Abstract
Numerous studies have documented that adolescents and young adults (AYAs) experience a significant cancer burden as well as significant cancer mortality compared with other age groups. The reasons for the disparate outcomes of AYAs and other age groups are not completely understood and are likely to be multifactorial, including a range of sociodemographic issues unique to these individuals as well as differences between adolescents, younger pediatric patients, and adults in the pharmacology of anticancer agents. Because adolescence is a period of transition from childhood to early adulthood, numerous physical, physiologic, cognitive, and behavioral changes occur during this time. In this review, we provide an overview of the unique developmental physiology of the adolescent and explain how these factors and the behavioral characteristics of adolescents may affect the pharmacology of anticancer agents in this patient population. Finally, we describe examples of studies that have assessed the relation between drug disposition and age, focusing on the AYA age group.
INTRODUCTION
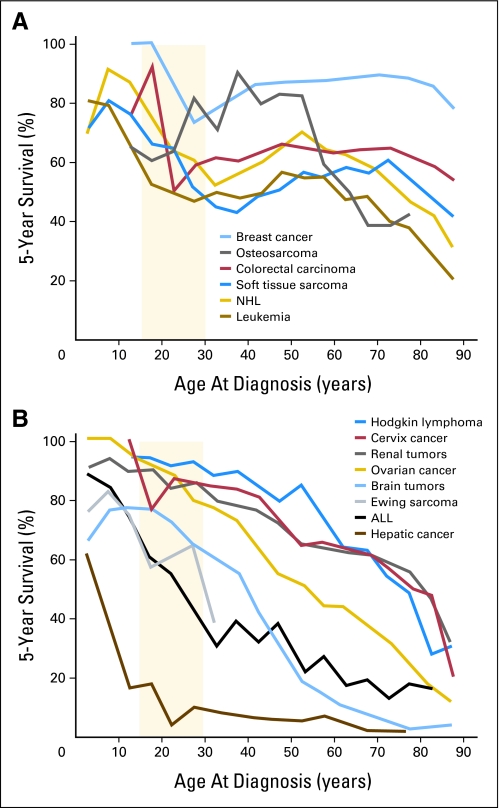
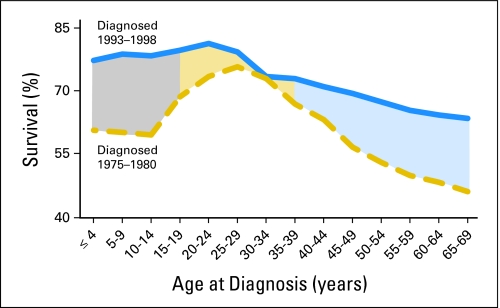
Adolescents (defined by PubMed as children who are 13 to 18 years of age) are unique in many ways. This is a statement few would disagree with, and it holds true within the field of oncology. From their most prevalent tumor types to their outcomes, adolescents are different from their younger pediatric counterparts.1–3 Adolescents and young adults (AYAs) experience a significant cancer burden and significant cancer mortality, and they generally fare poorly compared with other age groups.3,4 The second 15 years of life carries a cancer risk 2.7 times that of the first 15 years,5 and cancer is the second-most common cause of nonaccidental death among individuals age 15 to 24 years.4,6 It is now well documented that AYA patients with cancer have outcomes significantly worse than those of younger pediatric patients and, in some cancers, older adults as well. As depicted in Figure 1, AYAs have 5-year survival rates less than those of both younger pediatric patients and older adults for a wide variety of tumors, including non-Hodgkin's lymphoma and soft-tissue sarcomas, and have lower survival rates than younger patients for many malignancies, including brain tumors, renal tumors, Ewing sarcoma, and acute lymphoblastic leukemia (ALL).1,4 In addition, although overall cancer survival has increased dramatically in recent decades, patients within the AYA group have shown the least improvement.1,2,7 In the 1970s, cancer survival rates of AYAs were superior to those of both younger and older patients; however, this survival advantage decreased during subsequent years and was lost by the mid-1990s (Fig 2).4,7
Fig 1.
Five-year survival rates in adolescents and young adults compared with older and younger patients. The light orange background zone designates the age range of 15 to 29 years. (A) Cancers with survival rates that are lower in adolescents and young adults than in younger and older patients. (B) Cancers with survival rates that are lower in adolescents and young adults than in younger patients. Data adapted.4 NHL, non-Hodgkin's lymphoma; ALL, acute lymphocytic leukemia.
Fig 2.
Change in 5-year survival rates by age and treatment era. Data adapted.3 Gray, improvement in survival for patients age 0 to 14 years between 1975 and 1998; light orange, improvement in survival for patients age 15 to 39 years between 1975 and 1998; light blue, improvement in survival for patients older than 40 years between 1975 and 1998.
The reasons for the disparate outcomes of AYAs and other age groups are not completely understood and are likely to be multifactorial. A range of sociodemographic issues unique to individuals in this age group, including access to care and participation in clinical trials,6–9 are probable contributing factors, along with fundamental biologic differences in tumor and host biology.1 Differences between adolescents, younger pediatric patients, and adults in the pharmacology of anticancer agents may also influence outcomes, in terms of both the incidence and severity of adverse effects and tumor response. In addition, behavioral patterns within this age group, such as experimentation with alcohol and other drugs, can additionally influence drug disposition and outcome of treatment. In this review, we provide an overview of the unique developmental physiology of the adolescent and explain how these factors and the behavioral characteristics of adolescents may affect drug disposition. Finally, we describe examples of studies that have assessed the relation between age and anticancer drug disposition, focusing on studies in adolescents, when available.
DEVELOPMENTAL PHYSIOLOGY OF THE ADOLESCENT
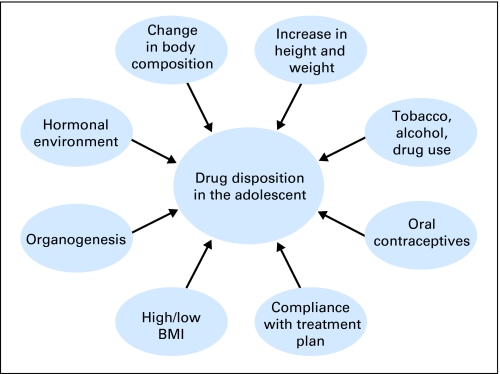
As part of the normal pubertal process, adolescents experience numerous physiologic and physical changes that can affect drug disposition (Fig 3). The onset and timing of each change varies widely within and between individuals, and groups of individuals, which emphasizes the variability in pharmacokinetics and pharmacodynamics observed during adolescence.
Fig 3.
During the normal pubertal process, adolescents experience numerous physiologic, physical, and psychosocial changes that can affect drug disposition. BMI, body mass index.
ANTHROPOMETRIC MEASURES
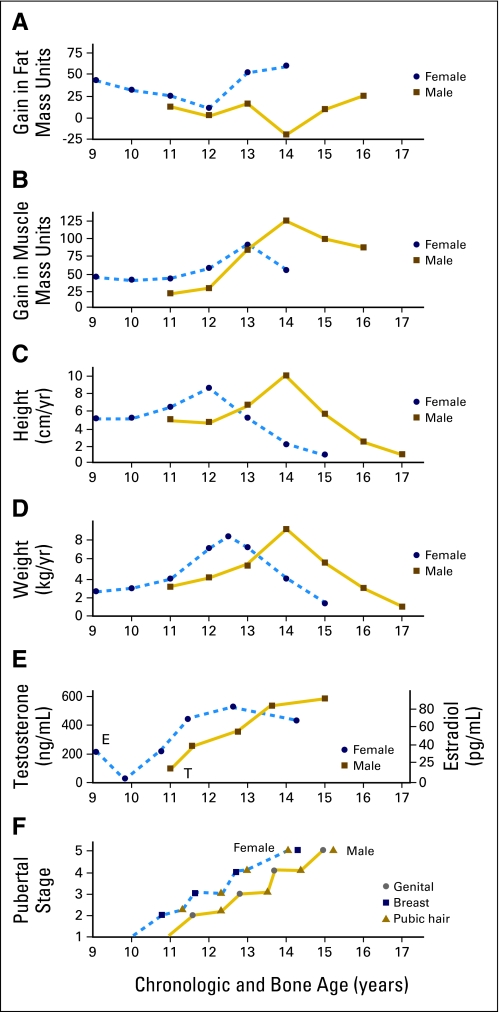
The gain in height and weight during the adolescent growth spurt accounts for approximately 25%10 and 50%,11 respectively, of adult values. As shown in Figure 4, this increase in size is accompanied by a noticeable, sex-specific change in body composition13,14 that can have important implications for drug disposition. Young women experience a greater increase in fat mass, whereas young men undergo a greater increase in fat-free mass. The end result is that adult women have a higher percentage of body fat than do men.13 In some adolescents, the increase in weight and body fat may be excessive. In a study of the prevalence of high body mass index (BMI) among children age 2 to 19 years during 2003 to 2006, those children age 12 to 19 years had the following frequency of high BMI: ≥ 97th percentile, 12.6%; greater than 95th percentile, 17.6%; and greater than 85th percentile, 34.1%.15
Fig 4.
