Abstract
Aberrant blood vessel growth in the retina that underlies the pathology of proliferative diabetic retinopathy and retinopathy of prematurity is the result of the ischemia-driven disruption of the normally antiangiogenic environment of the retina. In this study, we show that a potent inhibitor of angiogenesis found naturally in the normal eye, pigment epithelium-derived growth factor (PEDF), inhibits such aberrant blood vessel growth in a murine model of ischemia-induced retinopathy. Inhibition was proportional to dose and systemic delivery of recombinant protein at daily doses as low as 2.2 mg/kg could prevent aberrant endothelial cells from crossing the inner limiting membrane. PEDF appeared to inhibit angiogenesis by causing apoptosis of activated endothelial cells, because it induced apoptosis in cultured endothelial cells and an 8-fold increase in apoptotic endothelial cells could be detected in situ when the ischemic retinas of PEDF-treated animals were compared with vehicle-treated controls. The ability of low doses of PEDF to curtail aberrant growth of ocular endothelial cells without overt harm to retinal morphology suggests that this natural protein may be beneficial in the treatment of a variety of retinal vasculopathies.
The pathological growth of new blood vessels in the eye and the leaks and tissue disruption it causes are responsible for most cases of vision loss in the developed world. Abnormal vessels can originate from the choroid circulation in diseases like macular degeneration. It is not known what induces their growth. Abnormal vasculature can also develop from vessels within the retina. It is known that these irregular vessels are stimulated by ischemia and give rise to retinopathies that threaten sight in premature infants and many diabetic patients.
Ischemic retinopathies are often thought to be driven primarily by a rise in vascular endothelial cell growth factor (VEGF), a potent inducer of both neovascularization and vessel leakage, whose secretion by a variety of cells is stimulated by low oxygen (1). High levels of VEGF have been documented in the vitreous of human retinopathies (2, 3) and in animal models of ophthalmologic diseases involving neovascularization (4–7). But a change in VEGF seems unlikely to be the sole cause of retinopathies. Increases in VEGF can occur in the absence of retinopathy; decreases in the severity of retinopathies can occur without any change in VEGF levels (8, 9); and drugs that prevent a rise in VEGF can vary in their effects on retinopathy (10). In addition, therapies designed to interfere with the action of VEGF have not been able to completely halt vessel growth in a murine model of ischemia-induced retinopathy (11, 12). These discontinuities suggest that additional stimulatory angiogenic factors and/or natural inhibitors are also playing key roles in this disease process.
The major natural inhibitor in the vitreous and cornea of the eye is pigment epithelium-derived factor (PEDF; refs. 13 and 14), a protein produced by retinal pigment epithelial cells and found at high concentrations within the retina as well as in the vitreous, where it is responsible for the antiangiogenic activity of this fluid (14). PEDF is an exceptionally potent antiangiogenic factor that is regulated by oxygen in a pattern reciprocal to that of stimulatory VEGF in that it rises when oxygen is plentiful and falls when it is limiting (14, ¶). In this study, we use a well characterized mouse model of ischemic retinopathy to demonstrate the ability of this natural ocular inhibitor to control retinal neovascularization.
The elevation of a myriad of inducers of angiogenesis have been documented in the vitreous of eyes with proliferative diabetic retinopathy including VEGF (2, 3), bFGF (15), IGF-1 (16, 17), IL-8 (18, 19), angiogenin (20), placental growth factor (21), and hepatocyte growth factor (22). An advantage of natural inhibitors of angiogenesis like PEDF over those that specifically target single inducers or single classes of receptors is that they can prevent angiogenesis induced by any one of a wide variety of different stimulators (14). To suggest a mechanism by which PEDF might be preventing endothelial cells from responding to multiple inducers, we show that it stimulates apoptosis in vitro when given to cultured endothelial cells that are under stress and that it has similar effects on endothelial cells forming new vessels in situ in mice with retinopathy.
Methods
PEDF Purification.
A mammalian expression construct for full-length PEDF mRNA and six histidine residues at its carboxy terminus was engineered into pCEP4 (Invitrogen), and transfected into human embryonic kidney cells (14). Recombinant PEDF was purified from media conditioned by these cells by using the Xpress Protein Purification System (Invitrogen), dialyzed against PBS, filter-sterilized, and quantified by using a Coomassie protein reagent (Pierce). Purified PEDF ran as a single band on a silver stained SDS-polyacrylamide gel. Its antiangiogenic activity was verified by using the in vitro inhibition of endothelial cell migration (14).
