythrocyte is compatible with slow facilitated diffusion with symmetry for influex and efflux. The transmembrane chemical distribution ratio, and the equilibrium flux of Pi were not different from normal in the X-LH erythrocyte. Nor did the extracellular Pi concentration, arsenate, or temperature affect Pi entry differently in the two types of cells. We dedjce that different gene products serve the diffusional type of Pi transport in the erythrocyte membrane and the saturable component of transepithelial absorption in the gut and kidney. Only the latter is affected by the X-LH mutation. The former is apparently present not only in erythrocytes but also in epithelial tissue, where it can serve the absorption of pharmacologic amounts of Pi in the therapeutic repair of the depleted phosphate pools in X-LH.
Full text
PDF

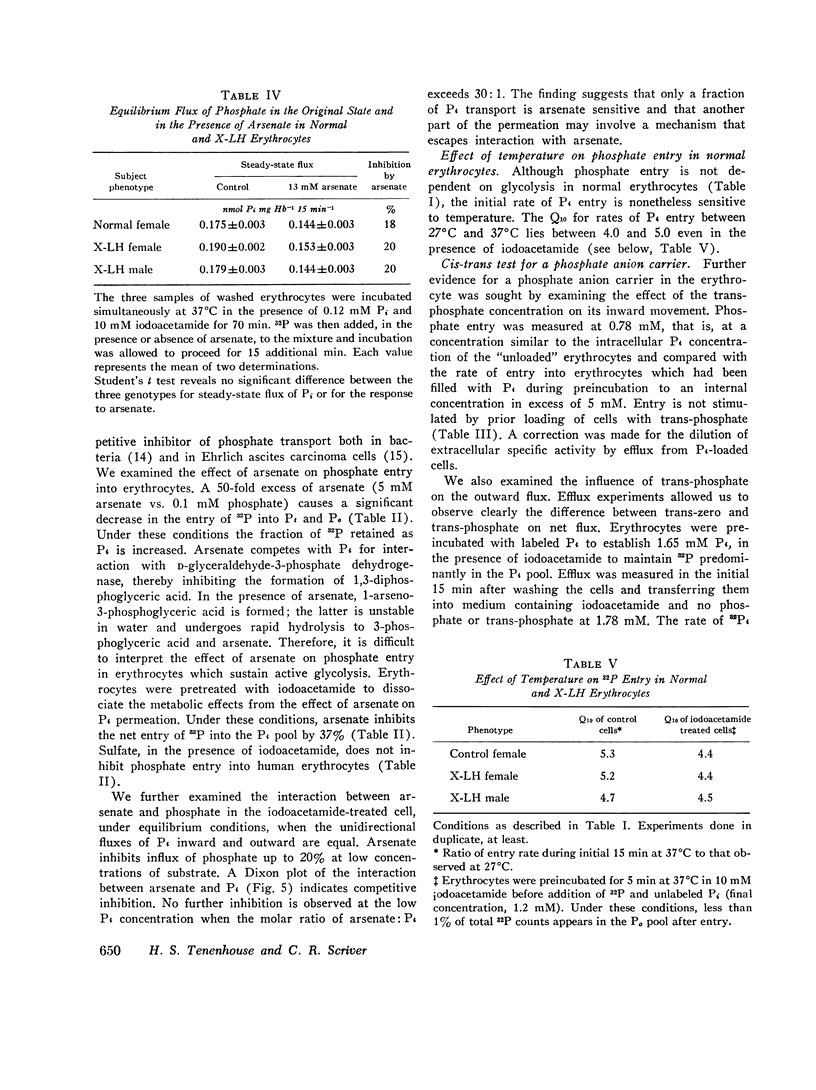



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
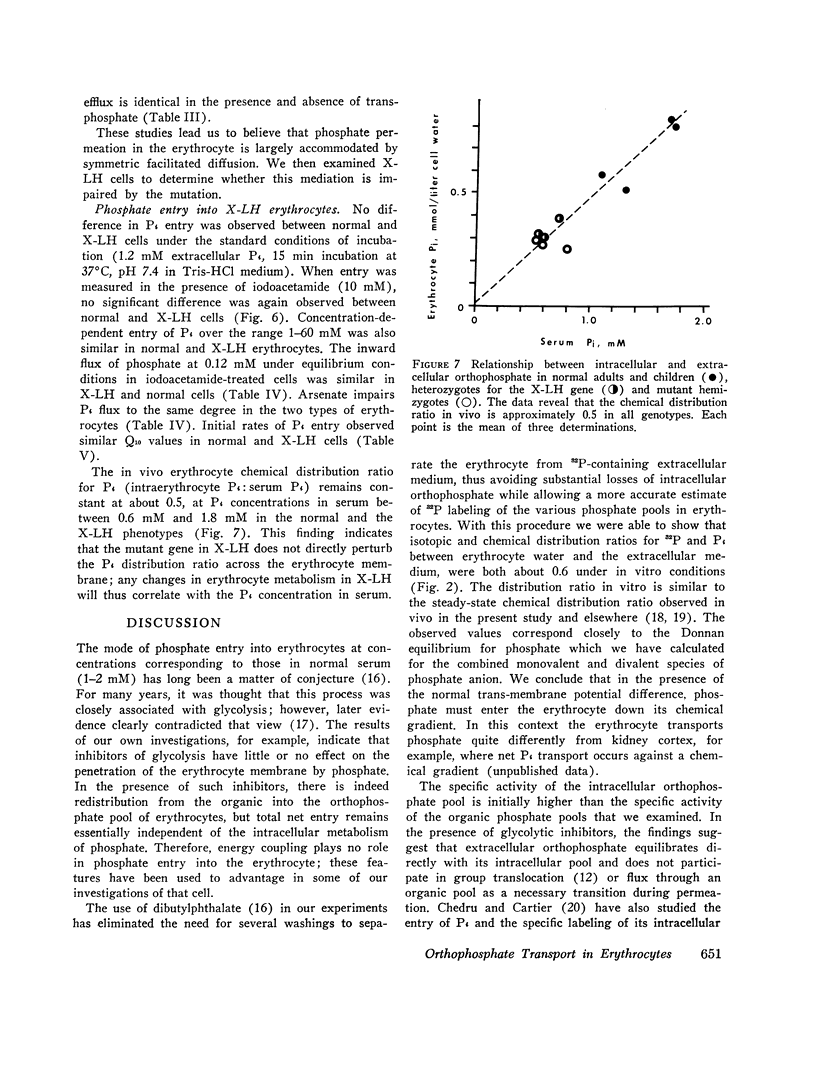
- BARTLETT G. R. Human red cell glycolytic intermediates. J Biol Chem. 1959 Mar;234(3):449–458. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- CHRISTENSEN H. N. Reactive sites and biological transport. Adv Protein Chem. 1960;15:239–314. doi: 10.1016/s0065-3233(08)60310-1. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- Chedru J., Cartier P. La perméabilité des globules rouges humains aux ions orthophosphates. Biochim Biophys Acta. 1966 Nov 8;126(3):500–512. [PubMed] [Google Scholar]
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuticke B. Anion permeability of the red blood cell. Naturwissenschaften. 1970 Apr;57(4):172–179. doi: 10.1007/BF00592968. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Uber die Kinetik der Phosphat-Permeation in den menschen-Erythrocyten bei Variation von extracellulärer Phosphat-Konzentration, anionen-Milieu and Zell-Volumen. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(1):21–38. [PubMed] [Google Scholar]
- Gardner J. D., Levy A. G. Transport of dibasic amino acids by human erythrocytes. Metabolism. 1972 May;21(5):413–431. doi: 10.1016/0026-0495(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Glorieux F. H., Scriver C. R., Reade T. M., Goldman H., Roseborough A. Use of phosphate and vitamin D to prevent dwarfism and rickets in X-linked hypophosphatemia. N Engl J Med. 1972 Sep 7;287(10):481–487. doi: 10.1056/NEJM197209072871003. [DOI] [PubMed] [Google Scholar]
