Abstract
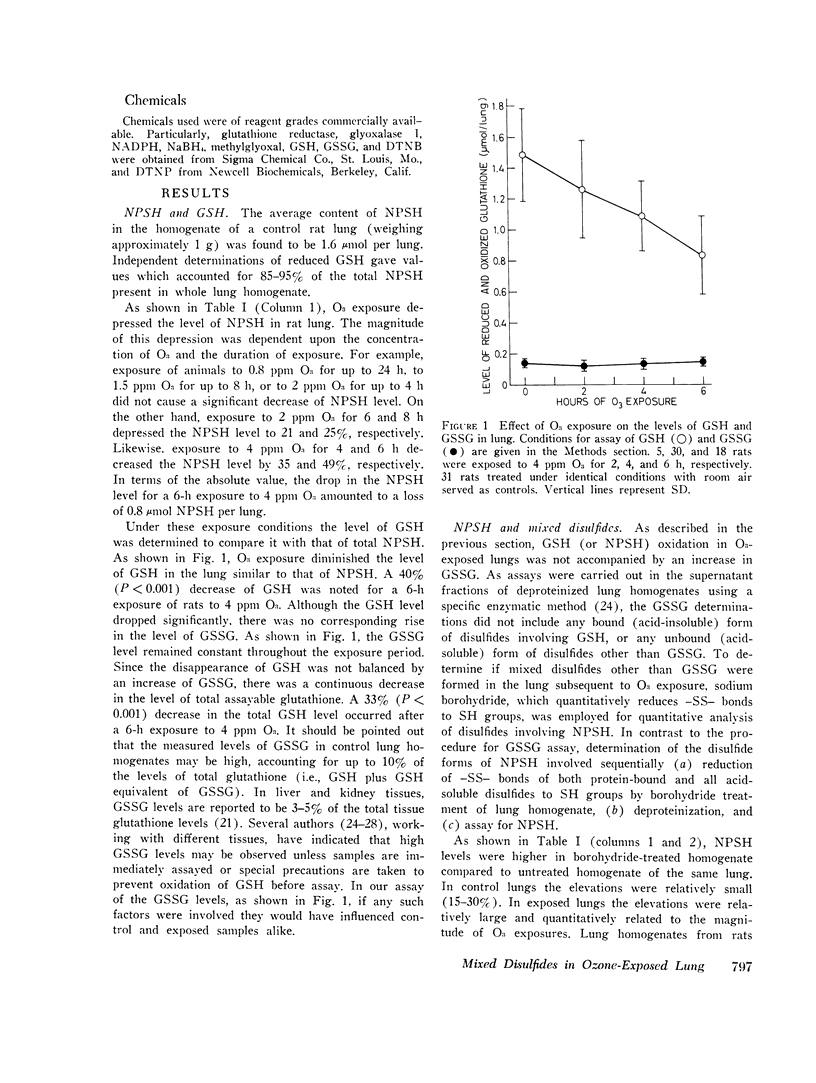
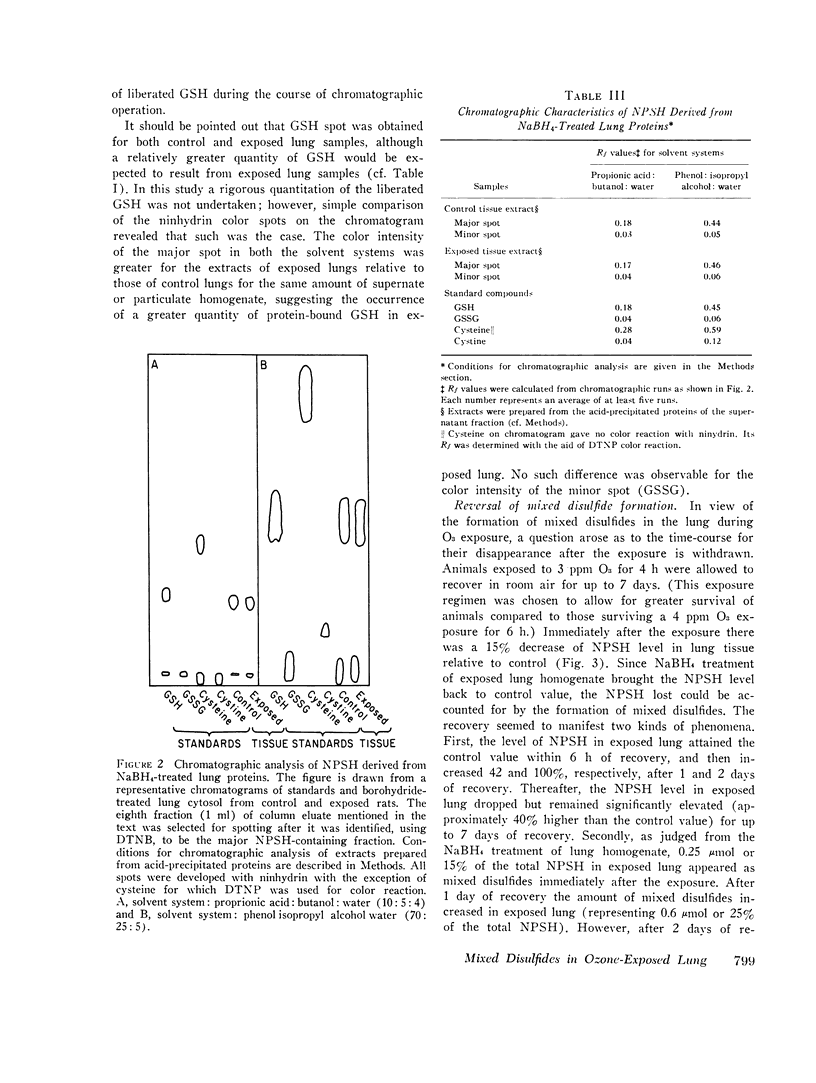
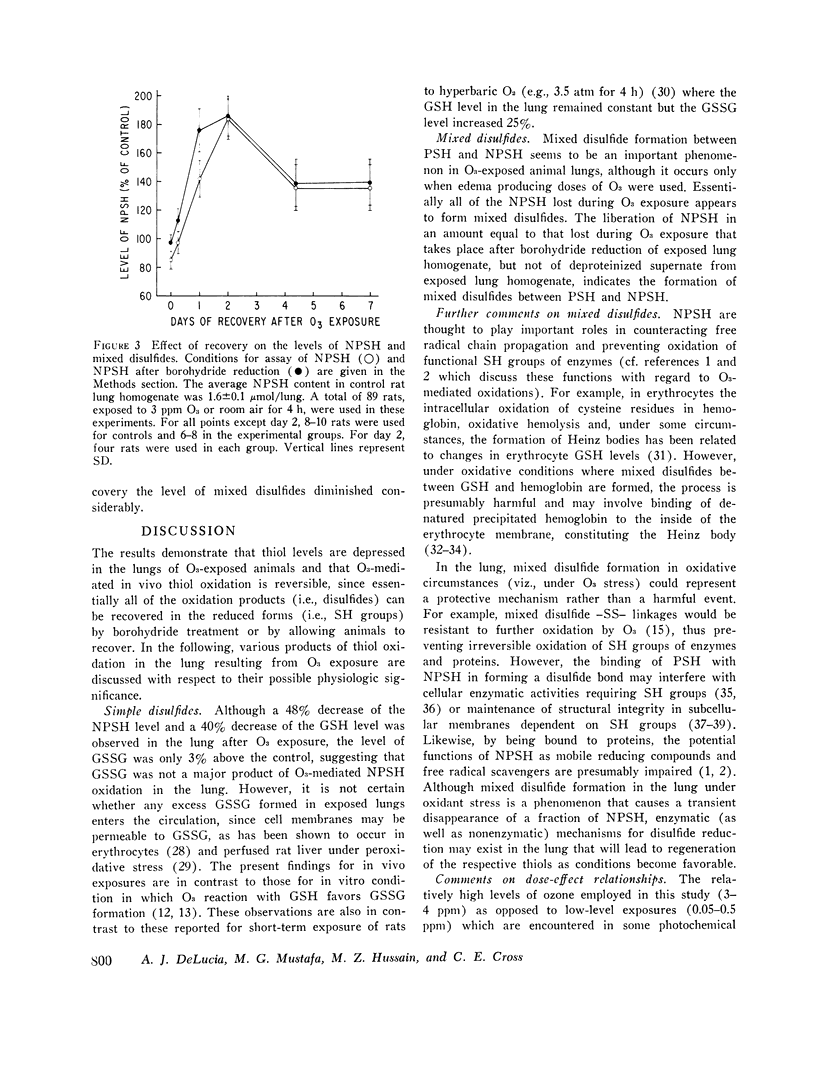
Nonprotein sulfhydryls (NPSH), a major source of cellular reducing substances, were examined in lung tissue after short-term exposure of rats to O3. While the NPSH level was unaffected by low-level exposures (e.g., 0.8 ppm for up to 24 h or 1.5 ppm for up to 8 h), it was significantly lowered by higher exposure regimens (e.g., 25 per cent after 2 ppm for 8 h and 49 per cent after 4 ppm for 6 h). After exposure to 4 ppm O3 for 6 h the level of reduced glutathione (GSH), which accounted for approximately 90 per cent of NPSH in the lung, decreased 40 per cent but without a rise in the level of oxidized gluathione (GSSG). Treatment of lung homogenate with borohydride led to recovery of NPSH in exposed lungs to control values, suggesting that NPSH or GSH oxidation during in vivo O3 exposure resulted in formation of mixed disulfides with other sulfhydryl (SH) groups of lung tissue. Extracts of borohydride-treated particulate and supernatant fractions of lung homogenate were analyzed for NPSH by paper chromatography. From this analysis GSH appeared to be the only NPSH bound to lung tissue proteins via mixed disulfide linkage. The formation of mixed disulfides appeared to be a transient phenomenon. Immediately after a 4-h exposure to 3 ppm O3 the level of mixed disulfides was small (15 per cent of the total NPSH) but attained a peak (equivalent to 0.6 mumol NPSH/lung) after a recovery for 24 h. However, the level diminished considerably within 48 h of recovery.