Care and Treatment of Mice.
A previously described murine model of ischemia-induced retinopathy (23) was set up by using dams and neonatal C57/BL6J mice (The Jackson Laboratory). Mice in the normoxia group were maintained in room air throughout the experiment. Mice in the hyperoxia group were exposed with their nursing dams to 75% oxygen/25% nitrogen from postnatal day 7 (P7) to P12, then removed to room air and injected i.p. each day from P12 through P16 with 100 μl of PBS (PBS vehicle) or PBS containing PEDF (P1 is the date of birth). At P17, all pups were weighed, killed, and perfused with 1 ml of fixative (4% paraformaldehyde/8% sucrose/sodium phosphate buffer, pH 7.2) through the left ventricle of the heart. Eyes were enucleated and placed in fixative. Four to five pups were treated at each dose in each experiment. All animals were treated according to Association for Research in Vision and Ophthalmology and National Institutes of Health guidelines for animal care and use, and protocols were approved by the Animal Care and Use Committee Northwestern University and Veterans Affairs Hospitals Chicago Health Care System–Lakeside Division.
Endothelial Cell Nuclei Counts.
Fixed tissues, identified only by code, were paraffin-embedded, 4-μm sagittal sections were cut, and slides were stained with hematoxylin-phloxine-saffron (HPS). Step sections of each eye were prepared and six consecutive sections at each of three levels spanning up to 75 microns were examined for a total of 18 sections per eye. One representative section that contained cornea, lens, and optic nerve and retina was chosen from each animal by a blinded investigator and all endothelial cell nuclei that crossed or extended beyond the internal limiting membrane were counted. In our hands, a very vigorous neovascularization response was induced by hyperoxia. This fact, along with our selection of sections from individual animals that traversed the globe at similar central points, made it possible to obtain highly significant data counting only a single slide from each animal. To validate this method, data derived from counting a single slide per animal were compared with those derived from counting five slides per pup. For the four experimental and four control animals examined in this way, no significant difference was seen. Tissues from PBS-treated controls and from normoxic controls were evaluated identically. Statistical analysis used the Student's t test and standard errors of the mean are reported.
In Situ Detection of Apoptotic Endothelial Cells.
Apoptotic cells were morphologically distinguished by nuclear fragmentation in HPS-stained tissue sections and quantified by terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) staining as described (24). Briefly, a minimum of three sections of each eye were deparaffinized, rehydrated, blocked with 1% normal serum for 20 min, and incubated 60 min in 1% blocking solution with goat antibody against mouse CD 31 (PECAM-1, Santa Cruz Biotechnology). After three 5-min washes with PBS, secondary antibodies were applied for 60 min at room temperature by using a 1:20 dilution of sheep antibody against mouse IgG conjugated with R-phycoerythrin (Sigma). An ApopTag kit (Intergen, Purchase, NY) was used according to the manufacturer's instructions. Slides were rinsed briefly in PBS, mounted with Prolong Antifade (Molecular Probes), and viewed with an Olympus Fluoview Confocal Laser Scanning Microscope. Slides were assigned a code and the number of apoptotic vascular endothelial cells per total endothelial cells in one representative sagittal section of the retina were counted in a blinded fashion. The range of total endothelial cells in the PBS group (n = 4) was 155–268, and in the PEDF-treated group (n = 4) was 78–94.
In Vitro Apoptosis Assay.
Human dermal microvascular endothelial cells (Clonetics, San Diego) were maintained in EGM-MV media (endothelial basal media plus additives) (BioWhittaker) and used between passages 4 and 10. Cells were seeded onto coverslips in wells of a 24-well plate at 5 × 104 cells/well, allowed to attach overnight, and refed with endothelial basal media containing 0.5% FCS with or without PEDF. Although apoptosis was also induced by PEDF when cells were incubated in 2% FCS, the lowest serum concentration compatible with good survival of untreated controls was routinely used in an effort to more closely approximate the plasma that endothelial cells are exposed to as a rule in vivo. A time course measuring apoptosis induced after treatment of endothelial cells with 10 nM PEDF for 6, 12, 18, 24, and 30 h showed that maximal apoptosis was reached by 18 h. Although this maximal level was also observed at 24 and 30 h, at later time points apoptosis also increased in untreated cells. Thus, in the experiments reported here, after 18 h an equal volume of 4% paraformaldehyde was added to the media to fix the cells. Apoptotic cells were detected by TUNEL using Apoptosis Detection kit (Intergen) and counterstained with propidium iodide. Three to five random fields ranging from 90–300 cells per field were counted for each treatment group.