- Glorieux F., Scriver C. R. Loss of a parathyroid hormone-sensitive component of phosphate transport in X-linked hypophosphatemia. Science. 1972 Mar 3;175(4025):997–1000. doi: 10.1126/science.175.4025.997. [DOI] [PubMed] [Google Scholar]
- Groth U., Rosenberg L. E. Transport of dibasic amino acids, cystine, and tryptophan by cultured human fibroblasts: absence of a defect in cystinuria and Hartnup disease. J Clin Invest. 1972 Aug;51(8):2130–2142. doi: 10.1172/JCI107020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber W., Deuticke B. Comparative aspects of phosphate transfer across mammalian erythrocyte membranes. J Membr Biol. 1973 Aug 30;13(1):19–36. doi: 10.1007/BF01868218. [DOI] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepner G. R., Tosteson D. C. Incubation of HK and LK sheep red cells in vitro for long periods. Biochim Biophys Acta. 1972 May 9;266(2):471–483. doi: 10.1016/0005-2736(72)90103-4. [DOI] [PubMed] [Google Scholar]
- Latzkovits L., Szentistványi I., Fajszi C. Tracer kinetic analysis of phosphate incorporation into erythrocytes in vitro. I. A simple model for simultaneous investigation of phosphate transport and exchange in erythrocytes. Acta Biochim Biophys Acad Sci Hung. 1972;7(1):55–66. [PubMed] [Google Scholar]
- Levinson C. Phosphate transport in Ehrlich ascites tumor cells and the effect of arsenate. J Cell Physiol. 1972 Feb;79(1):73–77. doi: 10.1002/jcp.1040790108. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Medveczky N., La Nauze J. M. Phosphate transport in Bacillus cereus. Biochim Biophys Acta. 1969 Oct 14;193(1):159–167. doi: 10.1016/0005-2736(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Schnell K. F. On the mechanism of inhibition of the sulfate transfer across the human erythrocyte membrane. Biochim Biophys Acta. 1972 Sep 1;282(1):265–276. doi: 10.1016/0005-2736(72)90333-1. [DOI] [PubMed] [Google Scholar]
- Short E. M., Binder H. J., Rosenberg L. E. Familial hypophosphatemic rickets: defective transport of inorganic phosphate by intestinal mucosa. Science. 1973 Feb 16;179(4074):700–702. doi: 10.1126/science.179.4074.700. [DOI] [PubMed] [Google Scholar]
- VESTERGAARD-BOGIND B. DETERMINATION ON A MICRO SCALE OF CONCENTRATION AND SPECIFIC RADIOACTIVITY OF INORGANIC PHOSPHATE IONS IN WHOLE BLOOD AND PACKED RED CELLS. Scand J Clin Lab Invest. 1964;16:457–464. doi: 10.3109/00365516409060539. [DOI] [PubMed] [Google Scholar]
- ZIPURSKY A., ISRAELS L. G. Transport of phosphate into the erythrocyte. Nature. 1961 Mar 25;189:1013–1015. doi: 10.1038/1891013a0. [DOI] [PubMed] [Google Scholar]


