Abstract
The distal-less homeobox gene (dlx) 5 encodes a transcription factor that controls jaw formation and appendage differentiation during embryonic development. We previously found that Dlx5 is overexpressed in an Akt2 transgenic model of T-cell lymphoma. To investigate if DLX5 is involved in human cancer, we screened its expression in the NCI 60 cancer cell line panel. DLX5 was frequently up regulated in cell lines derived from several tumor types, including ovarian cancer. We next validated its up regulation in primary ovarian cancer specimens. Stable knockdown of DLX5 by lentivirus-mediated transduction of short hairpin RNAi (shRNA) resulted in reduced proliferation of ovarian cancer cells due to inhibition of cell cycle progression in connection with down regulation of cyclins A, B1, D1, D2 and E and decreased phosphorylation of AKT. Cell proliferation resumed following introduction of a DLX5 cDNA harboring wobbled mutations at the shRNA-targeting sites. Cell proliferation was also rescued by transduction of a constitutively active form of AKT. Intriguingly, down regulation of IRS-2 and MET contributed to the suppression of AKT signaling. Moreover, DLX5 was found to directly bind to the IRS-2 promoter and augmented its transcription. Knockdown of DLX5 in xenografts of human ovarian cancer cells resulted in markedly diminished tumor size. In addition, DLX5 was found to cooperate with HRAS in the transformation of human ovarian surface epithelial cells. Together, these data suggest that DLX5 plays a significant role in the pathogenesis of some ovarian cancers.
Keywords: Ovarian Cancer, DLX5, AKT, IRS-2, MET
Introduction
Cancer is often regarded as the consequence of developmental dysregulation underlaid by aberrant expression of transcription factors that are normally involved in embryonic development (1). The homeobox gene superfamily encodes transcription factors that act as master controllers of development by regulating a diverse range of target genes. For example, TAL1 and HOX11 are essential transcription factors involved in early hematopoiesis, but their misexpression in thymocytes causes T cell acute lymphoblastic leukemia (T-ALL) by blocking thymocyte differentiation (2). In addition to HOX11, homeobox genes are widely implicated in various human cancers, by acting as either an oncogene or as a tumor suppressor. For example, in rhabdomyosarcoma, an oncogenic chromosomal translocation results in the fusion of the DNA binding domain of the PAX3 homeobox gene with the FKHR transcription factor gene (3). The homeobox gene NKX3-1 plays an important role in normal differentiation of the prostatic epithelium, while its loss of function initiates prostate carcinogenesis (4). In breast cancer, HOXA5 expression is frequently lost due to gene deletion or promoter methylation (5).
We previously identified a chromosomal abnormality in thymic tumor cells from a transgenic mouse model driven by a myristoylated (myr), constitutively active form of Akt2. Tumor cells from these mice often harbor an inversion of chromosome 6 that juxtaposes an evolutionally conserved homeobox bi-gene, Dlx5/6, to the enhancer of the Tcrb gene (6). Moreover, clonogenic assays revealed oncogenic cooperativity when both Dlx5 and activated Akt2 were co-expressed in mammalian cells. The Dlx gene family is related to the Drosophila distal-less (dll) gene and is mainly expressed in the developing forebrain and craniofacial structures. More recently, Dlx genes have also been found to play broader roles ranging from neurogenesis to hematopoiesis (7). Sonic hedgehog, BMP4, FGF and Wnt, among others, can induce Dlx expression in a tissue-specific manner (8).
Dlx5 is expressed in adult bone marrow cells and fetal liver cells, but its expression is suppressed in Thy1-positive T cells (9). In thymic lymphoma cells observed in myr-Akt2 transgenic mice, Dlx5 protein levels are highly elevated, which may facilitate tumor development by interfering with T-cell differentiation (6). Another DLX family member, DLX7 (now known as DLX4), has also been implicated in human hematopoietic neoplasms. DLX4 is expressed in normal hematopoietic cells and leukemia cell lines with erythroid characteristics, and its knockdown induced apoptosis via downregulation of MYC and GATA1 (10). DLX4 was also found to be frequently overexpressed in acute myeloid leukemia and T-ALL (11), and expression of DLX4 has been implicated in breast cancer progression and choriocarcinoma cell survival (12). The role of DLX5 in tumor development, however, is only beginning to emerge. Kato et al. reported that overexpression of DLX5 in non-small cell lung cancer is linked to tumor size and is predictive of a poor prognosis (13). They also showed that knockdown of DLX5 suppresses lung cancer cell viability and colony formation. Here, we report that DLX5 is frequently overexpressed in human ovarian cancer cells, and that depletion of DLX5 by RNA interference (RNAi) inhibits cell proliferation, in part by attenuating AKT signaling.