Results
A murine model of ischemia-induced retinopathy (23) was used to determine whether treatment with recombinant PEDF could inhibit pathological retinal angiogenesis. In this well characterized model, neonatal mice are exposed to hyperoxic conditions for 5 days from P7 to P12 during the time their retinal vasculature is developing. As a result, many vessels are obliterated as the vasculature accommodates to high oxygen levels. When the mice are returned to the lower oxygen of room air at P12, the retina becomes ischemic and in response extensive neovascularization occurs in all animals. By P17, vessels have proliferated and crossed the internal limiting membrane into the vitreous, creating a retinopathy that is similar to that seen in human retinopathy of prematurity and also shares many of the features of diabetic retinopathy.
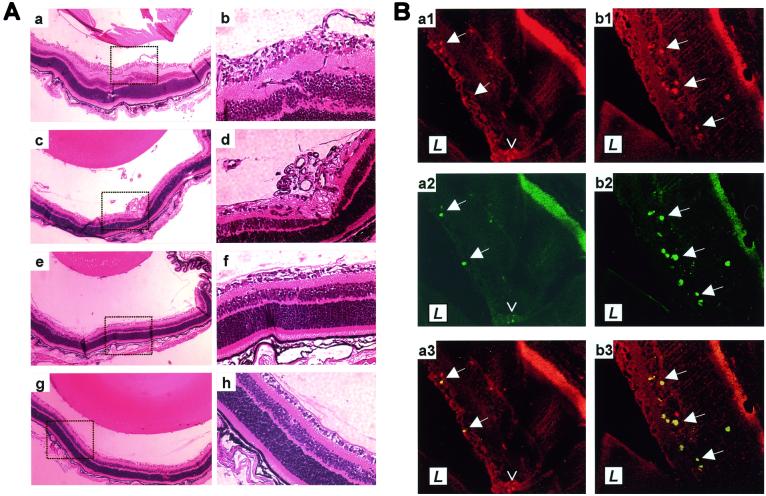
To test the effect of PEDF on such retinopathy, mice were exposed to hyperoxia, returned to room air, and injected i.p. once a day from P12 through P16 with various doses of recombinant PEDF. On P17, eyes were harvested. In two separate experiments, each using independently purified recombinant PEDF protein, the treated pups displayed no gross signs of toxicity and their body weights were similar to those of their vehicle-treated littermates (6.76 ± 0.16 g vs. 6.73 ± 0.28 g, respectively, for the highest dose). Retinopathy with clear vascular tufts extending into the vitreous was observed in all pups exposed to hyperoxia that had been treated with PBS vehicle (Fig. 1 Aa–Ad). This was in sharp contrast to the retinas of PEDF-treated pups, in which vascular tufts were reduced or not observed (Fig. 1 Ae–Ah).
Figure 1.
In situ angiogenesis and apoptosis of endothelial cells in PEDF-treated retinas. (A) Examples shown at low (Left) and high (Right) power of retinas of mice exposed to hyperoxia and subsequently treated with vehicle (Aa–Ad), with 11.2 μg/day PEDF (Ae and Af) or with 22.4 μg/day PEDF (Ag and Ah). (B) Sections of retinas from mice treated with vehicle (Ba) or with 11.2 μg/day PEDF (Bb) were stained with PECAM-1 to detect endothelial cells (Ba1 and Bb1) and with TUNEL to detect apoptotic cells (Ba2 and Bb2), and the images merged (Ba3 and Bb3). L indicates the position of the lens. Solid arrows are reference points within each retina. The open arrowhead in panel Ba1–Ba3 points to a vascular tuft of predominantly TUNEL-negative endothelial cells. Note that the retinal pigment epithelial layer autofluoresces.