Relation between developmental changes and chronologic and bone age. Data adapted.12
The challenges of treating obese adolescents extend to the field of oncology. Obese pediatric patients with ALL16 or acute myeloid leukemia (AML)17 have outcomes inferior to those of nonobese children. Not only can obesity influence drug disposition, primarily by affecting volume of distribution and drug clearance,18,19 but it also adds a complicating factor to drug administration. For an obese patient, it must be decided whether to calculate doses on the basis of ideal body weight, adjusted body weight, or actual body weight and whether to cap doses at a predetermined maximum. Although the use of alternative measures of weight and dose caps may reduce the risk of adverse drug effects, the possibility exists that the patient's cancer will be undertreated. However, the data regarding the influence of BMI on outcomes in pediatric patients with cancer is ultimately inconclusive. Although the studies mentioned demonstrate inferior outcomes among obese patients, a large study of pediatric patients with ALL found no association between BMI and survival, toxicity, or the pharmacokinetics of a subset of anticancer drugs.20
Another change during childhood and adolescence is the size and maturity of organs that play a role in drug metabolism. The liver and kidney increase in absolute size during adolescence,10,21 although their size in relation to total body weight decreases throughout childhood.22 In a study of 16 pediatric patients with cancer who were age 3 to 18 years, a linear increase in absolute liver weight and volume (as measured by magnetic resonance imaging) was observed as age increased23; however, liver volume relative to body weight decreased with age. The effect of the change in liver volume on the metabolism of substrates of three hepatic metabolism pathways (cytochrome P450 oxidation, glucuronidation, and biliary secretion) revealed differential effects, indicating that age-related changes in hepatic drug metabolism vary depending on the metabolic pathway. It has been suggested that not only the size of the liver but also its capacity to metabolize drugs continue to increase throughout adolescence.24 In addition, the secretory capacity of the kidney, which may also influence drug disposition, is thought to mature during this period.25
BIOCHEMICAL MEASURES
The increase in growth hormone (GH)/insulin-like growth factor 1 (IGF-1) secretion and the interplay of these hormones with sex steroids26,27 cause many physical changes during adolescence, including the obvious dramatic increase in height. During early childhood, pulsatile GH secretion occurs primarily during sleep, with only low-magnitude and low-frequency pulses during waking hours.27 As puberty progresses, the amplitude and duration of these pulses increases during both sleep and wakefulness, resulting in an increase in mean 24-hour GH levels.28 Sex differences in the timing of change in GH secretion rate have been demonstrated.28 GH secretion increases earlier in girls than in boys (pubertal stage 2 v 4) and also reaches maximal values earlier in girls than in boys (pubertal stages 3 to 4 v stage 4). These patterns are similar to the patterns of change in growth velocity and height noted in the Anthropometric Measures section, in which girls begin the growth spurt and attain peak height velocity earlier than boys (12 years v 14 years of age).11
Sex steroids play an important role in the secretion of and response to GH in both young men and young women. Testosterone augments GH secretion centrally and enhances GH activity peripherally, which leads to increased release of IGF-1 in response to GH.29 Estrogen acts to increase GH secretion by decreasing the negative-feedback inhibition of IGF-1.29 The effects of estrogen on GH action are dose dependent, with IGF-1 secretion by the liver stimulated by low doses and inhibited by high doses.26,29
Although the effect of GH on drug metabolism is not completely understood, studies have shown that exogenous GH can alter drug metabolism. One hypothesis is that this occurs by affecting the expression of drug-metabolizing enzymes.30 In one study, administration of exogenous human GH prolonged the half-life of antipyrine (a cytochrome P450 substrate) to 128% to 176% of control values in four of eight children receiving long-term GH therapy.31 Another study involving children age 10 to 14 years receiving human GH replacement indicated that the half-life of amobarbital increased, whereas that of theophylline decreased.32 Although administration of exogenous GH has been shown to affect drug metabolism, additional studies are needed to determine how the metabolism of cancer drugs is affected by the natural increase in endogenous human GH during puberty.
Adolescents experience numerous hormonal changes during puberty. These include not only changes in patterns of GH/IGF-1 secretion but also an increase in the amplitude and a change in the pattern of secretion of the gonadotropins and, hence, in sex steroid secretion.26,27 Secretion of gonadotropins, like that of GH, is pulsatile. During early puberty, the amplitude of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) night-time pulses begins to increase. During later stages of pubertal development, the pulse amplitude is increased during both sleep and waking hours, and mean LH and FSH levels increase accordingly in both young men and young women as puberty progresses.33 The increase in LH and FSH stimulates an increase in both testosterone and estradiol, leading to the development of secondary sex characteristics. These changes, like those in the GH/IGF-1 axis, begin earlier in young women than in young men. The first evidence of gonadarche is evident at an average age of 11 years in young women and 11.5 to 12 years in young men.11
As noted earlier in this section, the timing of the changes in hormone secretion, body size, and body composition during puberty is highly variable.10 Intra- and inter-individual variability in the onset and termination of these changes, in addition to the events of puberty, render adolescents a highly complex population in terms of drug disposition.
SOCIODEMOGRAPHIC CONSIDERATIONS
Sociodemographic and behavioral changes during adolescence are perhaps as dramatic as the observed physical changes and, depending on their nature and extent, can substantially affect drug disposition (Fig 3). For some, adolescence is a period of experimentation with tobacco, alcohol, and illicit drugs. These agents can cause drug-drug interactions that may alter the metabolism of anticancer drugs, potentially attenuating the antitumor effect and/or increasing the risk of adverse effects. Tobacco smoke can influence the cytochrome P450 enzyme system (inducing CYP1A1, 1A2, and possibly 2E1) and some UDP-glucuronosyltransferase isoforms.34,35 Induction of these enzymes can alter drug pharmacokinetics, whereas nicotine can affect the adrenergic system and interfere with the pharmacodynamics of other drugs. In addition, as adolescents become sexually active, the potential adverse effects of oral contraceptives must be considered. The primary estrogen in many oral contraceptives is 17α-ethinylestradiol, which is a substrate of CYP3A4, undergoes glucuronidation by UGT1A1, and is an inhibitor of CYP2B6 and CYP2C19, among others, thereby providing the potential for multiple drug interactions.36 Without specific studies examining the effects of these agents on the pharmacokinetics of each anticancer agent, it is difficult to recommend a general implementation of these principles. However, clinicians should be aware of the potential for drug interactions and should consider monitoring drug levels (when possible), with the potential for dose adjustments to be made in clinical scenarios when these interactions may impact on response or toxicity.