Materials and Methods
Cell lines, tumor specimens and reagents
Ovarian cancer cell lines IGROV1, OVCAR3, OVCAR4, OVCAR5, OVCAR8, OVCAR10, SKOV3 and A2780 were from Fox Chase Cancer Center and maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. Primary and SV40-immortalized non-tumorigenic human ovarian surface epithelial (HOSE) cells were maintained in 1:1 M199 and MCBD-105 media, respectively, supplemented with 5% FBS and 0.2 IU/mL insulin, 100 μg/mL penicillin and streptomycin, and 2 mM L-glutamine. Primary ovarian tumor specimens were obtained from patients who underwent surgery at Fox Chase Cancer Center. Antibodies against DLX5, DLX6, cyclin A, cyclin B1/2, cyclin D1/2/3, cyclin E1/2, IGF1Rβ, MET, IRS-1/2, PI3K p110β, β-actin and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated (p)-PDK1, total PDK1, p-AKT/AKT, p-GSK3β/GSK3β, p-p70S6K/p70S6K, p-ERK/ERK, HER2, EGF and p-MET were from Cell Signaling Technology (Danvers, MA).
Cell proliferation assay
Cell proliferation was measured by WST-1 assay (Clontech, Mountain View, CA). In brief, cells were seeded at 3×103 cells/well in a 96-well plate 5 days after initiating knockdown of DLX5. OD values at 450 nm were measured 2 hr after incubation with WST-1, using a 96-well micro-plate reader (BioRad, Hercules, CA). Also, cell growth curves were measured over a 5-day period. Cells were seeded in 24-well plates at 3×104 cells/well and were collected at days 0, 3 and 5. Viable cell numbers were estimated based on measurements of total cell protein at OD 595, using Bradford reagent (BioRad, Hercules, CA).
Cell cycle analysis
Cells were fixed and permeabilized with cold ethanol for 30 min on ice. After washing twice with cold PBS, cells were stained with staining solution (40 μg/mL Propidium Iodide and 50 μg/mL RNase A) at 37°C for 15 min. Samples were processed by using FACScan (BD, San Jose, CA), and data were analyzed by using FlowJo software (Tree Star, Ashland, OR).
Gene knockdown by lentivirus-mediated short-hairpin RNAi
Sequences used for DLX5 knockdown were selected by using siRNA Design Tools (Ambion, Austin, TX). The short-hairpin oligos were synthesized, annealed and inserted into pLVTHM (a gift of D. Trono, University of Geneva, Geneva, Switzerland). Among 11 lentivial constructs tested, two with the best knockdown efficiency were used for the experiments presented here. The human DLX5 sequence used for construct sh2 was TGG TGA ATG GCA AAC CAA A, and the sequence for construct sh3 was AGC TTA TGC CGA CTA TAG C. Control sequence against LacZ gene was GGA TCA GTC GCT GAT TAA A. Short hairpin sequences against human DLX6 were AAA GGG AAT GCT GCA TGT TTT (sh4) and AAG AAT CTG CAC AAA CTT GGC (sh10). Viruses were produced as previously reported (14). In brief, 293T cells were co-transfected with the lentiviral vector, packaging plasmid, and envelope plasmid. Virus supernatant was collected 24 h after transfection. Ovarian cancer cells were then infected with virus at an MOI of 1.5 for 6 h. Cell proliferation rates and relevant signaling pathways were measured 3-5 days after transduction of the shRNA.
Retroviral transduction of a DLX5 cDNA with wobble mutations
A Flag-tagged full length human DLX5 cDNA was amplified from human reference cDNA (Clontech) by using Pfx polymerase (Invitrogen, Carlsbad, CA) and then cloned into pMSCV vector (Clontech). The sequence targeted by lentiviral DLX5-sh2 was altered from TGG TGA ATG GCA AAC CAA A to TGG TCA ACG GGA AAC CAA A by using a site-directed mutagenesis kit (Stratagene, Cedar Creek, TX). Retrovirus containing the wobbled DLX5 was pseudotyped with pVSV-G by co-transfecting packaging cells. Supernatant was collected after 24 h, and cells were infected for 5 h at a MOI of 2. Puromycin at 2 μg/ml was used 48 h post-transduction to select cells.