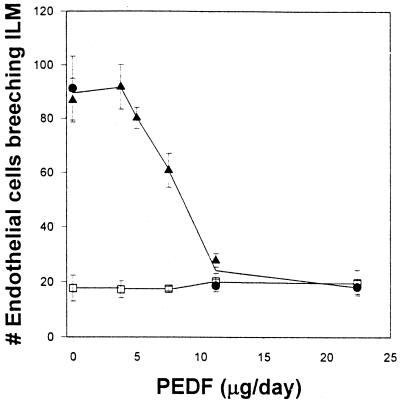
To quantify the retinopathy, the number of endothelial nuclei at or beyond the retina's internal limiting membrane was counted in sections of eyes that included the cornea, lens, and optic nerve. In mice not exposed to hyperoxia, PEDF had no effect on endothelial cell counts (Fig. 2). The constant background level of endothelial cells seen in these control animals represents endothelial cells present in vessels that normally enter the retina along the optic nerve and cross the internal limiting membrane before spreading under it. In mice exposed to hyperoxia, a clear dose-dependent inhibition of angiogenesis was seen (Fig. 2). At the two highest doses of PEDF used, 11.2 μg/day and 22.4 μg/day, complete inhibition of aberrant retinal angiogenesis was achieved, resulting in numbers of endothelial cells in the vitreous cavity that were not significantly different from those observed in age-matched pups that were housed at ambient oxygen levels. In a third experiment performed to compare native PEDF protein (kindly provided by Pat Becerra, National Eye Institute) with that made in our laboratory that contained a histidine tag, no difference between the two preparations was detected in vitro or in vivo. Pups treated with the highest dose of either PEDF showed 67% inhibition of the induced neovascularization (data not shown).
Figure 2.
Effect of PEDF treatment on hyperoxia-induced retinal neovascularization. Mice exposed to hyperoxia to induce retinopathy (experiment 1, circles; experiment 2, triangles) and control mice maintained in ambient room air (squares) were treated systemically for 5 days with PEDF, and the endothelial cells at or beyond the internal limiting membrane (ILM) counted. (Error bars, SEM.)
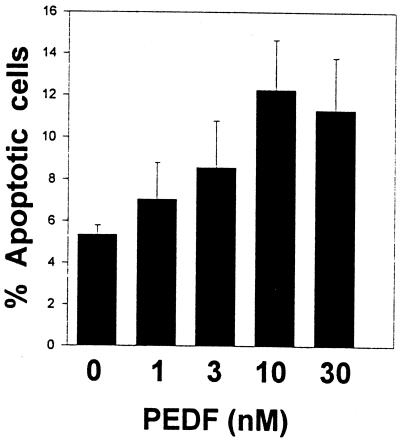
Seeking a mechanism by which PEDF might act on endothelial cells to prevent their responding to ischemic signals that stimulate angiogenesis, cultured human endothelial cells were put under mild stress by transferring them to low serum and the effect of PEDF on their apoptotic rate was measured. Increasing doses of PEDF increased the number of TUNEL-positive apoptotic cells (Fig. 3). PEDF also induced apoptosis in ischemic retinas in vivo. The endothelial cells undergoing apoptosis in situ were easily seen as their morphology changed from a slender, elongated form characteristic of the normal endothelial cell to the more rounded shape of an apoptotic cell and often expanded into the lumen of the vessel. Apoptotic endothelial cells were relatively rare in retinas of animals exposed to hyperoxia and treated with PBS, whereas they were common in the retinas of littermates that had been treated with PEDF (Fig. 1B; Table 1). Fig. 1B depicts tissue taken from a retina where PEDF had successfully inhibited aberrant endothelial cell growth so apoptotic endothelial cells are found only within the retina. These cells are presumably in the process of being attracted toward the vitreous, but have been pushed by the PEDF into apoptosis. At PEDF doses that are only partially effective at preventing retinopathy, many apoptosing endothelial cells that breached the inner limiting membrane were seen (data not shown).
Figure 3.
PEDF induction of endothelial cell apoptosis in vitro. Increasing concentrations of PEDF were added to human microvascular endothelial cells and the proportion of apoptotic cells scored 18 h later. (Error bars, SEM.)
Table 1.