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., JANDL J. H. Oxidative hemolysis and precipitation of hemoglobin. II. Role of thiols in oxidant drug action. J Clin Invest. 1961 Mar;40:454–475. doi: 10.1172/JCI104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Abnormalities of the hexose monophosphate shunt. Semin Hematol. 1971 Oct;8(4):311–347. [PubMed] [Google Scholar]
- Carter J. R., Jr Role of sulfhydryl groups in erythrocyte membrane structure. Biochemistry. 1973 Jan 2;12(1):171–176. doi: 10.1021/bi00725a028. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- DeLucia A. J., Hoque P. M., Mustafa M. G., Cross C. E. Ozone interaction with rodent lung: effect on sulfhydryls and sulfhydryl-containing enzyme activities. J Lab Clin Med. 1972 Oct;80(4):559–566. [PubMed] [Google Scholar]
- FAIRCHILD E. J., 2nd, MURPHY S. D., STOKINGER H. E. Protection by sulfur compounds against the air pollutants ozone and nitrogen dioxide. Science. 1959 Oct 2;130(3379):861–862. doi: 10.1126/science.130.3379.861. [DOI] [PubMed] [Google Scholar]
- Fairchild E. J. Tolerance mechanisms. Determinants of lung responses to injurious agents. Arch Environ Health. 1967 Jan;14(1):111–126. doi: 10.1080/00039896.1967.10664702. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K., Gaull G. E. A procedure for the quantitative analysis of the sulphur amino acids of rat tissues. Biochem J. 1967 Mar;102(3):959–975. doi: 10.1042/bj1020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Smith E. C., White J. M. Oxidative haemolysis and Heinz body haemolytic anaemia. Br J Haematol. 1974 Apr;26(4):513–517. doi: 10.1111/j.1365-2141.1974.