Abstract
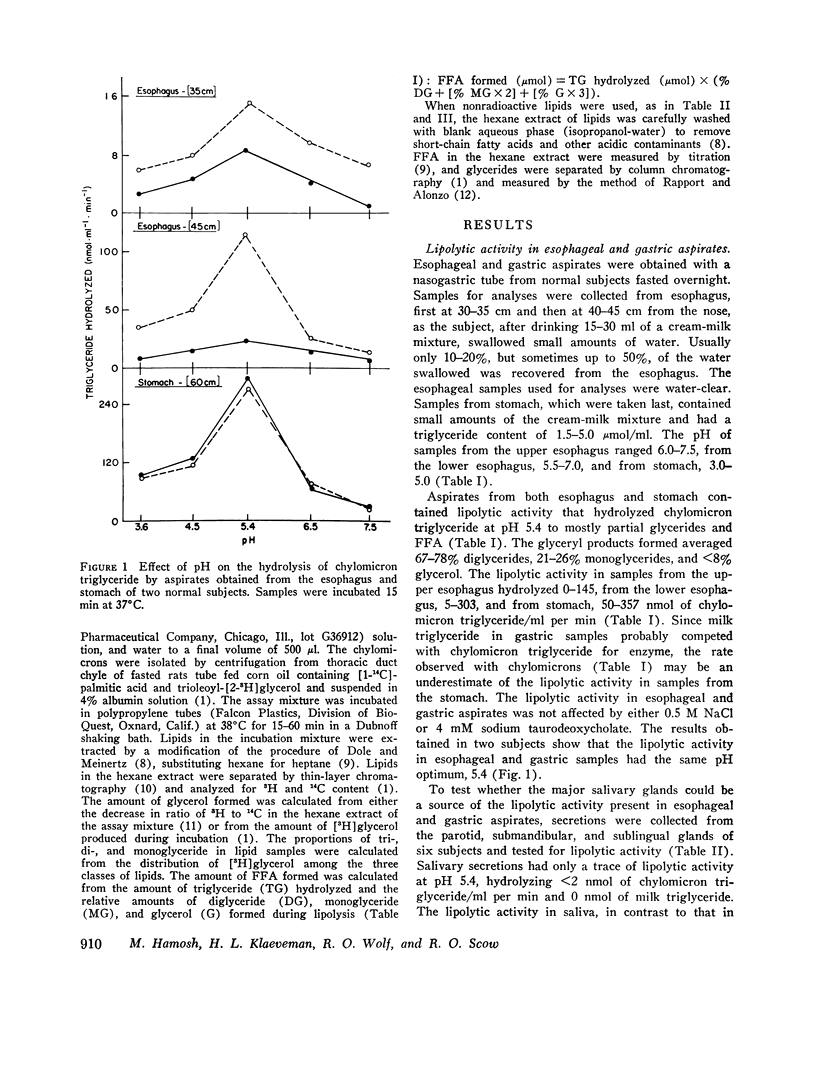
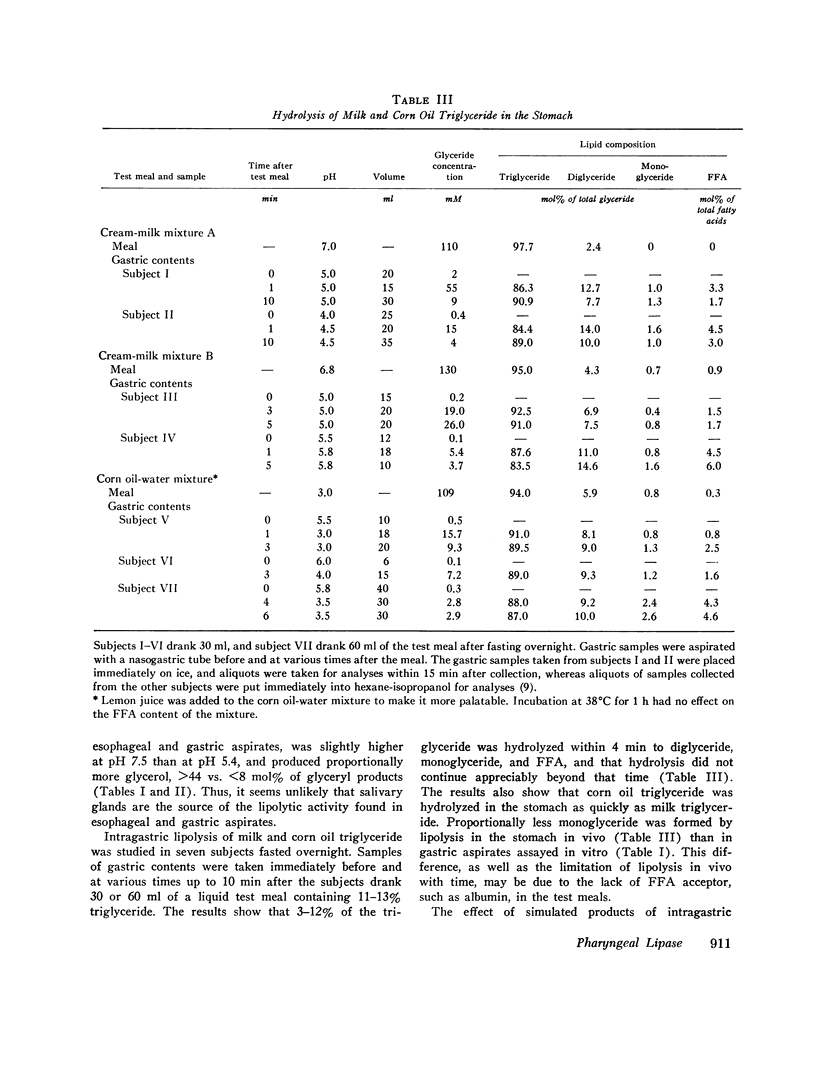
Lipolytic activity was studied in esophageal and gastric aspirates obtained with a nasogastric tube from 14 healthy adult subjects. Samples were collected from esophagus, first at 30-35 cm and then at 40-45 cm from the nose, as the subject, after drinking 15-30 ml of a cream-milk mixture, swallowed small amounts of water. The samples from stomach were taken last and usually contained a small amount of cream-milk mixture. Lipolytic activity was assayed using chylomicron, milk, and corn oil triglyceride as substrate. Esophageal and gastric samples both contained lipolytic activity which hydrolyzed long-chain triglyceride to diglyceride, monoglyceride, and FFTA, had a pH optimum of 5.4, and was not affected by either had a pH optimum of 5.4, and was not affected by either 0.5 M NaCl or 4 mM sodium taurodexycholate. The activity, expressed as nanomoles of chylomicron triglyceride hydrolyzed per milliter per minute, ranged from 0 to 145 in upper esophageal, 5 to 303 in lower esophageal, and 50 to 357 in gastric samples. Only a trace of lipolytic activity was found at pH 5.4 in saliva collected from the parotid, submandibular, and sublingual glands, thus excluding those tissues as a source of the activity found in esophageal and gastric aspirates. The findings suggest that in man glands in or near the pharynx secrete a lipase that acts in the stomach to hydrolyze long-chain triglyceride to partial glycerides and FFA. It is proposed this reaction is the first step in the digestion of dietary fat and that the amphiphilic lipids formed by lipolysis facilitate the emulsification of triglyceride in the stomach.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORGSTROM B., DAHLQVIST A., LUNDH G., SJOVALL J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957 Oct;36(10):1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank S., Krut L. H., Marks I. N., Bronte-Stewart B., Uys P. J. Hydrolysis of fat by human gastric juice. Gut. 1964 Oct;5(5):480–484. doi: 10.1136/gut.5.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman J. A., Darnton S. J. The lipase of rat gastric mucosa. A histochemical demonstration of the enzymatic activity against a medium chain triglyceride. Gastroenterology. 1970 Jul;59(1):13–21. [PubMed] [Google Scholar]
