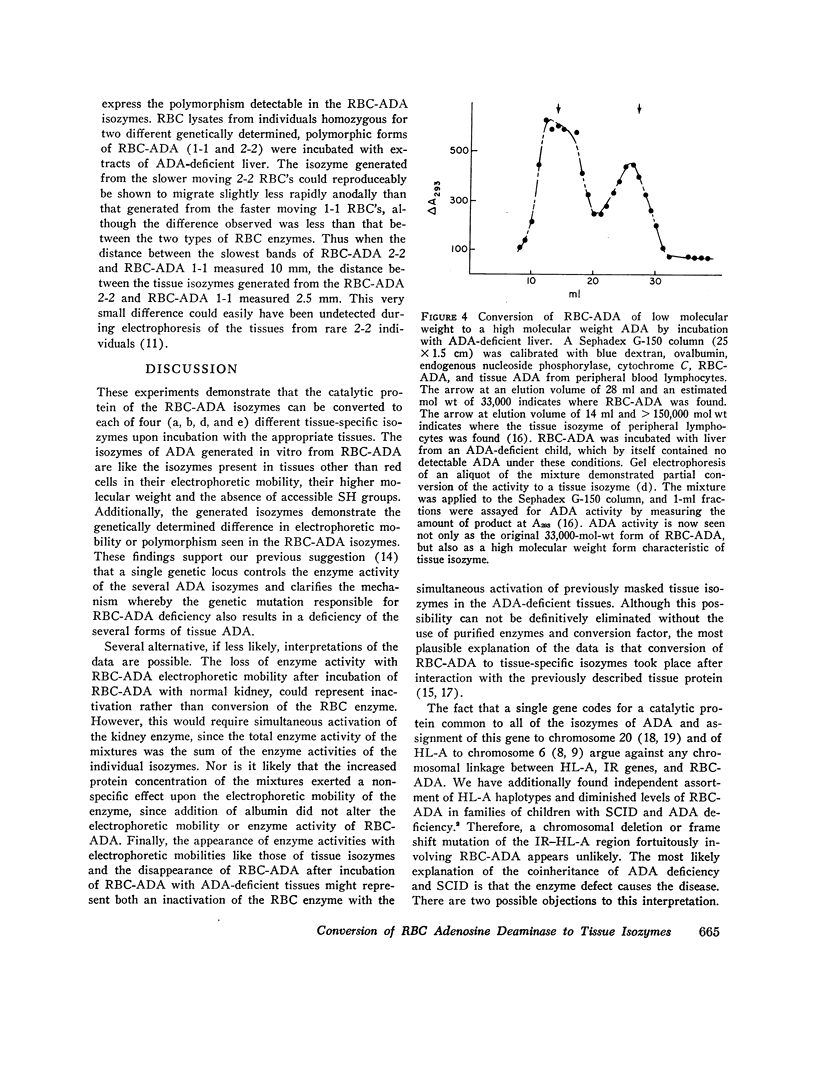
Abstract
Adenosine deaminase activity resides in various characteristic isozymes in red blood cells (RBC-ADA) and other tissues. Absence of RBC-ADA has been reported in a proportion of patients with autosomally inherited severe combined immunodeficiency (SCID). We have previously reported that the tissue isozymes of ADA are also deficient in children with SCID and RBC-ADA deficiency, although these isozymes differ from RBC-ADA in molecular weight, accessible SH groups, and electrophoretic mobility. The deficiency of all types of ADA in SCID implies that a catalytic unit of ADA in each isozyme is coded by the same structural gene. The relationship of RBC-ADA and the different tissue ADA isozymes is the subject of this paper. Incubation of RBC-ADA with ADA-deficient liver, kidney, and fibroblast extracts resulted in the appearance of new isozymes of ADA. These newly generated isozymes had the physicochemical and electrophoretic characteristics of the tissue-specific isozymes obtained from normal tissues. The electrophoretic mobility of the isozyme generated appeared to depend upon the tissue utilized and corresponded to the electrophoretic mobilities of the ADA isozymes found naturally in each of the different tissues. Additionally, the genetically determined polymorphism exhibited by RBC-ADA could be detected in the isozyme generated. Incubation with normal kidney also caused conversion of the RBC isozyme to the kidney form. These findings further support the concept that the catalytic activity of each of the several forms of the ADA enzyme resides in a single molecule coded at the same genetic locus as is defective in one form of SCID. The tissue-specific isozymes, which differ in electrophoretic mobility and molecular weight, are generated by interaction of the RBC catalytic unit with tissue-specific factors present in the different tissues of normal humans and patients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akedo H., Nishihara H., Shinkai K., Komatsu K., Ishikawa S. Multiple forms of human adenosine deaminase. I. Purification and characterization of two molecular species. Biochim Biophys Acta. 1972 Jul 13;276(1):257–271. doi: 10.1016/0005-2744(72)90028-9. [DOI] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Adenosine deaminase isozymes in human tissues. Ann Hum Genet. 1971 Oct;35(2):207–219. doi: 10.1111/j.1469-1809.1956.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Green H., Chan T. Pyrimidine starvation induced by adenosine in fibroblasts and lymphoid cells: role of adenosine deaminase. Science. 1973 Nov 23;182(4114):836–837. doi: 10.1126/science.182.4114.836. [DOI] [PubMed] [Google Scholar]
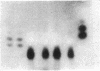
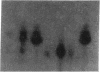