Quantification of in vivo endothelial cell apoptosis in retinas of mice with ischemic retinopathy
Proportion of TUNEL positive endothelial cells in the retina in one representative sagittal section. Range of total endothelial cells in PBS group was 155–268 and in PEDF group was 78–94.
P < 0.000005.
Discussion
In this study, we show that systemic treatment with a natural angiogenesis inhibitor found in the normal eye can block the development of the aberrant vasculature that develops in response to an ischemic stimulus within the retina. In the normal adult eye, PEDF is found at high, antiangiogenic concentrations in the interphotoreceptor matrix, and is also present in the vitreous and the cornea (13), where depletion studies show it is the primary antiangiogenic factor (14). But it is not detectable in unperturbed neonatal mice retinas during the establishment of retinal vessels (25, 26), becoming visible by immunohistochemistry by P21 (14). However, in the model used here, PEDF is prematurely induced in the retina by high oxygen by P12 (14) and may be partially responsible for the paucity of vessels that cause the retina to become ischemia when such mice return to ambient conditions where oxygen is lower.
We were able to prevent the formation of aberrant vessels induced by this ischemia with a relatively low systemic dose of PEDF. If the lowest completely effective dose partitioned evenly in the mouse it would result in a maximum level of 44.8 nM PEDF, which is less than the roughly 90 nM found in the normal adult mouse vitreous (O. Volpert, personal communication). This suggests that during ischemic retinopathy, the effective PEDF level in the eye does indeed fall, as might be expected in light of the fact that its production decreases in low oxygen (14, ¶). It remains to be determined whether a fall in PEDF is essential for ischemic retinopathy to proceed.
The effective systemic dose of PEDF expressed in mg/kg per day (2.2 mg/kg per day is equivalent to 11.2 μg/5 g mouse per day), is low when compared with amounts of other antiangiogenic factors needed to halt experimental neovascularization in other models. It requires 100 mg/kg per day of systemic angiostatin and 150 mg/kg of 2-methoxyestradiol to see some inhibition of angiogenesis in the cornea micropocket assay (27, 28), whereas only 2.8 mg/kg per day of PEDF is needed (O. Volpert and N.B., unpublished data). The high in vivo activity of PEDF is in keeping with its exceptional potency when compared with other antiangiogenic agents using in vitro tests (14).
PEDF appears to have blocked abnormal neovascularization without overt harm to established retinal vessels, because even the highest doses of PEDF did not reduce the number of endothelial cells at the inner limiting membrane below the values seen in the retinas of age-matched control pups maintained in room air. In addition, retinas of normoxic animals that had been treated with PEDF were histologically similar to and had numbers of endothelial cells similar to vehicle-treated littermates suggesting that it is vessels undergoing pathological neovascularization that are harmed by PEDF. Other antiangiogenic agents have been used in this model, including those interfering with VEGF (11, 12), with integrins (29–31) or intracellular signaling via NF-κB (32), as well as more broadly acting pharmacological agents, such as dexamethsone (33), indomethacin (34), or the calcium channel blocker Diltiazem (35); but none of these were able to completely inhibit aberrant vessel growth. Only the tyrosine kinase inhibitors, CGP 441251 (36) and PTK787 (37), have been as effective as PEDF. But 50–600 mg/kg per day were required, and these doses also resulted in the absence of endothelial cells in inner retinal layers, suggesting that, in contrast to PEDF, these drugs may be toxic to the retinal vascular development.
Data presented here indicate that PEDF acts to halt angiogenesis by inducing apoptosis in endothelial cells cultured in vitro or undergoing pathological neovascularization in vivo. In vitro, it is not possible to determine whether PEDF is initiating apoptosis or enhancing a tendency already present, because the cells are in the unnatural environment of a culture dish where some background level of TUNEL-positive cells is always present. In vivo endothelial cells in established vessels seem immune to PEDF, suggesting that some destabilization, possibly associated with activation, may be a prerequisite for sensitivity to PEDF. Several other natural, broad-acting inhibitors have similar activities in vitro, including thrombospondin-1 (24, 38), angiostatin (39, 40), endostatin (41), and 2-methoxyestradiol (42). Thrombospondin-1 also induces apoptosis in developing tumor vessels in vivo (24). This mechanism helps to explain why PEDF, as well as these other inhibitors, can antagonize a variety of inducers. The fact that many inducers are also survival factors for endothelial cells raises the possibility that an endothelial cell weighs how it will respond to the conflicting signals it receives simultaneously from natural inducers and inhibitors by using its apoptotic machinery. The relative level of pro- and antiapoptotic mediators within the cell would then determine whether it survives to respond to inducers or dies in response to the inhibitors.