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Harding J. J. Glutathione-protein mixed disulphides in human lens. Biochem J. 1969 Oct;114(4):88P–89P. doi: 10.1042/bj1140088pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrap K. R., Jackson R. C., Riches P. G., Smith C. A., Hill B. T. The occurrence of protein-bound mixed disulfides in rat tissues. Biochim Biophys Acta. 1973 May 17;310(1):104–110. doi: 10.1016/0005-2795(73)90012-3. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Haugaard N., Lee N. H., Kostrzewa R., Horn R. S., Haugaard E. S. The role of sulfhydryl groups in oxidative phosphorylation and ion transport by rat liver mitochrondia. Biochim Biophys Acta. 1969 Feb 25;172(2):198–204. doi: 10.1016/0005-2728(69)90063-2. [DOI] [PubMed] [Google Scholar]
- Heitmann P. A model for sulfhydryl groups in proteins. Hydrophobic interactions of the cystein side chain in micelles. Eur J Biochem. 1968 Jan;3(3):346–350. doi: 10.1111/j.1432-1033.1968.tb19535.x. [DOI] [PubMed] [Google Scholar]
- Jacob H. S. Mechanisms of Heinz body formation and attachment to red cell membrane. Semin Hematol. 1970 Jul;7(3):341–354. [PubMed] [Google Scholar]
- Little C., O'Brien P. J. The effectiveness of a lipid peroxide in oxidizing protein and non-protein thiols. Biochem J. 1968 Jan;106(2):419–423. doi: 10.1042/bj1060419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUNTAIN J. T. Detecting hypersusceptibility to toxic substances. An appraisal of simple blood test. Arch Environ Health. 1963 Mar;6:357–365. doi: 10.1080/00039896.1963.10663406. [DOI] [PubMed] [Google Scholar]