Finally, the rebellious nature of some adolescents and their need to demonstrate independence may reduce compliance with cancer treatment plans. A study of 46 patients with cancer who were age 2.5 to 23 years demonstrated that compliance was significantly correlated with age and that adolescent patients were less compliant compared with younger pediatric patients.37 At 20 weeks into therapy, the mean age of those who frequently missed doses of oral medications was 17.4 years, compared with 10.5 years among those who only occasionally missed doses and 9.5 years among those who never missed. Poor adherence can have serious consequences. Studies in patients with ALL have demonstrated that decreased doses of medications during maintenance therapy can lead to an increased risk of relapse.38 As more anticancer agents for oral administration become available and move to the outpatient setting, the need for oncologists to address this issue may take on greater urgency and may require creative techniques, such as that studied by Kato et al.39 In their study of AYAs with cancer, the use of a video game that addressed various behavioral issues important to participation in treatment was shown to result in improved adherence to oral medications.39
INFLUENCE OF DEVELOPMENTAL CHANGES ON DRUG DISPOSITION
In the previous section, the normal physiologic processes adolescents experience during puberty were summarized. From this section, it is clear that many of these changes have the potential to alter drug disposition (Fig 3). However, it is also clear that they occur variably among and within individuals. Therefore, a more specific discussion of the influence of developmental changes on drug disposition during adolescence will provide an insight into how the pharmacology of anticancer agents may influence outcomes in this patient population.
ABSORPTION
Drug absorption can be influenced by a number of factors that may change during the transition from childhood to adulthood, especially those that affect the gastrointestinal tract environment, including gastric pH, bile acid secretion, intestinal motility, and mucosal membrane permeability. Because adolescents and teenagers generally have a lower gastric pH than younger children, their absorption of acidic drugs, such as melphalan and chlorambucil, may be higher.40,41 Conversely, a lower pH may inhibit the active uptake of other acidic drugs, such as methotrexate. In this respect, we need to consider not only anticancer drugs themselves but also supportive ancillary agents, including antiemetics and analgesics. For example, in the case of a basic drug such as metoclopramide, older children with a lower gastric pH may experience decreased absorption. However, considering all the processes that affect drug absorption, it is reasonable to conclude that oral absorption of most drugs is similar to adults by 5 to 10 years of age.
DISTRIBUTION
The distribution of drugs given to treat cancer is largely determined by age-dependent changes in body composition. The increased water-to-fat ratio observed in infants can impact the volume of distribution, and this ratio is relatively stable afterward until puberty. Percent body fat usually declines in boys between the age of 13 to 14 years and adulthood (Fig 4), whereas an increase is seen in teenage girls; this difference may affect the distribution of anticancer drugs into adipose tissue, potentially leading to variable apparent volumes of distribution and plasma concentrations.42
Drug distribution can also be markedly influenced by the composition of circulating plasma proteins, which may play an important role in the intravascular transport of drugs to tumor cells.43 Although adolescence per se is not associated with any well-defined change in plasma proteins, both hormonal and behavioral changes, including an increased susceptibility to eating disorders, may affect drug disposition via this route. Malnutrition associated with anorexia and bulimia nervosa, most commonly seen in adolescent girls, can result in a reduced rate of protein synthesis, a decreased concentration of plasma proteins (eg, albumin and lipoprotein), and a higher plasma concentration of unbound drug.44 This factor may be particularly important in the case of cytotoxic drugs, such as cisplatin and etoposide, which are highly protein bound, because small changes in protein binding potentially lead to large changes in the fraction of free drug.45,46 In contrast, synthesis of the protein α-1-acid glycoprotein (AAG) can actually be increased in malnourished patients, which may reduce the percentage of unbound drug and possibly reduce the antitumor activity of AAG-bound drugs, such as imatinib and docetaxel.47–49
As discussed in the Anthropometric Measures section, the recent increase in teenage obesity may cause clinically relevant changes in drug disposition. The distribution of lipophilic drugs with high affinity for adipose tissue may be increased in obese (but clinically malnourished) patients, and elevated serum AAG concentrations could increase the binding of many nonacidic anticancer drugs.50,51 Altered distribution of the oxazaphosphorine drug ifosfamide has previously been reported in overweight patients.52
METABOLISM
The continued development of the liver and the maturation of drug-metabolizing enzymes during childhood and adolescence suggest that drug disposition may be significantly affected by changes in drug metabolism in this population. The expression of several drug-metabolizing enzymes has been shown to change markedly during the early stages of development, increasing from low levels in newborn children to levels in young children that may surpass those in adults, although results are less well defined in adolescents. Currently available information suggests that changes in drug-metabolizing enzyme activity are related to developmental changes, as characterized by the Tanner staging system, so that drug-metabolizing enzyme activity gradually increases during adolescence in conjunction with corresponding increases in levels of GH.24 Reports on the impact of ontogeny of drug-metabolizing enzymes have focused predominantly on those responsible for the functionalization of a drug (eg, oxidation, reduction, hydrolysis) or the so-called phase I enzymes. These reports showed that increased levels of hormones, such as estrogen and androgens, are correlated with reduced levels of CYP1A2, CYP2B6, and CYP2C19.24,53,54 CYP1A2 provides a good example of a phase I enzyme for which activity during adolescence has been studied: variation in the extent of CYP1A2-mediated demethylation of caffeine has been correlated with differences in stage of sexual maturity between teenage girls and boys.55 This finding may have implications for other drugs metabolized by CYP1A2, including the anticancer drug dacarbazine. A recent examination of methods designed to predict hepatic clearance in children as a function of age, using in vitro findings on cytochrome maturation, highlights the difficulty of predicting clearance values in older children and emphasizes the need for additional data from clinical studies.56 The inability to predict hepatic drug clearance may be particularly pertinent in adolescents, in whom a host of factors, including hormone-related variations in drug-metabolizing enzyme activity, changes in body composition, and increased liver size, may significantly affect drug disposition.
The activity of many phase II enzymes involved in drug conjugation are also noticeably reduced early in life, but teenagers and adolescents do not seem to differ significantly from adults in terms of the activity of these enzymes.57 Like differences in plasma protein concentrations, differences in the activity of drug-metabolizing enzymes may be most readily observed in adolescents affected by eating disorders and severe weight loss or gain. Increased phase I enzyme activity has been reported in patients with occurrences of chronic weight loss commonly associated with eating disorders, such as anorexia and bulimia,58 whereas the activity of phase II glucuronidation and sulfation conjugation pathways is increased with occurrences of obesity.50 Such changes may be particularly relevant in patients receiving anticancer drugs, such as irinotecan, as glucuronidation of the active metabolite plays a key role in its metabolism and is likely to influence the risk of toxicity.59 Similarly, the bifunctional alkylating agent busulfan, which is eliminated primarily via phase II glutathione conjugation, exhibits a higher apparent oral clearance in obese adolescent patients and adult patients with cancer than in those with normal body weight.60
An additional consideration is the potential impact of chronic use of anabolic steroids and other performance-enhancing drugs to increase body weight and muscle mass, a trend observed in adolescent and young adult athletes. Studies have suggested the potential for both inhibitory and inductory effects of anabolic steroids on various CYP enzymes, including CYP3A4 and CYP2C9. Indeed, in the case of CYP3A4, both inhibition and induction of activity have been reported depending on the particular anabolic steroid investigated and the CYP substrate being used.61–63 This area of research warrants additional investigation.
ELIMINATION
Plasma clearance of renally eliminated drugs is closely correlated with maturation of kidney function in children, and the age-associated relation between increasing tubular secretion and glomerular filtration rate (GFR) is well established in younger children.22,25 This factor can play a major role in determining appropriate dosing regimens; the platinum agent carboplatin provides a good example of the importance of renal function–based dosing in a pediatric setting.64 However, small differences between adolescents and adults in renal function and GFR are unlikely to significantly affect drug disposition. Therefore, although differences in drug elimination between adults and children have been reported, for predominantly renally eliminated drugs such as cyclophosphamide, these findings are likely to be explained by differences in renal function between younger children and adults as opposed to significant differences between adolescents and adults.