Results
DLX5 is frequently up regulated in cell lines derived from human cancers of various origins, including ovarian cancer
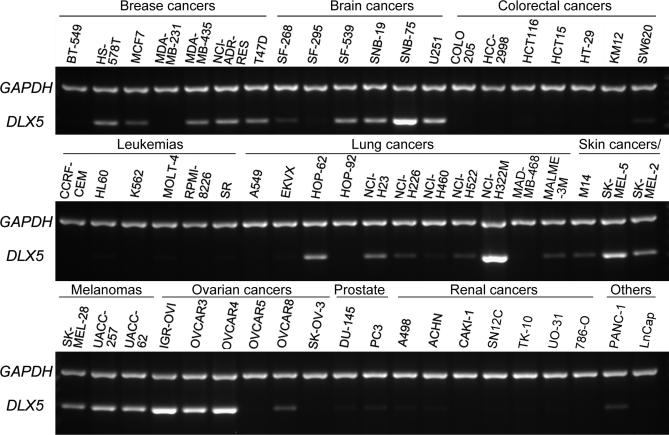
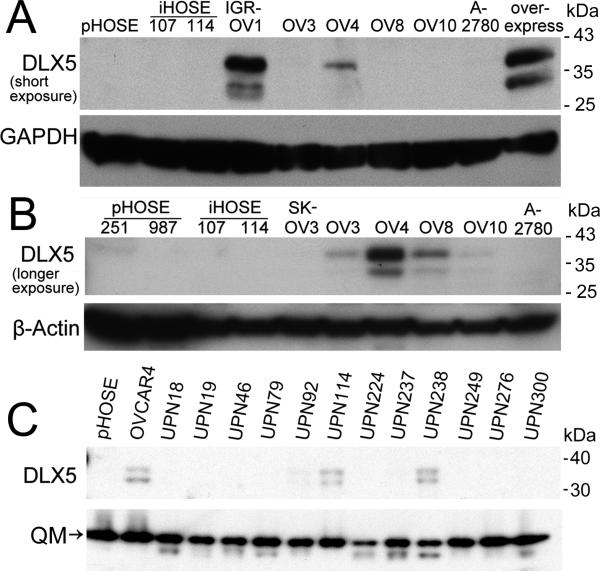
To test whether DLX5 expression is deregulated in human cancers, we initially screened the NCI 60 cancer cell line panel for DLX5 transcript levels. Semi-quantitative RT-PCR revealed that DLX5 mRNA is abundantly expressed in many cancer cell lines derived from malignant tissues of breast, brain, lung, skin and ovary, but expression of DLX5 was low or undetectable in tumor cells from patients with leukemia or colorectal, prostate and kidney cancers (Fig. 1). To delineate the involvement of various DLX family genes in ovarian oncogenesis, we next compared the mRNA expression of all six DLX members in ovarian cancer cells versus that observed in primary (pHOSE) and SV40-immortalized (iHOSE) ovarian epithelial cells. DLX1 and DLX2 were found to be equally expressed in primary and immortalized HOSE cells and malignant ovarian cells. DLX3 and DLX4 were up regulated in immortalized non-tumorigenic and malignant cells. Interestingly, DLX5 and DLX6 were detected only in ovarian cancer cells, not in pHOSE or iHOSE cells (Fig. S1A). These observations suggest that expression of DLX5/6 occurs only in fully transformed cells, which prompted us to determine if DLX5 and DLX6 have a role in ovarian tumor maintenance. The up regulation of DLX5/6 appears to be due to an epigenetic alteration, because high level amplification was not observed in tumor cells, as shown by real-time PCR analysis (Fig. S1B). We next performed immunoblot analysis and found that DLX5 is expressed in IGR-OV1, OVCAR3, OVCAR4, OVCAR8 and OVCAR10 cells at variable levels, but it was not detected in pHOSE and iHOSE cells (Fig. 2A). The predicted molecular weight of DLX5 protein is 33 kDa, which corresponds to the lower of two bands observed in the immunoblot analysis. The upper band (~38 kDa) may be the result of a post-translational modification, not an isoform resulted from alternatively spliced mRNA, because overexpression of DLX5 by transfecting 293T with a full-length human DLX5 cDNA showed the same pattern (Fig. 2A, B). Alternatively, it is possible that the faster migrating band could represent a partially cleaved form of the 38-kDa DLX5 protein. Fractionation experiments revealed that both forms of DLX5 are located in the nucleus (supplemental Fig. S2A). Moreover, incubation of cells with the cell-permeable inhibitors of two major proteases, i.e., Caspase or Calpain, did not result in any obvious changes in the DLX5 immunoblot pattern, suggesting that the 33-kDa band is not a cleavage product of these proteases. Furthermore, global phosphatase inhibition by CalyculinA resulted in increased phosphorylation of both forms (Fig. S2B). Endogenous DLX6 protein could only be detected by IP/western blot analysis using total cell lysates at multi-milligram protein levels, suggesting a low expression level compared with that of DLX5, and thus may downplay its significance (Fig. S3). Because of the abundance of DLX5 seen in malignant cells, we focused our subsequent studies on this protein. To rule out the possibility that DLX5 expression only occurs in cancer cell lines during in vitro culture, we next examined the expression of DLX5 in tumor samples. We found that DLX5 is not expressed in non-malignant ovarian samples (Fig. S4A), but is expressed in a subset of late stage tumors (Fig. 2C, Fig. S4B).
Figure 1.
DLX5 is frequently expressed in human cancer cells. DLX5 transcript levels were assessed by using semi-quantitative RT-PCR in the NCI 60 cancer cell line panel. Expression of GAPDH was included as an internal control.
Figure 2.
Up regulation of DLX5 in ovarian cancer. (A, B) Expression of DLX5 protein in ovarian cancer cell lines. DLX5 protein was analyzed by immunoblotting at short (A) and longer (B) exposure. Lysate from 293T cell cells transfected with a pcDNA3.1-human DLX5 plasmid (lane designated as Overexpress) was loaded as a positive control (A). (C) Expression of DLX5 in human malignant ovarian cancer specimens. DLX5 protein levels in ovarian carcinoma samples were determined by immunoblot analysis.