Our finding that PEDF induced apoptosis in endothelial cells indicates that the response to PEDF treatment must vary with cell type. When PEDF is applied at concentrations similar to those used here to neuronal cells, it blocks rather than induces apoptosis (43–45). The PEDF survival signal to cerebellar granule cells appears to be mediated by an 80-kDa receptor (46). PEDF's action on endothelial cells is also likely to be receptor-mediated, but perhaps by way of a different receptor.
Just as multiple inducers of angiogenesis may contribute to ocular angiogenesis, factors in addition to PEDF may counterbalance these inducers. Two additional proteins with inhibitory activity have been reported in the vitreous, thrombospondin-1 (47), and IP-10 (19). Thrombospondin-1 seems likely to play some role in the control of ocular vessels, because the 17-day-old thrombospondin-1 null mice have aberrant vessels in their eye that extend beyond the internal limiting membrane of the retina (48) and in adult rats thrombospondin-1 can be down-regulated by high glucose (47). Recently, thrombospondin-1 has been reported to inhibit angiogenesis by about 50% in a rat model of retinal ischemia (49). However, it is not yet possible to sort out the direct effects of thrombospondin-1 on endothelial cells from the indirect effects it may have by virtue of its ability to activate latent TGF-β (50).
Current therapies for retinopathy include laser photocoagulation or cryoablation that kill small areas of cells and slow the deterioration of vision, but both therapies result in limited vision loss due to the nature of the treatment (51). In addition, their effectiveness is reduced by the recurrence of abnormal neovascularization. PEDF, as well as the many additional antiangiogenic compounds becoming available, offers the hope of making current treatment more effective, and the possibility of providing primary therapy without attendant vision loss.
Acknowledgments
We thanks Xiamin Wu for protein purification, Mona Cornwell for histology, Xuemei Huang for technical assistance, Keith Cook for assistance with oxygen monitoring, Patricia Becerra for providing a sample of native PEDF, and the National Cancer Institute for support through Grants CA52750 and CA64239.
Abbreviations
- PEDF
pigment epithelium-derived factor
- VEGF
vascular endothelial growth factor
- P
postnatal day
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 2122.
Miller, A. D., Robinson, G. S., Smith, L. E. H. & Becerra, S. P. (2000) Invest. Ophthalmol. Visual Sci. 41, 4619 (abstr.).
References
- 1.Miller J W. Am J Pathol. 1997;151:13–23. [PMC free article] [PubMed] [Google Scholar]
- 2.Adamis A P, Miller J W, Bernal M T, D'Amico D J, Folkman J, Yeo T-K, Yeo K-T. Am J Ophthalmol. 1994;118:445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 3.Aiello L P, Avery R L, Arrigg P G, Keyt B A, Jampel H D, Shah S T, Pasquale L R, Thieme H, Iwamoto M A, Park J E, et al. N Eng J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 4.Miller J W, Adamis A P, Shima D T, D'Amore P A, Moulton R S, O'Reilly M S, Folkman J, Dvorak H F, Brown L F, Berse B, et al. Am J Pathol. 1994;145:574–584. [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce E A, Avery R L, Foley E D, Aiello L P, Smith L E H. Proc Natl Acad Sci USA. 1995;92:905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone J, Chang-Ling T, Pe're J, Itin A, Gnessin H, Keshet E. Invest Ophthalmol Visual Sci. 1996;37:290–299. [PubMed] [Google Scholar]