Changes in renal elimination should be considered in obese or malnourished AYA patients. In obese patients, cisplatin but not carboplatin clearance is increased, suggesting an increase in tubular secretion but not GFR.65 Other studies have found decreased clearance of the toxic metabolite doxorubicinol in children with greater than 30% body fat after treatment with doxorubicin.66 Malnutrition associated with eating disorders, which are most prevalent in teenage girls, can reduce perfusion of the kidneys and liver. This can lead to a reduction in drug elimination, the extent of which may depend on the free fraction of drug in the blood. Diminished hepatic blood flow can therefore attenuate the clearance of drugs for which blood flow rather than protein binding influences unbound drug clearance.44
Future studies of developmental changes in the expression of drug transporters, such as P-glycoprotein, may also be important in optimizing dosage regimens in adolescents with cancer. Interestingly, a relationship between genetic polymorphisms in the ABCB1 gene and cyclosporine pharmacokinetics is related to age and, thus, to developmental stage in children older than age 8 years.67
In summary, developmental changes in physiologic processes occur during adolescence and lead to highly variable alterations in drug disposition in this age group. Prominent differences between adolescents and younger children exist for absorption and distribution, but the differences between the two groups is less for metabolism and elimination. However, eating disorders, such as anorexia and bulimia, in addition to obesity, which are all commonly seen in the adolescent age group, may have a prominent effect on drug distribution, phase I enzyme activity, and renal elimination. Thus, it is important to perform well-designed pharmacokinetic studies of anticancer drugs in this patient population.
SELECTED EXAMPLES
The previous sections have provided a rational physiologic and sociodemographic basis for differences that have been observed in the pharmacokinetics of anticancer drugs between adolescents and young children or adults. However, it is also clear that wide inter- and intrapatient variabilities exist in the factors that affect disposition of drugs in the adolescent patient population. In this section, selected examples are presented of investigations that have studied the pharmacokinetics of anticancer drugs in children with cancer and have examined the relationship between age and drug disposition. Key findings from these studies are summarized in Table 1.
Table 1.
Summary of the Pharmacology Studies Published in Adolescents
| Drug and Study | No. of Patients | Age Range (years) | Sex |
Summary of Findings | |
|---|---|---|---|---|---|
| M | F | ||||
| Temozolomide68 | 39 | 0.7-21.9 | 20 | 19 | No difference in Cl between adolescents (older than 10 years of age) and younger children |
| Dexamethasone69 | 214 | 1.0-18.8 | 115 | 99 | Age (younger or older than 10 years) a significant predictor of Cl in children with low albumin; lower apparent oral Cl in adolescents consistent with increased toxicity |
| Topotecan70 | 162 | 0.04-22 | 110 | 52 | No differences in systemic Cl between adolescents and younger children |
| Vincristine71 | 54 | 0.2-18 | NA | NA | Cl normalized to BW differed significantly between adolescents and children younger than 10 years of age |
| Vincristine72 | 98 | 1.3-17.3 | NA | NA | No relationship between age and pharmacokinetics |
| Imatinib73 | 26 | 7.0-24 | 17 | 9 | Median Cl greater in children older than 12 years of age, but no statistically significant correlation with age observed |
| Imatinib73 | 15 | 6.0-22 | NA | NA | |
| Etoposide86 | 29 | 1.58-23.9 | NA | NA | No correlation between age and etoposide Cl after adjusting for body size |
| Etoposide76 | 16 | 0.3-22 | NA | NA | AUC normalized to BW correlated to age |
| Etoposide75 | 31 | 0.8-23.7 | NA | NA | No correlation between Cl normalized for BSA and age, but significant inverse relationship between Cl normalized for BW and age |
| Etoposide74 | 18 | 1.1-17 | 10 | 8 | Inverse relationship between Cl and age |
| Etoposide77 | 109 | 0.4-18.7 | NA | NA | Age negatively correlated with Cl normalized for BSA |
| Methotrexate79 | 134 | 0.33-18.5 | 76 | 58 | Age negatively correlated with plasma concentration |
| Methotrexate80 | 122 | 0.25-15 | NA | NA | Cl faster in children younger than 10 years of age than in adolescents |
| Methotrexate81 | 49 | 0.5-17 | NA | NA | Cl in children younger than 10 years of age two-fold greater than in those older than 10 years of age |
| Actinomycin D82 | 31 | 1.0-20 | 18 | 13 | Higher AUC in younger children (< 40 kg) than in adolescents |
| Busulfan83 | 27 | 1.3-50 | 10 | 17 | Shorter elimination half-life and higher clearance values in children younger than 5 years of age versus children age 5-16 years |
| Busulfan96 | 25 | 0.5-54 | 15 | 10 | Significant increase in busulfan apparent oral clearance expressed relative to body surface area in children age 0.5-4 years versus older children (≥ 12 years of age) |
Abbreviations: NA, not available; Cl, clearance; BW, body weight; AUC, area under the curve; BSA, body-surface area.
Temozolomide
Panetta et al68 reported the population pharmacokinetics of temozolomide, a highly bioavailable methylating agent that undergoes spontaneous base-catalyzed hydrolysis to form the methyl triazene, MTIC. They studied 39 children with primary CNS tumors treated with various temozolomide dosages. The pharmacokinetics of temozolomide and its active metabolite MTIC were modeled by using linear mixed-effects modeling. Although temozolomide apparent oral clearance was directly related to body-surface area (BSA) and was positively correlated with age, no apparent difference was noted between adolescents (> 10 years of age) and younger children.
Dexamethasone
In a recent study, Yang et al69 studied 214 children with ALL enrolled on an institutional protocol (Total XV) that included therapy with oral dexamethasone (8 mg/m2). Pharmacokinetic studies evaluated numerous covariates using linear mixed-effects modeling. The relation between patient covariates and dexamethasone apparent oral clearance was evaluated additionally by using a classification and regression-tree analysis. Serum albumin was found to be the most significant predictor of dexamethasone apparent clearance, and age (less than v greater than 10 years) was a significant predictor of dexamethasone apparent oral clearance in children with low albumin. Although the treatment group (low risk v standard/high-risk) complicated the interpretation of the effect of age on clearance, the results of the multivariate analyses suggested that age had an independent effect. This model predicted a dexamethasone apparent oral clearance of 7.8 L/h/m2 for an adolescent age 19 years versus 15.8 L/h/m2 for a child age 5 years, which is consistent with a greater incidence of toxicity in the older children.
Topotecan
Schaiquevich et al70 reported a population pharmacokinetic analysis of topotecan, which is primarily (60% to 70%) cleared through renal excretion. The remainder of topotecan elimination occurs primarily through biliary or hepatic mechanisms. These investigators studied topotecan lactone disposition in 162 children enrolled on six phase I/II clinical trials. They found that BSA was related to topotecan clearance, which explained 54% of the inter-individual variability. Other significant covariates included GFR, phenytoin coadministration, and age only when analyzed as a categoric variable (ie, < 0.5 v > 0.5 years). Thus, for topotecan, a renally eliminated drug, no differences in systemic clearance were noted between adolescents and younger children.
Vincristine
Although results from early studies suggested that vincristine clearance differed in adolescents versus other pediatric age groups, more recent studies have shown otherwise. Biliary excretion is the primary route of elimination for vincristine, and some metabolism is via the cytochrome P450 system (eg, CYP3A4). Crom et al71 studied 54 children with ALL enrolled on an institutional clinical trial (Total XI) that included vincristine administered at a dosage of 1.5 mg/m2. Plasma concentrations of vincristine were analyzed by using a specific high-performance liquid chromatography method, and the data were analyzed by using a MAP-Bayesian approach. Mean vincristine clearance normalized to body weight and BSA was compared among infants, children, and adolescents. Although no difference was noted among the three groups in clearance normalized to BSA, clearance normalized to body weight differed significantly between adolescents and children younger than age 10 years. However, a more recent study in 98 children age 1.3 to 17.3 years with ALL showed no relation between age and vincristine pharmacokinetics.72
Imatinib
The population pharmacokinetics of imatinib mesylate and its active metabolite (CGP/74,588) were investigated in 41 children and young adults receiving oral imatinib (260 to 570 mg/m2).73 Body weight was the only covariate found to be significantly related to imatinib apparent clearance. The median apparent clearance value was 5.1 L/h for children younger than 12 years of age, and it was 10.3 L/h for those older than 12 years of age. However, no statistically significant association with age was observed, and no difference between the two age groups was noted.