- Bieberdorf F. A., Chernick S. S., Scow R. O. Effect of insulin and acute diabetes on plasma FFA and ketone bodies in the fasting rat. J Clin Invest. 1970 Sep;49(9):1685–1693. doi: 10.1172/JCI106386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. B., Brause B., Holt P. R. Lipolysis and absorption of fat in the rat stomach. Gastroenterology. 1969 Feb;56(2):214–222. [PubMed] [Google Scholar]
- Cohen M., Morgan R. G., Hofmann A. F. Lipolytic activity of human gastric and duodenal juice against medium and long chain triglycerides. Gastroenterology. 1971 Jan;60(1):1–15. [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- DOUGLAS G. J., Jr, REINAUER A. J., BROOKS W. C., PRATT J. H. The effect on digestion and absorption of excluding the pancreatic juice from the intestine. Gastroenterology. 1953 Mar;23(3):452–459. [PubMed] [Google Scholar]
- HENRIQUES B. L., CHAUNCEY H. H. A modified method for the collection of human submaxillary and sublingual saliva. Oral Surg Oral Med Oral Pathol. 1961 Sep;14:1124–1129. doi: 10.1016/0030-4220(61)90506-0. [DOI] [PubMed] [Google Scholar]
- Hamosh M., Clary T. R., Chernick S. S., Scow R. O. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970 Sep 8;210(3):473–482. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- Hamosh M., Scow R. O. Lingual lipase and its role in the digestion of dietary lipid. J Clin Invest. 1973 Jan;52(1):88–95. doi: 10.1172/JCI107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPPORT M. M., ALONZO N. Photometric determination of fatty acid ester groups in phospholipides. J Biol Chem. 1955 Nov;217(1):193–198. [PubMed] [Google Scholar]
- SENIOR J. R. INTESTINAL ABSORPTION OF FATS. J Lipid Res. 1964 Oct;5:495–521. [PubMed] [Google Scholar]
- Small D. M. A classification of biologic lipids based upon their interaction in aqeous systems. J Am Oil Chem Soc. 1968 Mar;45(3):108–119. doi: 10.1007/BF02915334. [DOI] [PubMed] [Google Scholar]
- Stein O., Scow R. O., Stein Y. FFA-3H uptake by perfused adipose tissue: electron microscopic autoradiographic study. Am J Physiol. 1970 Aug;219(2):510–518. doi: 10.1152/ajplegacy.1970.219.2.510. [DOI] [PubMed] [Google Scholar]