DLX5 knockdown diminishes the proliferation of ovarian cancer cells in vitro and inhibits tumor growth in vivo
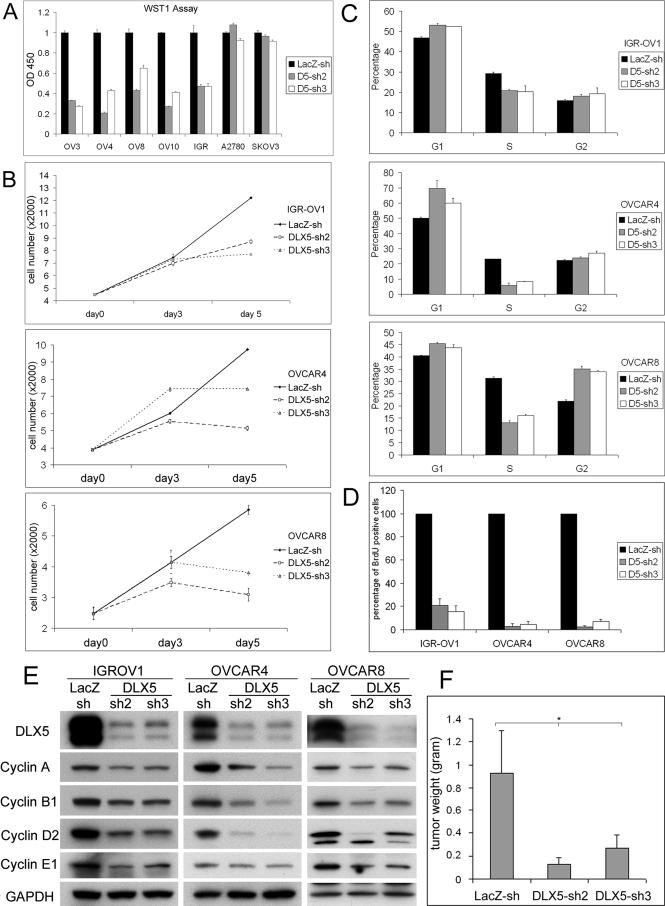
To assess the role of DLX5 in ovarian cancer, we used lentivirus-mediated RNAi knockdown to inhibit DLX5 expression in ovarian carcinoma cells. Two efficient knockdown constructs were selected for generating lentiviruses. WST-1 assays were performed to measure cell proliferation and viability 5 days after viral infection. We found that DLX5 shRNAs impaired the viability/proliferation of cell lines (OVCAR3, 4, 8, 10 and IGR-OV1) overexpressing DLX5 but had little or no effect on DLX5-negative cell lines (A2780 and SKOV3; Fig. 3A). Cell proliferation curves for IGR-OV1, OVCAR4 and OVCAR8 cells documented that proliferation was inhibited, suggesting that cell cycle arrest, rather than apoptosis, results from down regulation of DLX5 (Fig. 3B). This notion was validated by results of a FACS assay, which revealed that more cells are arrested in G1 and G2/M phases, with fewer cells in S phase (Fig. 3C). Similarly, DLX5 knockdown inhibited DNA replication as shown by BrdU incorporation (Fig. 3D). Consistent with the reduced cell division rate, knockdown of DLX5 resulted in decreased expression of multiple cyclin family members, including cyclins A, B1, D2 and E1 in IGR-OV1, OVCAR4 and OVCAR8 cells (Fig. 3E). Knockdown of DLX6 did not consistently affect cell proliferation, which may be due to its low expression level (Fig. S5A, B).
Figure 3.
DLX5 knockdown suppresses cell proliferation by inhibiting cell cycle progression. (A) lentiviruses harboring shRNA against LacZ or DLX5 were generated and used to infect DLX5-positive (IGR-OV1, OVCAR4, OVCAR8 and OVCAR10) or DLX5-negative (SKOV3 and A2780) ovarian cancer cell lines. Cell viability/proliferation was determined by WST-1 assay 5 days after viral infection. (B) Cell proliferation curves of IGR-OV1, OVCAR4 and OVCAR8 cell lines after virus infection at the indicated times. (C) Cell cycle analysis was performed on IGR-OV1, OVCAR4, and OVCAR8 cells with control or DLX5 knockdown. (D) Rates of DNA synthesis were determined by counting cells staining with BrdU. (E) DLX5 knockdown results in decreased expression of various cell cycle regulators, including cyclins A, B1, D2 and E1. (F) DLX5 knockdown reduces growth of xenografted OVCAR8 cells in SCID mice. Tumors were recovered 1 month after subcutaneous injection and weighted (* p<0.05).
To evaluate the in vivo effect of DLX5 knockdown on tumor progression, OVCAR8 cells infected with control lentivirus or lentivirus expressing shRNA against DLX5 were injected subcutaneously into SCID mice. Mice were sacrificed 1 month after injection, and the xenograft tumors were weighted. These studies revealed that tumors expressing DLX5 RNAi were significantly smaller (Fig. 3F).
