Abstract
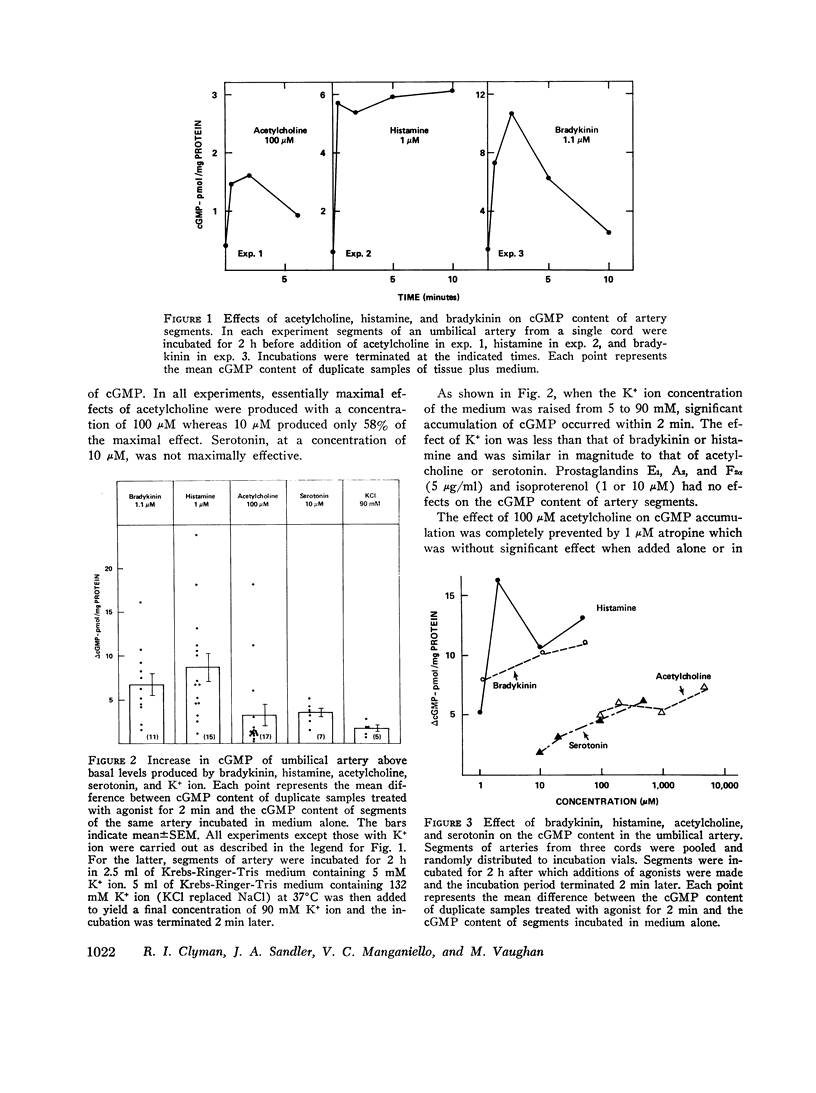
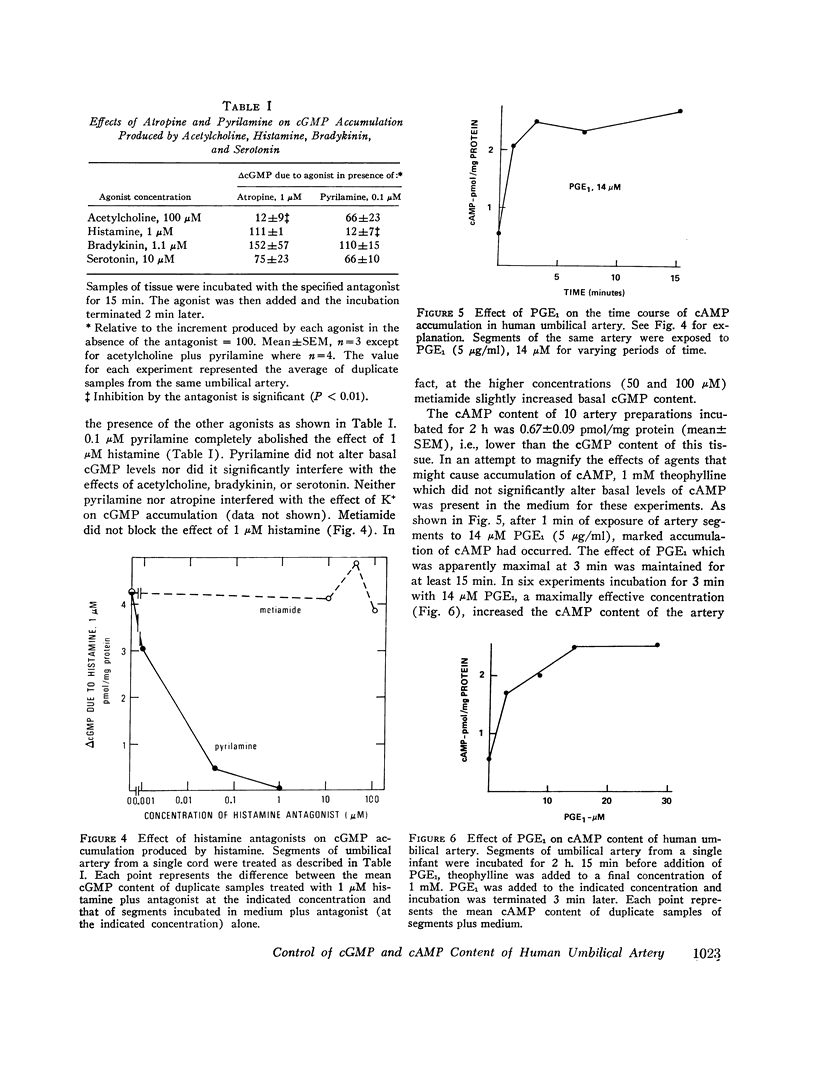
Human umbilical arteries are unique vessels in that they close quickly and completely at birth. It has been suggested that cyclic uanosine 3',5'-monophosphate (cAMP) in relaxation. This hypothesis has been evaluated in term gestational human umbilical artery segments incubated at 37 degrees C and in room air. (a) The basal cGMP content (1 pmol/mg protein) of artery segments incubated in room air was almost twice that of cAMP. (b) Bradykinin, histamine, serotonin, acetylcholine, and K+ ion, which cause umbilical artery constriction, can increase the cGMP content of the artery segments within 30 s of exposure without altering the cAMP content. (c) Prostaglandin E1, but not isoproterenol, caused accumulation of cAMP which is consistent with reports that umbilical arteries lack functional beta-receptors and that only prostaglandin E1 can bring about relaxation of umbilical arteries. (d) 1 muM atropine blocked the effect of 100 muM acetylcholine on cGMP content without altering the responses to histamine, bradykinin, serotonin, or K+ ion. (e) Pyrilamine (an H1 antagonist), but not metiamide (an H2 antagonist), blocked the effect of histamine on cGMP from which it is inferred that histamine causes accumulation of cGMP in umbilical artery via its interaction with H1 receptors. The results are consistent with the view that metabolism of the two cyclic nucleotides is independently controlled in the human umbilical artery and that cGMP is involved in contraction of the artery at birth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Malaviya D., Reich C. F., Orkin L. R. Effects of vasoactive agents on isolated human umbilical arteries and veins. Am J Physiol. 1972 Feb;222(2):345–355. doi: 10.1152/ajplegacy.1972.222.2.345. [DOI] [PubMed] [Google Scholar]