Etoposide
Numerous investigators have studied the disposition of etoposide, a topoisomerase II inhibitor.74-77,84-87 Etoposide is metabolized in the liver, and both parent compound and metabolites are primarily renally eliminated with some biliary excretion. Numerous investigators have reported etoposide pharmacokinetics in children with cancer, but few have reported data regarding the relationship between etoposide disposition and age. None of the pharmacokinetic studies were designed to specifically evaluate differences in etoposide clearance between young children and adolescents.
Rodman et al86 studied etoposide disposition in 29 pediatric patients undergoing autologous bone marrow transplantation given high-dose etoposide and carboplatin. Patients were treated with etoposide as a 6-hour infusion at escalating dosages (320, 400, and 500 mg/m2 daily for 3 days), and pharmacokinetic studies were conducted after the end of the infusion on days 2 and 3. No correlation was found between age and etoposide clearance after adjusting clearance for body size. Eksborg et al76 studied 16 pediatric patients (age 4 months to 22 years) treated with single-agent etoposide over a range of dosages (32 to 210 mg/m2) as a short infusion (1 to 3 hours). These investigators reported that AUC (area under the curve; dose normalized by BSA), which is equivalent to inverse clearance, was independent of age in this patient population. However, AUC normalized to body weight was directly correlated to age. Wurthwein et al75 reported etoposide disposition in 31 children and young adults (age 0.8 to 23.7 years) undergoing bone marrow transplantation and receiving a multidrug preparation regimen that included high-dosage etoposide (40 mg/kg). These investigators reported no correlation between etoposide systemic clearance normalized for BSA and age; however, as Ekborg et al76 reported, they found a significant inverse relationship between etoposide clearance normalized for body weight and age.
Two other studies have reported an inverse relationship between etoposide clearance and age, with a higher etoposide clearance observed in younger compared with older patients.74,77 Sonnichsen et al74 studied the disposition of an intravenous preparation of etoposide administered orally in 18 children with solid tumors. They performed exploratory regression analyses to determine if patient characteristics were associated with etoposide clearance, and they found both age and albumin to be inversely related. Kishi et al77 studied 109 patients enrolled on an institutional clinical trial (Total XIIIB) that included a 2-hour intravenous infusion of etoposide (300 mg/m2). Pharmacokinetic covariates were evaluated by using linear mixed-effects modeling. Age was negatively correlated with etoposide clearance normalized for BSA (ie, lower clearance in older children). Interestingly, the authors found that age was not related to clearance when the etoposide clearance was induced by glucocorticoids. The authors speculated that young children may have a greater capacity to clear etoposide and may be less susceptible than adolescents and adults to induction of etoposide clearance by glucocorticoids.
Methotrexate
The folate analog methotrexate inhibits dihydrofolate reductase, which leads to a decrease in the production in tetrahydrofolic acid, which interferes with DNA, RNA, and protein synthesis. Methotrexate is excreted mainly as unchanged drug in the urine, with small amounts excreted in the bile and feces.78 Its disposition has been extensively studied in populations of patients with different tumors (eg, ALL, osteosarcoma), receiving various dosages, and in different age groups (eg, infants, children, adolescents).79–81,88–93 However, limited data exist pertaining to the relationship between methotrexate clearance and age, and pharmacokinetic studies designed to evaluate whether AYAs have different methotrexate clearances than children or older adults have not been reported.
Of those published pharmacokinetic studies that have reported age as related to methotrexate clearance, the overall impression is that clearance is inversely related to age (ie, clearance is faster in younger than in older patients). Relling et al79 were the first to report this in a study, in which they examined factors associated with high-risk methotrexate plasma concentration (defined as 42-hour concentration > 1 μmol/L) and toxicity in 134 children with ALL. Among children receiving high-dose methotrexate (ie, 0.9 to 3.7 g/m2 intravenously over 24 hours), age was negatively correlated with 42-hour methotrexate plasma concentration (ie, younger children had faster clearances than older children). Donelli et al80 studied methotrexate pharmacokinetics in 122 children with non–B-cell ALL receiving methotrexate 5 g/m2 as a 24-hour infusion. They reported that methotrexate clearance was faster in children younger than 10 years of age than in adolescents older than 10 years. Moreover, they investigated this relationship as a continuous variable and found that methotrexate clearance decreased with increasing age. Aumente et al81 studied the population pharmacokinetics of methotrexate in 49 children with ALL receiving methotrexate 3 g/m2 as a 24-hour infusion. Their final population model included both age and total body weight as covariates related to methotrexate clearance. As with the study by Donelli et al80, these investigators used 10 years of age as a cut point for their analysis and found that the methotrexate clearance in the younger patients (< 10 years) was approximately two-fold greater than in the older patients (> 10 years). Colom et al89 studied the population pharmacokinetics of methotrexate in 14 patients with osteosarcoma receiving 209 cycles of methotrexate therapy. These patients received an average methotrexate dosage of 11.2 g/m2 over 4 hours, and limited sampling was performed during and after the infusion. The final population model for methotrexate clearance included both age and body weight; on the basis of this model, a patient age 15 years weighing 50 kg would have a methotrexate clearance of 4.8 L/h (or approximately 54 mL/min/m2). Although this was a small study for a population analysis, the authors did validate their results in a separate population of 10 new patients. A number of studies have found that children exhibit more rapid methotrexate clearance than adults, and a general trend toward decreasing clearance with increasing age has been observed. However, as with many anticancer drugs, substantial inter- and intra-individual variability has been observed, and significant overlap in clearance values has been determined in children and adults.
Dactinomycin
The antitumor antibiotic dactinomycin has been the focus of a limited number of pharmacokinetic studies, but only one study was published in a pediatric patient population.82 This study involved 31 children receiving dactinomycin (0.7 to 1.5 mg/m2), including 12 patients age 10 to 20 years. Although insufficient data were generated in this study to fully characterize the three-compartment model used to fit the data, a wide range of dactinomycin plasma concentrations were observed. Exposure to dactinomycin was generally lower in the larger adolescent patients than in younger children, which indicated that the practice of capping the dactinomycin dose at 2 mg may result in underdosing in older children.
Busulfan
Several studies published over a number of years have investigated the clinical pharmacology of the bifunctional alkylating agent busulfan in a pediatric setting.60,83,94,95 Of particular relevance to drug disposition in adolescents, Hassan et al83 described the relationship between busulfan pharmacokinetics and age in three patient groups: children age ≤ 5 years, children age 5 to 16 years, and adults age older than 16 to 50 years. Data from 27 patients determined a shorter elimination half-life in children age younger than 5 years compared with older children and adults (2.05 hours v 2.79 hours and 2.59 hours, respectively). Similarly, older children exhibited total body clearances comparable to adult patients (3.02 and 2.7 mL/min/kg, respectively), and significantly higher clearance values were determined in the younger group of children (7.3 mL/min/kg). These findings are supported by results from a study involving 14 children (age 0.5 to 4 years) and 11 older children and adults (age 12 to 54 years), which showed a significant increase in busulfan apparent oral clearance expressed relative to BSA in the group of younger children.96 This study proposed that the higher busulfan clearance observed in younger children could be explained by an enhanced ability to metabolize the drug through glutathione conjugation. More recently, studies involving larger patient numbers have also highlighted statistically significant correlations between age and pharmacokinetic parameters, including busulfan AUC, clearance, and half-life.95
As noted in the Selected Examples section, the pharmacokinetics of many anticancer drugs have been studied in different settings (eg, different tumors, ranges of dosages, different age groups). The relation between age and drug clearance has been reported for several drugs (eg, dexamethasone, methotrexate), but the results are often conflicting (eg, vincristine). For most anticancer drugs, pharmacokinetic studies are conducted during early drug development (ie, phase I studies); typically, these studies are small and are not designed to evaluate the relationship between age and drug clearance. More recently, modifications to the phase I study design have enabled investigators to study more children of specified age ranges, which ultimately will enhance the ability to detect relationships between age and drug disposition.