DLX5 knockdown attenuates AKT signaling by down regulating upstream signaling
To investigate the mechanism involved in cell cycle arrest following knockdown of DLX5, we next examined whether a specific signaling pathway is compromised upon DLX5 knockdown. Interestingly, AKT activity was found to be suppressed in IGR-OV1 and OVCAR4 cells after expression of DLX5 shRNA (Fig. 4A). The decreased p-AKT levels appeared to result from insufficient upstream signaling, since total AKT protein levels were not altered, whereas p-PDK1 levels were suppressed. The total protein levels of PDK1 and PI3K subunits were unaffected, suggesting that decreased AKT pathway activation arises from the upstream signal-initiating complex, rather than the protein levels of components of the PI3K-AKT pathway itself (Fig. 4A). In fact, AKT pathway inhibition by the inhibitors LY294002, GSK690693 and RAD001 appeared to mimic DLX5 knockdown in these cells, as shown by decreased cell proliferation and downregulation of cyclin levels (Fig. S6A, B).
Figure 4.
DLX5 knockdown attenuates AKT signaling pathway by down regulating upstream effectors. (A) AKT signaling is compromised by DLX5 knockdown in IGR-OV1 and OVCAR4 cells. Expression of p-PDK1/PDK1, p-AKT/AKT, p-GSK3, p-p70S6K and p-S6 were analyzed by Western blotting. (B) DLX5 knockdown reduces MET and IRS-2 expression. Levels of membrane receptor kinases MET, EGFR, HER2 and IGFR1, and adaptor proteins IRS-1 and IRS-2 were determined by immunoblotting. (C) DLX5 knockdown reduces MET/HGFR and IGFR signaling to AKT. IGR-OV1 and OVCAR4 cells with DLX5 knockdown were starved and treated with 10 ng/ml HGF or 50 ng/ml IGFI for 10 min. p-AKT/AKT levels were analyzed by immunoblotting. (D) myr-AKT protects cells from DLX5 knockdown-induced inhibition of cell proliferation. Ovarian cancer cells were co-transduced by viruses containing DLX5 shRNA and myr-AKT, and cell viability/proliferation was analyzed by WST-1 assay 5 days after infection. (E) Cyclin levels and Akt signaling diminished by DLX5 knockdown are restored by expression of myr-AKT.
We thus decided to investigate the effects of DLX5 knockdown on major upstream tyrosine kinase receptors and their adaptor proteins. We found that knockdown of DLX5 in IGR-OV1 and OVCAR4 cells consistently resulted in decreased total protein levels of MET and IRS-2 but not EGFR, HER2 or IGFR (Fig. 4B). We also found that expression of MET, IRS-1/2, EGFR, and HER2 are up regulated in many ovarian cancer cell lines and that the cell lines that overexpress DLX5 consistently had increased IRS-2 protein levels (Fig. S7). Moreover, DLX5 knockdown impaired IGF- or HGF-induced AKT activation in IGR-OV1 and OVCAR4 cells (Fig. 4C). On the other hand, re-introduction of a myr-AKT2 cDNA antagonized the growth-inhibiting effect of DLX5 knockdown as shown by WST-1 assay (Fig. 4D). Phosphorylation of GSK3β and expression of various cyclins were also restored by expression of constitutively active AKT2 (Fig. 4E).
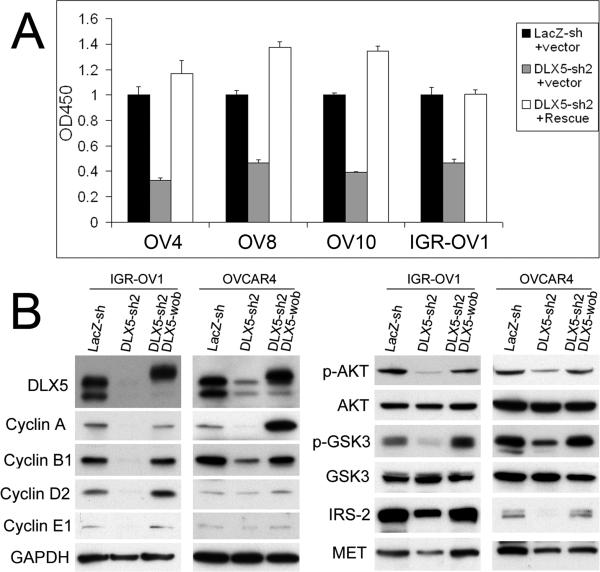
Re-introduction of a wobbled DLX5 cDNA restores cell proliferation and cell signaling in DLX5 knockdown cells
To rule out a possible off-target effect of the DLX5 shRNA, we introduced three different wobble mutations into the shRNA2-targeted sequence in a Flag-DLX5 cDNA. The wobbled DLX5 restored cell proliferation in cells expressing shRNA2 (Fig. 5A). The wobbled DLX5 was resistant to DLX5 shRNA2 in IGR-OV1 and OVCAR4 cells co-transduced with shRNA2, as shown by the expression of Flag-DLX5 protein (Fig. 5B). Thus, shRNA2 failed to diminish cyclin levels or to weaken AKT signaling in the wobbled DLX5-rescued cells likely due, at least in part, to unaffected IRS-2 and MET levels (Fig. 5B).
Figure 5.
A wobbled DLX5 cDNA prevents DLX5 knockdown-induced cell cycle arrest. (A) WST-1 assays were performed to determine cell viability/proliferation after transduction of DLX5 shRNA2 and a Flag-DLX5 cDNA containing three wobbled codons at the shRNA2 targeted sites. (B) The wobbled DLX5 cDNA restores cyclin levels as well as AKT signaling. Immunoblotting was performed to analyze expression levels of DLX5, cyclins A, B1 and D2, various AKT pathway members, as well as IRS-2 and MET.