- Ash A. S., Schild H. O. Receptors mediating some actions of histamine. Br J Pharmacol Chemother. 1966 Aug;27(2):427–439. doi: 10.1111/j.1476-5381.1966.tb01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor I., Guntheroth W. G. In vitro response to oxygen of human umbilical arteries and of animal ductus arteriosus. Can J Physiol Pharmacol. 1970 Jul;48(7):500–502. doi: 10.1139/y70-077. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Code C. F. Effect of histamine and its methyl derivatives on cyclic AMP metabolism in gastric mucosa and its blockade by an H2 receptor antagonist. J Clin Invest. 1974 Jan;53(1):334–337. doi: 10.1172/JCI107555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. C., Gant D. W., Park M. Actions of histamine on human, sheep and monkey umbilical vasculature. Pharmacology. 1972;7(2):101–108. doi: 10.1159/000136278. [DOI] [PubMed] [Google Scholar]
- Eltherington L. G., Stoff J., Hughes T., Melmon K. L. Constriction of human umbilical arteries. Interaction between oxygen and bradykinin. Circ Res. 1968 Jun;22(6):747–752. doi: 10.1161/01.res.22.6.747. [DOI] [PubMed] [Google Scholar]
- Fox H., Jacobson H. N. Innervation of the human umbilical cord and umbilical vessels. Am J Obstet Gynecol. 1969 Feb 1;103(3):384–389. doi: 10.1016/0002-9378(69)90498-0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S. M. The identification of prostaglandins in human umbilical cord. Br J Pharmacol Chemother. 1967 Feb;29(2):230–237. doi: 10.1111/j.1476-5381.1967.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lichtenstein L. M., Gillespie E. Inhibition of histamine release by histamine controlled by H2 receptor. Nature. 1973 Aug 3;244(5414):287–288. doi: 10.1038/244287a0. [DOI] [PubMed] [Google Scholar]