In conclusion, although much is known about the changes that occur leading up to and during adolescence in the physiologic and sociodemographic processes that impact drug disposition, the actual changes that occur in anticancer drug disposition during this time are less well documented. Although some anticancer drugs demonstrate a clear relationship between drug disposition and age, no such relationship has been observed for other drugs (eg, temozolomide, topotecan). However, data from these studies must be interpreted carefully, because most studies were not specifically designed to evaluate for this relationship. Moreover, many other aspects of study methodology must be carefully considered, including study population (eg, numbers of patients, disease state), drug dosage, drug administration (eg, route, duration of infusion, bolus v continuous infusion), pharmacokinetic analysis, and statistical analysis.
Only with carefully designed studies will investigators have the ability to assess the effect of age on anticancer drug disposition and then additionally on drug effect (eg, toxicity or antitumor effect). It is important to improve our knowledge in this area by coordinating well-planned clinical pharmacology studies in pediatric patient populations. Advances in limited sampling strategies, population pharmacokinetic modeling, and the development of assays that use minimal sample volume to maximize patient recruitment will facilitate such studies. We can then look forward to the development of more appropriate approaches to chemotherapy dosing in AYAs.
Supplementary Material
Footnotes
Supported by Public Health Service, National Institute of Health Grants No. CA23099 and CA21765; American Lebanese Syrian Associated Charities; Research Councils United Kingdom; and Cancer Research United Kingdom.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Manuscript writing: Gareth J. Veal, Christine M. Hartford, Clinton F. Stewart
Final approval of manuscript: Gareth J. Veal, Christine M. Hartford, Clinton F. Stewart
REFERENCES
- 1.Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8:288–298. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer A. Older adolescents with cancer in North America deficits in outcome and research. Pediatr Clin North Am. 2002;49:1027–1042. doi: 10.1016/s0031-3955(02)00035-4. [DOI] [PubMed] [Google Scholar]
- 3.Adolescent and Young Adult Oncology Progress Review Group: Closing the Gap. Research and Care Imperatives for Adolescents and Young Adults with Cancer. Bethesda, MD: Department of Health and Human Services; 2006. National Institutes of Health, National Cancer Institute, and the LiveStrong Young Adult Alliance. [Google Scholar]
- 4.Bleyer A. Young adult oncology: The patients and their survival challenges. CA Cancer J Clin. 2007;57:242–255. doi: 10.3322/canjclin.57.4.242. [DOI] [PubMed] [Google Scholar]
- 5.Bleyer A. Adolescent and young adult (AYA) oncology: The first A. Pediatr Hematol Oncol. 2007;24:325–336. doi: 10.1080/08880010701316850. [DOI] [PubMed] [Google Scholar]
- 6.Howell DL, Ward KC, Austin HD, et al. Access to pediatric cancer care by age, race, and diagnosis, and outcomes of cancer treatment in pediatric and adolescent patients in the state of Georgia. J Clin Oncol. 2007;25:4610–4615. doi: 10.1200/JCO.2006.07.6992. [DOI] [PubMed] [Google Scholar]
- 7.Albritton KH, Wiggins CH, Nelson HE, et al. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25:4616–4621. doi: 10.1200/JCO.2006.08.4103. [DOI] [PubMed] [Google Scholar]
- 8.Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110:2385–2393. doi: 10.1002/cncr.23060. [DOI] [PubMed] [Google Scholar]
- 9.Albritton KH, Eden T. Access to care. Pediatr Blood Cancer. 2008;50:1094–1098. doi: 10.1002/pbc.21461. [DOI] [PubMed] [Google Scholar]
- 10.Paul IM, Levine RL. Drug Therapy in the Adolescent. In: Yaffe SY, Aranda JV, editors. Neonatal and Pediatric Pharmacology: Therapeutic Principles in Practice (ed 3) Philadelphia, PA: Lippincott Williams and Wilkins; 2005. pp. 308–325. [Google Scholar]
- 11.Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002;31:192–200. doi: 10.1016/s1054-139x(02)00485-8. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein JW. The effect of developmental changes in adolescence on drug disposition. J Adolesc Health. 1994;15:612–618. doi: 10.1016/s1054-139x(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 13.Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–15. doi: 10.1097/med.0b013e328320d54c. [DOI] [PubMed] [Google Scholar]
- 14.Siervogel RM, Demerath EW, Schubert C, et al. Puberty and body composition. Horm Res. 2003;60:36–45. doi: 10.1159/000071224. [DOI] [PubMed] [Google Scholar]
- 15.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 16.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25:2063–2069. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 17.Lange BJ, Gerbing RB, Feusner J, et al. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293:203–211. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]
- 18.Blouin RA, Warren GW. Pharmacokinetic considerations in obesity. J Pharm Sci. 1999;88:1–7. doi: 10.1021/js980173a. [DOI] [PubMed] [Google Scholar]
- 19.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–231. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 20.Hijiya N, Panetta JC, Zhou Y, et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood. 2006;108:3997–4002. doi: 10.1182/blood-2006-05-024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane JR, Connor JD. The influence of endogenous and exogenous sex hormones in adolescents with attention to oral contraceptives and anabolic steroids. J Adolesc Health. 1994;15:630–634. doi: 10.1016/s1054-139x(94)90629-7. [DOI] [PubMed] [Google Scholar]
- 22.Chen N, Aleksa K, Woodland C, et al. Ontogeny of drug elimination by the human kidney. Pediatr Nephrol. 2006;21:160–168. doi: 10.1007/s00467-005-2105-4. [DOI] [PubMed] [Google Scholar]
- 23.Murry DJ, Crom WR, Reddick WE, et al. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab Dispos. 1995;23:1110–1116. [PubMed] [Google Scholar]
- 24.Kennedy M. Hormonal regulation of hepatic drug-metabolizing enzyme activity during adolescence. Clin Pharmacol Ther. 2008;84:662–673. doi: 10.1038/clpt.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linday LA. Developmental changes in renal tubular function. J Adolesc Health. 1994;15:648–653. doi: 10.1016/s1054-139x(94)90632-7. [DOI] [PubMed] [Google Scholar]
- 26.Clark PA, Rogol AD. Growth hormones and sex steroid interactions at puberty. Endocrinol Metab Clin North Am. 1996;25:665–681. doi: 10.1016/s0889-8529(05)70346-7. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein J. Endocrine physiology at puberty. In: Friedman SB FM, Schonberg SK, Alderman EM, editors. Comprehensive Adolescent Health Care (ed 2) S. Louis: MO, Mosby; 1998. pp. 23–28. [Google Scholar]
- 28.Albertsson-Wikland K, Rosberg S, Karlberg J, et al. Analysis of 24-hour growth hormone profiles in healthy boys and girls of normal stature: Relation to puberty. J Clin Endocrinol Metab. 1994;78:1195–1201. doi: 10.1210/jcem.78.5.8175978. [DOI] [PubMed] [Google Scholar]
- 29.Meinhardt UJ, Ho KK. Modulation of growth hormone action by sex steroids. Clin Endocrinol (Oxf) 2006;65:413–422. doi: 10.1111/j.1365-2265.2006.02676.x. [DOI] [PubMed] [Google Scholar]