DLX5 binds to the IRS-2 promoter and augments its transcription
To determine if DLX5 is directly responsible for up regulation of IRS-2 in DLX5-positive cancer cells, the IRS-2 promoter was cloned into pGL3 vector, and luciferase assays were performed. DLX5 was found to augment IRS-2 promoter activity (Fig. 6A-B). The DLX5 binding consensus sequence of the IRS-2 promoter was synthesized, biotin labeled, and then used for gel shift assays. DLX5 protein caused a band shift in the DNA retardation gel, and addition of DLX5 antibody imposed a super-shift (Fig. 6C). We next immunoprecipitated DLX5 protein from IGR-OV1 cells, and it produced the same band shift (Fig. 6D). Mutation in the DLX5 binding consensus of the IRS-2 promoter resulted in markedly diminished luciferase activity, as well as a gel shift (Fig. 6E, F). Moreover, ChIP assay showed that DLX5 binds to the IRS-2 promoter at the endogenous level in IGR-OV1 and OVCAR4 cells, but not in SKOV3 cells (Fig. 6G).
Figure 6.
DLX5 binds to and transactivates the IRS-2 promoter. (A) Luciferase assay was performed by co-transfecting pGL3-IRS-2 promoter with GFP or with pMSCV expressing FLAG tagged full-length (FL) DLX5 or DLX5 with truncated C terminus (ΔC’), N terminus (ΔN’) or terminal Homeobox domain-C (ΔH-C). (B) Western blot analysis demonstrates the expression of FLAG tagged full-length and truncated DLX5 by using a anti-FLAG antibody. (C) Gel shift assay was carried out to analyze the interaction between recombinant DLX5 protein and its binding consensus in the IRS-2 promoter. (D) DLX5 immunoprecipitated from nuclei of IGR-OV1 cells (IGR) also binds to IRS-2 promoter. IP from SKOV3 cells (SK) was used as the negative control. (E) Luciferase assay was performed by co-transfecting the mutant pGL3-IRS-2 promoter with DLX5. (F) Oligos with mutant DLX5 binding sequence was used for the gel shift assay. (G) Chromatin immunoprecipitation assay performed to determine the interaction of DLX5 and IRS-2 promoter at the endogenous level.
DLX5 promotes HRAS-induced HOSE transformation in vitro
To investigate if DLX5 can facilitate HRAS-initiated ovarian epithelial cell transformation in vitro, SV40-immortalized HOSE cells were transduced with retroviral HRAS(G12V) /GFP, retroviral HRAS(G12V) /DLX5 or retroviral Vector/DLX5. After double selection with hygromycin and puromycin, HOSE cells were seeded in soft agar plates, and colonies were counted four weeks later. DLX5 was found to enhance the transformation of HRAS-induced HOSE cells, when compared to oncogenic HRAS alone, suggesting oncogenic cooperativity between DLX5 and HRAS (Fig. S8A-C).
Discussion
Homeodomain transcription factors play important roles in directing cellular proliferation and differentiation, and their dysregulation has been implicated in various human cancers. In some ovarian cancers, HOXB7 is expressed at high levels, and overexpression of HOXB7 in normal HOSE cells enhances cell proliferation (15). Other homeobox proteins such as MEIS, PBX, and PAX8 are also frequently up regulated in ovarian carcinomas (16, 17). Moreover, up regulation of DLX4 has been observed in high grade ovarian cancers, and overexpression of DLX4 promotes ovarian cancer cell proliferation and increases clonogenicity in vitro, and it induces vascular endothelial growth factor transcription and enhances tumor vascularization in vivo (18).
Our findings suggest that overexpression of DLX5 promotes the proliferation of ovarian cancer cells and that knockdown of DLX5 causes cell cycle arrest in vitro and diminished tumor size in xenografts of ovarian cancer cells in SCID mice. The arrest appears to occur in both G1 and G2/M. Moreover, down regulation of cyclin proteins was shown to underlie the reduced cell proliferation rate seen in tumor cells following knockdown of DLX5. Thus. the primary consequence of DLX5 knockdown may be the inhibition of DNA synthesis and S phase entry, which may be related to the down regulation of Cyclin A and Cyclin E that we observed. At the same time, down regulation of Cyclin D2 as well as Cyclin B may cause the arrest of cells at G1 phase and G2/M phase, respectively.
We also found that overexpression of DLX5 increases HRAS-induced colony formation in HOSE cells. It has been reported that activated HRAS can transform SV40-imortalized HOSE in vitro (19). Our study implies that DLX5 is not only important in maintaining ovarian cancer cell proliferation but also participates in the transformation of SV40-imortalized HOSE cells by cooperating with activated HRAS, similar to the oncogenic cooperativity we observed between Dlx5 and activated Akt2 in Rat-1 fibroblasts (20).