- 7.Dorey C K, Aouididi S, Reynaud X, Dvorak H F, Brown L F. Arch Ophthalmol. 1996;114:1210–1217. doi: 10.1001/archopht.1996.01100140410008. [DOI] [PubMed] [Google Scholar]
- 8.Smith L E H, Kopchick J J, Chen W, Knapp J, Kinose F, Daley D, Foley E, Smith R G, Schaeffer J M. Science. 1997;276:1706–1709. doi: 10.1126/science.276.5319.1706. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto N, Tobe T, Hackett S F, Ozaki H, Vinores M A, LaRochelle W, Zack D J, Campochairo P A. Am J Pathol. 1997;151:281–291. [PMC free article] [PubMed] [Google Scholar]
- 10.Frank R N, Amin R, Kennedy A, Hohman T C. Arch Ophthalmol. 1997;115:1036–1047. doi: 10.1001/archopht.1997.01100160206011. [DOI] [PubMed] [Google Scholar]
- 11.Aiello L P, Pierce E A, Foley E D, Takagi H, Chen H, Riddle L, Ferrara N, King G L, Smith L E H. Proc Natl Acad Sci USA. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson G S, Pierce E A, Rook S L, Foley E, Webb R, Smith L E H. Proc Nat Acad Sci USA. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becerra S P. Adv Exp Med Biol. 1997;425:223–237. [PubMed] [Google Scholar]
- 14.Dawson D W, Volpert O V, Gillis P, Crawford S E, Xu H-J, Benedict W, Bouck N P. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 15.Sivalingam A, Kenney J, Brown G C, Benson W E, Donoso L. Arch Ophthalmol. 1990;108:869–872. doi: 10.1001/archopht.1990.01070080113046. [DOI] [PubMed] [Google Scholar]
- 16.Grant M, Russel B, Fitzgerald C, Merimee T J. Diabetes. 1986;35:416–420. doi: 10.2337/diab.35.4.416. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Schwickerath R, Pfeiffer A, Blum W F, Freyberger H, Klein M, Losche C, Rollmann R, Schatz H. J Clin Invest. 1993;92:2620–2625. doi: 10.1172/JCI116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida A, Yoshida S, Khalil A D, Ishibashi T, Inomata H. Invest Ophthalmol Visual Sci. 1998;39:1097–1106. [PubMed] [Google Scholar]
- 19.Elner S G, Strieter R, Bian Z M, Kunkel S, Mokhtarzaden L, Johnson M, Lukacs N, Elner V M. Arch Ophthalmol. 1998;116:1597–1601. doi: 10.1001/archopht.116.12.1597. [DOI] [PubMed] [Google Scholar]
- 20.Ozaki H, Hayashi H, Oshima K. Ophthalmol Res. 1996;28:356–360. doi: 10.1159/000267929. [DOI] [PubMed] [Google Scholar]
- 21.Khaliq A, Foreman D, Ahmed A, Weich H, Gregor Z, McLeod D, Boulton M. Lab Invest. 1998;78:109–116. [PubMed] [Google Scholar]
- 22.Katsura Y, Okano T, Noritake M, Kosano H, Nishigori H, Kado S, Matsuoka T. Diabetes Care. 1998;21:1759–1763. doi: 10.2337/diacare.21.10.1759. [DOI] [PubMed] [Google Scholar]
- 23.Smith L E H, Wesolowski E, McLennan A, Kostyk S K, D'Amato R, Sullivan R, D'Amore P A. Invest Ophthalmol Visual Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 24.Jimenez B, Volpert O V, Crawford S E, Febbraio M, Silverstein R L, Bouck N. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 25.Blanks J C, Johnson L V. J Comp Neurol. 1986;254:543–553. doi: 10.1002/cne.902540407. [DOI] [PubMed] [Google Scholar]
- 26.Connolly S E, Hores T A, Smith L E H, D'Amore P A. Microvasc Res. 1988;36:275–290. doi: 10.1016/0026-2862(88)90028-3. [DOI] [PubMed] [Google Scholar]
- 27.Drixler T A, Rinkes I H M, Ritchie E D, van Vroonhoven T J M V, Gebbink M F B J, Voest E E. Cancer Res. 2000;60:1761–1765. [PubMed] [Google Scholar]
- 28.Klauber N, Parangi S, Flynn E, Hamel E, D'Amato R J. Cancer Res. 1997;57:81–86. [PubMed] [Google Scholar]
- 29.Hammes H-P, Brownlee M, Jonczyk A, Sutter A, Preissner K T. Nat Med. 1996;2:529–533. doi: 10.1038/nm0596-529. [DOI] [PubMed] [Google Scholar]