- 30.Leeder JS, Kearns GL. Pharmacogenetics in pediatrics: Implications for practice. Pediatr Clin North Am. 1997;44:55–77. doi: 10.1016/s0031-3955(05)70463-6. [DOI] [PubMed] [Google Scholar]
- 31.Rifkind AB, Saenger P, Levine LS, et al. Effects of growth hormone on antipyrine kinetics in children. Clin Pharmacol Ther. 1981;30:127–132. doi: 10.1038/clpt.1981.137. [DOI] [PubMed] [Google Scholar]
- 32.Redmond GP, Bell JJ, Nichola PS, et al. Effect of growth hormone on human drug metabolism: Time course and substrate specificity. Pediatr Pharmacol (New York) 1980;1:63–70. [PubMed] [Google Scholar]
- 33.Oerter KE, Uriarte MM, Rose SR, et al. Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. 1990;71:1251–1258. doi: 10.1210/jcem-71-5-1251. [DOI] [PubMed] [Google Scholar]
- 34.Kroon LA. Drug interactions with smoking. Am J Health Syst Pharm. 2007;64:1917–1921. doi: 10.2146/ajhp060414. [DOI] [PubMed] [Google Scholar]
- 35.Zevin S, Benowitz NL. Drug interactions with tobacco smoking: An update. Clin Pharmacokinet. 1999;36:425–438. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Cui D, Wang B, et al. Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: A new look at an old drug. Clin Pharmacokinet. 2007;46:133–157. doi: 10.2165/00003088-200746020-00003. [DOI] [PubMed] [Google Scholar]
- 37.Tebbi CK, Cummings KM, Zevon MA, et al. Compliance of pediatric and adolescent cancer patients. Cancer. 1986;58:1179–1184. doi: 10.1002/1097-0142(19860901)58:5<1179::aid-cncr2820580534>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 38.Pritchard MT, Butow PN, Stevens MM, et al. Understanding medication adherence in pediatric acute lymphoblastic leukemia: A review. J Pediatr Hematol Oncol. 2006;28:816–823. doi: 10.1097/01.mph.0000243666.79303.45. [DOI] [PubMed] [Google Scholar]
- 39.Kato PM, Cole SW, Bradlyn AS, et al. A video game improves behavioral outcomes in adolescents and young adults with cancer: A randomized trial. Pediatrics. 2008;122:e305–e317. doi: 10.1542/peds.2007-3134. [DOI] [PubMed] [Google Scholar]
- 40.Mikkelsen RB, Asher C, Hicks T. Extracellular pH, transmembrane distribution and cytotoxicity of chlorambucil. Biochem Pharmacol. 1985;34:2531–2534. doi: 10.1016/0006-2952(85)90538-6. [DOI] [PubMed] [Google Scholar]
- 41.Skarsgard L, Skwarchuk M, Vinczan A, et al. The cytotoxicity of melphalan and its relationship to pH, hypoxia and drug uptake. Anticancer Res. 1995;15:219–223. [PubMed] [Google Scholar]
- 42.Kreipe R. Normal somatic adolescent growth and development. In: McAnarney E, Kreipe R, Orr D, editors. Textbook of Adolescent Medicine. Philadelphia, PA: Saunders WB; 1992. p. 57. [Google Scholar]
- 43.Ehrnebo M, Agurell S, Jalling B, et al. Age differences in drug binding by plasma proteins: Studies on human foetuses, neonates and adults. Eur J Clin Pharmacol. 1971;3:189–193. doi: 10.1007/BF00565004. [DOI] [PubMed] [Google Scholar]
- 44.Gura K, Chan L-N. Drug therapy and the role of nutrition. In: Walker W, Duggan C, Watkins J, editors. Nutrition in Pediatrics: Basic Science and Clinical Applications (ed 3) Lewiston, NY: BC Decker; 2003. p. 234. [Google Scholar]
- 45.Gullo JJ, Litterst CL, Maguire PJ, et al. Pharmacokinetics and protein binding of cis-dichlorodiammine platinum (II) administered as a one hour or as a twenty hour infusion. Cancer Chemother Pharmacol. 1980;5:21–26. doi: 10.1007/BF00578558. [DOI] [PubMed] [Google Scholar]
- 46.Liu B, Earl HM, Poole CJ, et al. Etoposide protein binding in cancer patients. Cancer Chemother Pharmacol. 1995;36:506–512. doi: 10.1007/BF00685801. [DOI] [PubMed] [Google Scholar]
- 47.Chan L-N. Drug-nutrient interactions in transplant recipients. J Parenter Enteral Nutr. 2001;25:132–141. doi: 10.1177/0148607101025003132. [DOI] [PubMed] [Google Scholar]
- 48.Bruno R, Olivares R, Berille J. Alpha-1-acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9:1077–1082. [PubMed] [Google Scholar]
- 49.Delbaldo C, Chatelut E, Ré M, et al. Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2006;12:6073–6078. doi: 10.1158/1078-0432.CCR-05-2596. [DOI] [PubMed] [Google Scholar]
- 50.Blouin RA, Kolpek JH, Mann HJ. Influence of obesity on drug disposition. Clin Pharm. 1987;6:706–714. [PubMed] [Google Scholar]
- 51.Baker SD, Grochow LB, Donehower RC. Should anticancer drug doses be adjusted in the obese patient? J Natl Cancer Inst. 1995;87:333–334. doi: 10.1093/jnci/87.5.333. [DOI] [PubMed] [Google Scholar]
- 52.Lind MJ, Margison JM, Cerny T, et al. Prolongation of ifosfamide elimination half-life in obese patients due to altered drug distribution. Cancer Chemother Pharmacol. 1989;25:139–142. doi: 10.1007/BF00692355. [DOI] [PubMed] [Google Scholar]
- 53.Hines R, McCarver D. The ontogeny of human drug-metabolizing enzymes: Phase I oxidative enzymes. J Pharmacol. 2002;300:355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- 54.Rogers AS. The role of cytochrome P450 in developmental pharmacology. J Adolesc Health. 1994;15:635–640. doi: 10.1016/s1054-139x(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 55.Lambert GH, Schoeller DA, Kotake AN, et al. The effect of age, gender, and sexual maturation on the caffeine breath test. Dev Pharmacol Ther. 1986;9:375–388. doi: 10.1159/000457262. [DOI] [PubMed] [Google Scholar]
- 56.Björkman S. Prediction of cytochrome P450-mediated hepatic drug clearance in neonates, infants and children: How accurate are available scaling methods? Clin Pharmacokinet. 2006;45:1–11. doi: 10.2165/00003088-200645010-00001. [DOI] [PubMed] [Google Scholar]
- 57.McCarver D, Hines R. The ontogeny of human drug-metabolizing enzymes: Phase II conjugation enzymes and regulatory mechanisms. J Pharmacol. 2002;300:361–366. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- 58.Oshikoya KA, Senbanjo IO. Pathophysiological changes that affect drug disposition in protein-energy malnourished children. Nutr Metab. 2009;6:50–57. doi: 10.1186/1743-7075-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iyer L, King CD, Whitington PF, et al. Genetic predisposition to the metabolism of irinotecan (CPT-11): Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest. 1998;101:847–854. doi: 10.1172/JCI915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibbs JP, Gooley T, Corneau B, et al. The impact of obesity and disease on busulfan oral clearance in adults. Blood. 1999;93:4436–4440. [PubMed] [Google Scholar]
- 61.Acharjee BK, Mahanta R. Enhanced hepatic and kidney cytochrome p-450 activities in nandrolone decanoate treated albino mice. Drug Metab Lett. 2009;3:120–124. doi: 10.2174/187231209788654054. [DOI] [PubMed] [Google Scholar]
- 62.Zhang YY, Yang L. Interactions between human cytochrome P450 enzymes and steroids: Physiological and pharmacological implications. Expert Opin Drug Metab Toxicol. 2009;5:621–629. doi: 10.1517/17425250902967648. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura H, Nakasa H, Ishii I, et al. Effects of endogenous steroids on CYP3A4-mediated drug metabolism by human liver microsomes. Drug Metab Dispos. 2002;30:534–540. doi: 10.1124/dmd.30.5.534. [DOI] [PubMed] [Google Scholar]
- 64.Thomas H, Boddy AV, English MW, et al. Prospective validation of renal function-based carboplatin dosing in children with cancer: A United Kingdom Children's Cancer Study Group trial. J Clin Oncol. 2000;18:3614–3621. doi: 10.1200/JCO.2000.18.21.3614. [DOI] [PubMed] [Google Scholar]