- Hirschhorn R., Beratis N., Rosen F. S., Parkman R., Stern R., Polmar S. Adenosine-deaminase deficiency in a child diagnosed prenatally. Lancet. 1975 Jan 11;1(7898):73–75. doi: 10.1016/s0140-6736(75)91075-2. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Grossman J., Weissmann G. Effect of cyclic 3',5'-adenosine monophosphate and theophylline on lymphocyte transformation. Proc Soc Exp Biol Med. 1970 Apr;133(4):1361–1365. doi: 10.3181/00379727-133-34690. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Levytaka V., Pollara B., Meuwissen H. J. Evidence for control of several different tissue-specific isozymes of adenosine deaminase by a single genetic locus. Nat New Biol. 1973 Dec 19;246(155):200–202. doi: 10.1038/newbio246200a0. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Levytska V. Alterations in isozymes of adenosine deaminase during stimulation of human peripheral blood lymphocytes. Cell Immunol. 1974 Jun;12(3):387–395. doi: 10.1016/0008-8749(74)90095-1. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Harris H. The investigation of reactive sulphydryls in enzymes and their variants by starch gel electrophoresis. Studies on red cell adenosine deaminase. Ann Hum Genet. 1969 Jul;33(1):81–87. doi: 10.1111/j.1469-1809.1969.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Jenkins T. Red-blood-cell adenosine deaminase deficiency in a "healthy" Kung individual. Lancet. 1973 Sep 29;2(7831):736–736. doi: 10.1016/s0140-6736(73)92568-3. [DOI] [PubMed] [Google Scholar]
- Jongsma A., van Someren H., Westerveld A., Hagemeijer A., Pearson P. Localization of genes on human chromosomes by studies of human-Chinese hamster somatic cell hybrids. Assignment of PGM3 to chromosome C6 and regional mapping of the PGD, PGM1 and pep-C genes on chromosome A1. Humangenetik. 1973 Dec 10;20(3):195–202. doi: 10.1007/BF00385730. [DOI] [PubMed] [Google Scholar]
- Knudsen B. B., Dissing J. Adenosine deaminase deficiency in a child with severe combined immunodeficiency. Clin Genet. 1973;4(4):344–347. doi: 10.1111/j.1399-0004.1973.tb01929.x. [DOI] [PubMed] [Google Scholar]
- Lamm L. U., Svejgaard A., Kissmeyer-Nielsen F. PGM3: HL-A is another linkage in man. Nat New Biol. 1971 May 26;231(21):109–110. doi: 10.1038/newbio231109a0. [DOI] [PubMed] [Google Scholar]
- Levine B. B., Stember R. H., Fotino M. Ragweed hay fever: genetic control and linkage to HL-A haplotypes. Science. 1972 Dec 15;178(4066):1201–1203. doi: 10.1126/science.178.4066.1201. [DOI] [PubMed] [Google Scholar]
- Nishihara H., Ishikawa S., Shinkai K., Akedo H. Multiple forms of human adenosine deaminase. II. Isolation and properties of a conversion factor from human lung. Biochim Biophys Acta. 1973 Apr 12;302(2):429–442. doi: 10.1016/0005-2744(73)90172-1. [DOI] [PubMed] [Google Scholar]
- Ochs H. D., Yount J. E., Giblett E. R., Chen S. H., Scott C. R., Wedgwood R. J. Adenosine-deaminase deficiency and severe combined immunodeficiency syndrome. Lancet. 1973 Jun 16;1(7816):1393–1394. doi: 10.1016/s0140-6736(73)91725-x. [DOI] [PubMed] [Google Scholar]
- Ruddle F. H. Linkage analysis in man by somatic cell genetics. Nature. 1973 Mar 16;242(5394):165–169. doi: 10.1038/242165a0. [DOI] [PubMed] [Google Scholar]
- Scott C. R., Chen S. H., Giblett E. R. Detection of the carrier state in combined immunodeficiency disease associated with adenosine deaminase deficiency. J Clin Invest. 1974 Apr;53(4):1194–1196. doi: 10.1172/JCI107658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield J. A., Creagan R. P., Nichols E. A., Ruddle F. H. Assignment of a gene for adenosine deaminase to human chromosome 20. Hum Hered. 1974;24(1):1–11. doi: 10.1159/000152631. [DOI] [PubMed] [Google Scholar]