- Manganiello V., Evans W. H., Stossel T. P., Mason R. J., Vaughan M. The effect of polystyrene beads on cyclic 3',5'-adenosine monophosphate concentration in leukocytes. J Clin Invest. 1971 Dec;50(12):2741–2744. doi: 10.1172/JCI106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhänsli-Weiss I., Heymann M. A., Rudolph A. M., Melmon K. L. The pattern and mechanisms of response to oxygen by the ductus arteriosus and umbilical artery. Pediatr Res. 1972 Sep;6(9):693–700. doi: 10.1203/00006450-197209000-00001. [DOI] [PubMed] [Google Scholar]
- SOMLYO A. V., WOO C. Y., SOMLYO A. P. RESPONSES OF NERVE-FREE VESSELS TO VASOACTIVE AMINES AND POLYPEPTIDES. Am J Physiol. 1965 Apr;208:748–753. doi: 10.1152/ajplegacy.1965.208.4.748. [DOI] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Davis J. W., Sutherland E. W. A new enzymatic assay for guanosine 3':5'-cyclic monophosphate and its application to the ductus deferens of the rat. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1721–1725. doi: 10.1073/pnas.70.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Stoner J. S., Manganiello V. C., Vaughan M. Guanosine cyclic 3',5'-monophosphate and guanylate cyclase activity in guinea pig lung: effects of acetylcholine and cholinesterase inhibitors. Mol Pharmacol. 1974 Jan;10(1):155–161. [PubMed] [Google Scholar]
- Stoner J., Manganiello V. C., Vaughan M. Effects of bradykinin and indomethacin on cyclic GMP and cyclic AMP in lung slices. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3830–3833. doi: 10.1073/pnas.70.12.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triner L., Vulliemoz Y., Verosky M., Habif D. V., Nahas G. G. Adenyl cyclase-phosphodiesterase system in arterial smooth muscle. Life Sci I. 1972 Sep 1;11(17):817–824. [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Elevation of cyclic guanosine 3',5'-monophosphate levels in dog thyroid slices caused by acetylcholine and sodium fluoride. J Biol Chem. 1972 Nov 10;247(21):7062–7066. [PubMed] [Google Scholar]