The receptor kinases EGFR, HER2, MET and IGF1R are often overexpressed in ovarian cancers and may contribute to tumor progression (21, 22). Inhibition of IGF1R function induces apoptosis in ovarian cancer cells (23-25). Its adaptor proteins IRS-1 and IRS-2 have also been shown to be oncogenic. IRS proteins have been shown to be overexpressed in hepatic and pancreatic cancers and to possess constitutive activity in breast cancer, myosarcoma, and multiple endocrine neoplasia, among others (26). Furthermore, transgenic mice overexpressing IRS-1 or IRS-2 develop breast cancer (27). Interestingly, IRS-2 has been implicated in positive feedback regulation of IGFR specifically through the mTOR pathway (28). The elevated IRS levels observed in certain cancers are related to activation of the AKT pathway, and dephosphorylation of IRS protein has been shown to inhibit AKT signaling (29-31). Similarly, inhibition of MET represses AKT signaling and abolishes tumor cell invasion (22). Importantly, our findings indicate that knockdown of DLX5 reduces the expression of IRS-2 and MET in ovarian cancer cells overexpressing DLX5, which in turn resulted in decreased AKT activity.
The basal activity of IRS-2 promoter is maintained by the transcription factor AP1 but is susceptible to certain oncogenic stimuli. In breast cancer cells, IRS-2 transcription can be augmented by EGFR signaling through the JNK-AP1 pathway (32). Moreover, in some prostate cancer cells, the steroid receptor coactivator-3 binds to the IRS-2 promoter and enhances IRS-2 transcription (33). Our studies have revealed that IRS-2 protein levels are up regulated in ovarian cancer cells overexpressing DLX5 and that DLX5 can bind to the IRS-2 promoter and augment its activity and downstream AKT signaling. Interestingly, the truncated DLX5 forms also retained partial activity on IRS-2 promoter, possibly because shorter mRNAs having a higher translation rate than the full-length mRNA and/or that truncated DLX5 proteins are more stable. Identification of other potential targets of DLX5 participating in oncogenic signaling is worthy of investigation.
Supplementary Material
Acknowledgments
The following Fox Chase Cancer Center shared facilities were used in the course of this work: Cell Culture, Biosample Repository, Flow Cytometry, and Laboratory Animal Facilities. Y.T. was supported in part by a William J. Avery Postdoctoral Fellowship.
Grant support: NCI CA77429, P50 CA083638 and CA06927 and an appropriation from the Commonwealth of Pennsylvania.
References
- 1.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–85. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 2.O'Neil J, Look AT. Mechanisms of transcription factor deregulation in lymphoid cell transformation. Oncogene. 2007;26:6838–49. doi: 10.1038/sj.onc.1210766. [DOI] [PubMed] [Google Scholar]
- 3.Galili N, Davis RJ, Fredericks WJ, et al. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5:230–5. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- 4.Abate-Shen C, Shen MM, Gelmann E. Integrating differentiation and cancer: the Nkx3.1 homeobox gene in prostate organogenesis and carcinogenesis. Differentiation. 2008;76:717–27. doi: 10.1111/j.1432-0436.2008.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raman V, Martensen SA, Reisman D, et al. Compromised HOXA5 function can limit p53 expression in human breast tumours. Nature. 2000;405:974–8. doi: 10.1038/35016125. [DOI] [PubMed] [Google Scholar]
- 6.Tan Y, Timakhov RA, Rao M, et al. A novel recurrent chromosomal inversion implicates the homeobox gene Dlx5 in T-cell lymphomas from Lck-Akt2 transgenic mice. Cancer Res. 2008;68:1296–302. doi: 10.1158/0008-5472.CAN-07-3218. [DOI] [PubMed] [Google Scholar]
- 7.Merlo GR, Zerega B, Paleari L, Trombino S, Mantero S, Levi G. Multiple functions of Dlx genes. Int J Dev Biol. 2000;44:619–26. [PubMed] [Google Scholar]
- 8.Panganiban G, Rubenstein JL. Developmental functions of the Distal-less/Dlx homeobox genes. Development. 2002;129:4371–86. doi: 10.1242/dev.129.19.4371. [DOI] [PubMed] [Google Scholar]
- 9.Woodside KJ, Shen H, Muntzel C, Daller JA, Sommers CL, Love PE. Expression of Dlx and Lhx family homeobox genes in fetal thymus and thymocytes. Gene Expr Patterns. 2004;4:315–20. doi: 10.1016/j.modgep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Shimamoto T, Nakamura S, Bollekens J, Ruddle FH, Takeshita K. Inhibition of DLX-7 homeobox gene causes decreased expression of GATA-1 and c-myc genes and apoptosis. Proc Natl Acad Sci U S A. 1997;94:3245–9. doi: 10.1073/pnas.94.7.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haga SB, Fu S, Karp JE, et al. BP1, a new homeobox gene, is frequently expressed in acute leukemias. Leukemia. 2000;14:1867–75. doi: 10.1038/sj.leu.2401912. [DOI] [PubMed] [Google Scholar]