- 30.Friedlander M, Theesfeld C L, Sugita M, Fruttiger M, Thomas M A, Chang S, Cheresh D A. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luna J, Tobe T, Mousa S A, Reilly T M, Campochiaro P A. Lab Invest. 1996;75:563–573. [PubMed] [Google Scholar]
- 32.Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H. Invest Ophthalmol Visual Sci. 1999;40:1624–1629. [PubMed] [Google Scholar]
- 33.Rotschild T, Nandgaonkar B N, Yu K, Higgins R D. Pediatr Res. 1999;46:94–100. doi: 10.1203/00006450-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Nandgaonkar B N, Rotschild T, Yu K, Higgins R D. Pediatr Res. 1999;46:184–188. doi: 10.1203/00006450-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Higgins R D, Yu K, Sanders R J, Nandgaonkar B N, Rotschild T, Rifkin D B. Curr Eye Res. 1999;18:20–27. doi: 10.1076/ceyr.18.1.20.5390. [DOI] [PubMed] [Google Scholar]
- 36.Seo M S, Kwak N, Ozaki H, Yamada H, Okamoto N, Yamada E, Fabbro D, Hofmann F, Wood J M, Campochiaro P A. Am J Pathol. 1999;154:1743–1753. doi: 10.1016/S0002-9440(10)65430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozaki H, Seo M-S, Ozaki K, Yamada H, Yamada E, Okamoto N, Hofmann F, Wood J M, Campochairo P A. Am J Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo N, Krutzsch H C, Inman J K, Roberts D D. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- 39.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O'Reilly M, Folkman J. Proc Natl Acad Sci USA. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucas R, Holmgren L, Garcia I, Jimenez V, Mandroita S J, Borlat F, Sim B K, Wu Z, Grau G E, Shing Y, et al. Blood. 1998;92:4730–4741. [PubMed] [Google Scholar]
- 41.Dhanabal M, Ramchandran R, Waterman M J, Lu H, Knebelmann B, Segal M, Sukhatme V P. J Biol Chem. 1999;274:11721–11726. doi: 10.1074/jbc.274.17.11721. [DOI] [PubMed] [Google Scholar]
- 42.Yue T L, Wang X, Louden C S, Gupta S, Pillarisetti K, Gu J L, Hart T K, Lysko P G, Feuerstein G Z. Mol Pharmacol. 1997;51:951–962. doi: 10.1124/mol.51.6.951. [DOI] [PubMed] [Google Scholar]
- 43.Araki T, Taniwaki T, Becerra S P, Chader G J, Schwartz J P. J Neurosci Res. 1998;53:7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 44.Houenou L J, D'Costa A P, Li L, Turgeon V L, Enyadike C, Alberdi E, Becerra S P. J Comp Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- 45.Taniwaki T, Hirashima N, Becerra S P, Chader G J, Etcheberrigaray R, Schwartz J P. J Neurochem. 1997;68:26–32. doi: 10.1046/j.1471-4159.1997.68010026.x. [DOI] [PubMed] [Google Scholar]
- 46.Alberdi E, Aymerich M S, Becerra S P. J Biol Chem. 1999;274:31605–31612. doi: 10.1074/jbc.274.44.31605. [DOI] [PubMed] [Google Scholar]
- 47.Shebani N, Sorenson C M, Cornelius L A, Frazier W A. Biochem Biophys Res Commun. 2000;267:257–261. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 48.Stellmach V, Volpert O V, Crawford S E, Lawler J, Hynes R O, Bouck N. Eur J Cancer. 1996;32A:2394–2400. doi: 10.1016/s0959-8049(96)00385-1. [DOI] [PubMed] [Google Scholar]
- 49.Shafiee A, Penn J S, Krutzsch H C, Inman J K, Roberts D D, Blake D A. Invest Ophthalmol Visual Sci. 2000;41:2378–2388. [PubMed] [Google Scholar]
- 50.Crawford S E, Stellmach V, Murphy-Ullrich J E, Ribeiro S M, Lawler J, Hynes R O, Boivin G P, Bouck N. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 51.Carney M D, Sanborn G E, Tabassian A R. In: Clinical Guide to Comprehensive Ophthalmology. Lee D A, Higginbotham E J, editors. New York: Thieme; 1999. pp. 396–418. [Google Scholar]