- Menzel D. B. Oxidation of biologically active reducing substances by ozone. Arch Environ Health. 1971 Aug;23(2):149–153. doi: 10.1080/00039896.1971.10665973. [DOI] [PubMed] [Google Scholar]
- Menzel D. B. Toxicity of ozone, oxygen, and radiation. Annu Rev Pharmacol. 1970;10:379–394. doi: 10.1146/annurev.pa.10.040170.002115. [DOI] [PubMed] [Google Scholar]
- Modig H. Cellular mixed disulphides between thiols and proteins, and their possible implication for radiation protection. Biochem Pharmacol. 1968 Feb;17(2):177–186. doi: 10.1016/0006-2952(68)90321-3. [DOI] [PubMed] [Google Scholar]
- Mudd J. B., Leavitt R., Ongun A., McManus T. T. Reaction of ozone with amino acids and proteins. Atmos Environ. 1969 Nov;3(6):669–682. doi: 10.1016/0004-6981(69)90024-9. [DOI] [PubMed] [Google Scholar]
- Mustafa M. G., Cross C. E. Effects of short-term ozone exposure on lung mitochondrial oxidative and energy metabolism. Arch Biochem Biophys. 1974 Jun;162(2):585–594. doi: 10.1016/0003-9861(74)90219-7. [DOI] [PubMed] [Google Scholar]
- Nasr A. N. Biochemical aspects of ozone intoxication: a review. J Occup Med. 1967 Dec;9(12):589–597. [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Accurate measurement of oxidized glutathione content of human, rabbit, and rat red blood cells and tissues. Anal Biochem. 1968 Oct 24;25(1):70–76. doi: 10.1016/0003-2697(68)90082-1. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Willis R. J., Kratzing C. C. Changes in levels of tissue nucleotides and glutathione after hyperbaric oxygen treatment. Aust J Exp Biol Med Sci. 1972 Dec;50(6):725–729. doi: 10.1038/icb.1972.65. [DOI] [PubMed] [Google Scholar]