- 12.Samuel S, Naora H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005;41:2428–37. doi: 10.1016/j.ejca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Kato T, Sato N, Takano A, et al. Activation of placenta-specific transcription factor distal-less homeobox 5 predicts clinical outcome in primary lung cancer patients. Clin Cancer Res. 2008;14:2363–70. doi: 10.1158/1078-0432.CCR-07-1523. [DOI] [PubMed] [Google Scholar]
- 14.Wiznerowicz M, Trono D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J Virol. 2003;77:8957–61. doi: 10.1128/JVI.77.16.8957-8961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naora H, Yang YQ, Montz FJ, Seidman JD, Kurman RJ, Roden RB. A serologically identified tumor antigen encoded by a homeobox gene promotes growth of ovarian epithelial cells. Proc Natl Acad Sci U S A. 2001;98:4060–5. doi: 10.1073/pnas.071594398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crijns AP, de Graeff P, Geerts D, et al. MEIS and PBX homeobox proteins in ovarian cancer. Eur J Cancer. 2007;43:2495–505. doi: 10.1016/j.ejca.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Bowen NJ, Logani S, Dickerson EB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–7. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 18.Hara F, Samuel S, Liu J, Rosen D, Langley RR, Naora H. A homeobox gene related to Drosophila distal-less promotes ovarian tumorigenicity by inducing expression of vascular endothelial growth factor and fibroblast growth factor-2. Am J Pathol. 2007;170:1594–606. doi: 10.2353/ajpath.2007.061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Yang G, Thompson-Lanza JA, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64:1655–63. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 20.Mabuchi S, Altomare DA, Connolly DC, et al. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res. 2007;67:2408–13. doi: 10.1158/0008-5472.CAN-06-4490. [DOI] [PubMed] [Google Scholar]
- 21.van Dam PA, Vergote IB, Lowe DG, et al. Expression of c-erbB-2, c-myc, and c-ras oncoproteins, insulin-like growth factor receptor I, and epidermal growth factor receptor in ovarian carcinoma. J Clin Pathol. 1994;47:914–9. doi: 10.1136/jcp.47.10.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou HY, Wong AS. Activation of p70S6K induces expression of matrix metalloproteinase 9 associated with hepatocyte growth factor-mediated invasion in human ovarian cancer cells. Endocrinology. 2006;147:2557–66. doi: 10.1210/en.2005-1404. [DOI] [PubMed] [Google Scholar]
- 23.Hongo A, Yumet G, Resnicoff M, Romano G, O'Connor R, Baserga R. Inhibition of tumorigenesis and induction of apoptosis in human tumor cells by the stable expression of a myristylated COOH terminus of the insulin-like growth factor I receptor. Cancer Res. 1998;58:2477–84. [PubMed] [Google Scholar]
- 24.Maloney EK, McLaughlin JL, Dagdigian NE, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63:5073–83. [PubMed] [Google Scholar]
- 25.Hongo A, Kuramoto H, Nakamura Y, et al. Antitumor effects of a soluble insulin-like growth factor I receptor in human ovarian cancer cells: advantage of recombinant protein administration in vivo. Cancer Res. 2003;63:7834–9. [PubMed] [Google Scholar]
- 26.Dearth RK, Cui X, Kim HJ, Hadsell DL, Lee AV. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle. 2007;6:705–13. doi: 10.4161/cc.6.6.4035. [DOI] [PubMed] [Google Scholar]
- 27.Dearth RK, Cui X, Kim HJ, et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 2006;26:9302–14. doi: 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon J, Stephan S, Mukhopadhyay A, et al. Insulin receptor substrate-2 mediated insulin-like growth factor-I receptor overexpression in pancreatic adenocarcinoma through protein kinase Cdelta. Cancer Res. 2009;69:1350–7. doi: 10.1158/0008-5472.CAN-08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCampbell AS, Walker CL, Broaddus RR, Cook JD, Davies PJ. Developmental reprogramming of IGF signaling and susceptibility to endometrial hyperplasia in the rat. Lab Invest. 2008;88:615–26. doi: 10.1038/labinvest.2008.29. [DOI] [PubMed] [Google Scholar]
- 30.Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–53. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 31.Dromard M, Bompard G, Glondu-Lassis M, Puech C, Chalbos D, Freiss G. The putative tumor suppressor gene PTPN13/PTPL1 induces apoptosis through insulin receptor substrate-1 dephosphorylation. Cancer Res. 2007;67:6806–13. doi: 10.1158/0008-5472.CAN-07-0513. [DOI] [PubMed] [Google Scholar]
- 32.Cui X, Kim HJ, Kuiatse I, Kim H, Brown PH, Lee AV. Epidermal growth factor induces insulin receptor substrate-2 in breast cancer cells via c-Jun NH(2)-terminal kinase/activator protein-1 signaling to regulate cell migration. Cancer Res. 2006;66:5304–13. doi: 10.1158/0008-5472.CAN-05-2858. [DOI] [PubMed] [Google Scholar]
- 33.Yan J, Yu CT, Ozen M, Ittmann M, Tsai SY, Tsai MJ. Steroid receptor coactivator-3 and activator protein-1 coordinately regulate the transcription of components of the insulin-like growth factor/AKT signaling pathway. Cancer Res. 2006;66:11039–46. doi: 10.1158/0008-5472.CAN-06-2442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.