- 65.Sparreboom A, Wolff AC, Mathijssen RH, et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol. 2007;25:4707–4713. doi: 10.1200/JCO.2007.11.2938. [DOI] [PubMed] [Google Scholar]
- 66.Thompson P, Rosner G, Matthay K, et al. Impact of body composition on pharmacokinetics of doxorubicin in children: A Glaser Pediatric Research Network study. Cancer Chemother Pharmacol. 2009;64:243–251. doi: 10.1007/s00280-008-0854-z. [DOI] [PubMed] [Google Scholar]
- 67.Hesselink DA, van Schaik RH, Nauta J, et al. A drug transporter for all ages? ABCB1 and the developmental pharmacogenetics of cyclosporine. Pharmacogenomics. 2008;9:783–789. doi: 10.2217/14622416.9.6.783. [DOI] [PubMed] [Google Scholar]
- 68.Panetta JC, Kirstein MN, Gajjar A, et al. Population pharmacokinetics of temozolomide and metabolites in infants and children with primary central nervous system tumors. Cancer Chemother Pharmacol. 2003;52:435–441. doi: 10.1007/s00280-003-0670-4. [DOI] [PubMed] [Google Scholar]
- 69.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26:1932–1939. doi: 10.1200/JCO.2007.13.8404. [DOI] [PubMed] [Google Scholar]
- 70.Schaiquevich P, Panetta JC, Iacono LC, et al. Population pharmacokinetic analysis of topotecan in pediatric cancer patients. Clin Cancer Res. 2007;13:6703–6711. doi: 10.1158/1078-0432.CCR-07-1376. [DOI] [PubMed] [Google Scholar]
- 71.Crom WR, de Graaf SS, Synold T, et al. Pharmacokinetics of vincristine in children and adolescents with acute lymphocytic leukemia. J Pediatr. 1994;125:642–649. doi: 10.1016/s0022-3476(94)70027-3. [DOI] [PubMed] [Google Scholar]
- 72.Frost BM, Lönnerholm G, Koopmans P, et al. Vincristine in childhood leukaemia: No pharmacokinetic rationale for dose reduction in adolescents. Acta Paediatr. 2003;92:551–557. [PubMed] [Google Scholar]
- 73.Menon-Andersen D, Mondick JT, Jayaraman B, et al. Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults. Cancer Chemother Pharmacol. 2009;63:229–238. doi: 10.1007/s00280-008-0730-x. [DOI] [PubMed] [Google Scholar]
- 74.Sonnichsen DS, Ribeiro RC, Luo X, et al. Pharmacokinetics and pharmacodynamics of 21-day continuous oral etoposide in pediatric patients with solid tumors. Clin Pharmacol Ther. 1995;58:99–107. doi: 10.1016/0009-9236(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 75.Würthwein G, Klingebiel T, Krümpelmann S, et al. Population pharmacokinetics of high-dose etoposide in children receiving different conditioning regimens. Anticancer Drugs. 2002;13:101–110. doi: 10.1097/00001813-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Eksborg S, Söderhäll S, Frostvik-Stolt M, et al. Plasma pharmacokinetics of etoposide (VP-16) after i.v. administration to children. Anticancer Drugs. 2000;11:237–241. doi: 10.1097/00001813-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Kishi S, Yang W, Boureau B, et al. Effects of prednisone and genetic polymorphisms on etoposide disposition in children with acute lymphoblastic leukemia. Blood. 2004;103:67–72. doi: 10.1182/blood-2003-06-2105. [DOI] [PubMed] [Google Scholar]
- 78.Schmiegelow K. Advances in individual prediction of methotrexate toxicity: A review. Br J Hem. 2009;146:489–503. doi: 10.1111/j.1365-2141.2009.07765.x. [DOI] [PubMed] [Google Scholar]
- 79.Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12:1667–1672. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 80.Donelli MG, Zucchetti M, Robatto A, et al. Pharmacokinetics of HD-MTX in infants, children, and adolescents with non-B acute lymphoblastic leukemia. Med Pediatr Oncol. 1995;24:154–159. doi: 10.1002/mpo.2950240303. [DOI] [PubMed] [Google Scholar]
- 81.Aumente D, Buelga DS, Lukas JC, et al. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45:1227–1238. doi: 10.2165/00003088-200645120-00007. [DOI] [PubMed] [Google Scholar]
- 82.Veal GJ, Cole M, Errington J, et al. Pharmacokinetics of dactinomycin in a pediatric patient population: A United Kingdom Children's Cancer Study Group Study. Clin Cancer Res. 2005;11:5893–5899. doi: 10.1158/1078-0432.CCR-04-2546. [DOI] [PubMed] [Google Scholar]
- 83.Hassan M, Oberg G, Bekassy AN, et al. Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol. 1991;28:130–134. doi: 10.1007/BF00689702. [DOI] [PubMed] [Google Scholar]
- 84.Sinkule JA, Hutson P, Hayes FA, et al. Pharmacokinetics of etoposide (VP16) in children and adolescents with refractory solid tumors. Cancer Res. 1984;44:3109–3113. [PubMed] [Google Scholar]
- 85.Lowis SP, Pearson AD, Newell DR, et al. Etoposide pharmacokinetics in children: The development and prospective validation of a dosing equation. Cancer Res. 1993;53:4881–4889. [PubMed] [Google Scholar]
- 86.Rodman JH, Murry DJ, Madden T, et al. Altered etoposide pharmacokinetics and time to engraftment in pediatric patients undergoing autologous bone marrow transplantation. J Clin Oncol. 1994;12:2390–2397. doi: 10.1200/JCO.1994.12.11.2390. [DOI] [PubMed] [Google Scholar]
- 87.Palle J, Frost BM, Britt-Marie F, et al. Etoposide pharmacokinetics in children treated for acute myeloid leukemia. Anticancer Drugs. 2006;17:1087–1094. doi: 10.1097/01.cad.0000231470.54288.49. [DOI] [PubMed] [Google Scholar]
- 88.Plard C, Piard C, Bressolle F, et al. A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;60:609–620. doi: 10.1007/s00280-006-0394-3. [DOI] [PubMed] [Google Scholar]
- 89.Colom H, Farré R, Soy D, et al. Population pharmacokinetics of high-dose methotrexate after intravenous administration in pediatric patients with osteosarcoma. Ther Drug Monit. 2009;31:76–85. doi: 10.1097/FTD.0b013e3181945624. [DOI] [PubMed] [Google Scholar]
- 90.Thompson PA, Murry DJ, Rosner GL, et al. Methotrexate pharmacokinetics in infants with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;59:847–853. doi: 10.1007/s00280-006-0388-1. [DOI] [PubMed] [Google Scholar]
- 91.Rau T, Erney B, Göres R, et al. High-dose methotrexate in pediatric acute lymphoblastic leukemia: Impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther. 2006;80:468–476. doi: 10.1016/j.clpt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Skärby T, Jönsson P, Hjorth L, et al. High-dose methotrexate: On the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol. 2003;51:311–320. doi: 10.1007/s00280-002-0552-1. [DOI] [PubMed] [Google Scholar]
- 93.Rousseau A, Sabot C, Delepine N, et al. Bayesian estimation of methotrexate pharmacokinetic parameters and area under the curve in children and young adults with localised osteosarcoma. Clin Pharmacokinet. 2002;41:1095–1104. doi: 10.2165/00003088-200241130-00006. [DOI] [PubMed] [Google Scholar]
- 94.Vassal G, Gouyette A, Hartmann O, et al. Pharmacokinetics of high-dose busulfan in children. Cancer Chemother Pharmacol. 1989;24:386–390. doi: 10.1007/BF00257448. [DOI] [PubMed] [Google Scholar]
- 95.Bostrom B, Enockson K, Johnson A, et al. Plasma pharmacokinetics of high-dose oral busulfan in children and adults undergoing bone marrow transplantation. Pediatr Transplant. 2003;7( suppl 3):12–18. doi: 10.1034/j.1399-3046.7.s3.2.x. [DOI] [PubMed] [Google Scholar]
- 96.Gibbs JP, Murray G, Risler L, et al. Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan. Cancer Res. 1997;57:5509–5516. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.