Abstract
Hyperphosphorylation of tau protein is associated with neurofibrillary lesion formation in Alzheimer’s disease and other tauopathic neurodegenerative diseases. It fosters lesion formation by increasing the concentration of free tau available for aggregation and by directly modulating the tau aggregation reaction. To clarify how negative charge incorporation into tau directly affects aggregation behavior, the fibrillization of pseudophosphorylation mutant T212E prepared in a full-length four-repeat tau background was examined in vitro as a function of time and submicromolar tau concentrations using electron microscopy assay methods. Kinetic constants for nucleation and extension phases of aggregation were then estimated by direct measurement and mathematical simulation. Kinetic analysis revealed that pseudophosphorylation increased tau aggregation rate by increasing the rate of filament nucleation. In addition, it increased aggregation propensity by stabilizing mature filaments against disaggregation. The data suggest that incorporation of negative charge into the T212 site can directly promote tau filament formation at multiple steps in the aggregation pathway.
Keywords: Aggregation, Alzheimer’s disease, Tau protein, phosphorylation, kinetics
1. Introduction
The neurofibrillary lesions of Alzheimer’s disease develop intracellular aggregates of the microtubule-associated protein tau [1]. Although certain familial tauopathies result from mutations in the tau gene (MAPT), the pathogenesis of Alzheimer’s disease is not associated with changes in tau amino acid sequence. Rather, lesion formation is accompanied by a 3–4 fold increase in tau phosphorylation stoichiometry [2, 3]. The covalently bound phosphate is distributed among ~40 sites within and adjacent to the tau microtubule-binding domain [4–6]. Occupancy of these sites may influence tau aggregation in two ways. First, occupancy of certain sites modulates tau-tubulin affinity [7], fostering an increase in the levels of free cytoplasmic tau available to nucleate and support the aggregation reaction [8–11]. Second, hyperphosphorylation can increase tau aggregation propensity directly [12, 13]. However, the precise mechanism of these direct effects has been difficult to establish. Challenges to overcome include the difficulties of recapitulating the complex phosphorylation patterns observed in disease tissue and of quantifying the aggregation reaction under controlled conditions. The challenge of site occupancy has been addressed through phosphorylation mimicry, where phosphorylatable hydroxy-amino acids are converted to negatively-charged Asp or Glu residues. The approach fosters site-specific incorporation of negative charge at full occupancy. Resultant pseudophosphorylation mutants have been shown to mimic phosphorylation-induced changes in tau structure and function [14–16], and to be recognized by phosphorylation-sensitive anti-tau antibodies [17]. The challenge of aggregation kinetics has been addressed by the development of agents that drive efficient aggregation in vitro over tractable time periods and near physiological concentrations of tau protein[18].
Despite these advances, aggregation kinetics in the presence of exogenous inducers can be difficult to analyze with explicit models. For example, the effects of some inducers, such as heparin, depend on the concentration ratio between inducer and tau protein [19]. Other inducers, such as anionic surfactants, micellize on contact with tau [20]. When aggregation reactions are initiated with sodium octadecyl sulfate (ODS), for example, the rate of micellization is slow relative to aggregation, and so the early stages of aggregation may be obscured [21, 22].
Recently we found that aggregation of full-length tau at submicromolar concentrations can be achieved with Thiazine red [23]. Thiazine red mediated aggregation can be explicitly modeled as a homogeneous nucleation scheme involving the formation of an unstable dimeric nucleus followed by monomer addition to growing filament ends [24]. Under these conditions, the nucleation and extension phases of aggregation can be assessed and quantified. Thus, the inherent aggregation propensity of pseudophosphorylated tau can be quantified and compared to that of wild-type tau.
Here, we examine the aggregation propensity of a tau mutant pseudophosphorylated at residue T212 in a full-length four-repeat tau background. This site composes part of the AT100 epitope [25, 26], which is recognized by multiple protein kinases [27–31], and is selectively occupied in disease [32]. The results show that the introduction of negative charge at this position directly promotes tau fibrillization by acting at multiple points along the aggregation pathway.
2. Materials and Methods
2. 1 Materials
Recombinant polyhistidine-tagged 2N4R tau and pseudophosphorylation mutant 2N4R-T212E were prepared as described previously [21, 33]. Aggregation inducer Thiazine red (Chemical Abstract Service registry number 2150-33-6) was obtained from TCI America (Portland, OR, USA). Formvar/carbon-coated copper grids, glutaraldehyde, and uranyl acetate were obtained from Electron Microscopy Sciences (Fort Washington, PA, USA). Primary mouse monoclonal Tau5 [34] was the gift of L. I. Binder (Northwestern University), whereas HRP-linked goat anti-mouse IgG was from Kirkegaard and Perry (Gaithersburg, MD). Nitrocellulose membranes (0.45 μm) were from Bio-Rad Laboratories (Hercules, CA).
2.2 Tau fibrillization assay
Tau filaments were formed from purified tau incubated without agitation in assembly buffer (10 mM HEPES, pH 7.4, 100 mM NaCl, and 5 mM dithiothreitol) for up to 24 h at 37°C. Aggregation was initiated with Thiazine red (100 μM final concentration). For samples analyzed by electron microscopy, reactions were terminated with 2% glutaraldehyde, adsorbed to Formvar/carbon-coated copper grids, stained with 2% uranyl acetate, and viewed in a Tecnai G2 Spirit BioTWIN transmission electron microscope (FEI, Hillsboro, OR, USA) operated at 80 kV and 23,000–49,000x magnification. At least three viewing fields were captured for each reaction condition in which filaments >10 nm in length were counted and quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA). Total filament length is defined as the sum of the lengths of all resolved filaments per field and is reported as ± SD.
For quantification by immuno-dot blot, reactions were centrifuged at 200,000g for 1 h at 16°C, after which time aliquots of the resultant supernatants were spotted onto nitrocellulose membranes. Membranes were blocked in 4% nonfat dry milk dissolved in blocking buffer (100 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 0.5% Tween 20) for 2 h, and then incubated with mouse monoclonal antibody Tau5 at 1:1000 dilution for 2 h. The membrane was washed three times in blocking buffer and incubated with HRP-linked goat anti-mouse IgG for 2 h. The membrane was then washed three times in blocking buffer and developed with the Enhanced Chemiluminescence Western Blotting Analysis System (GE Healthcare, Buckinghamshire, UK). Chemiluminescence was recorded on an Omega 12iC Molecular Imaging System and quantified using UltraQuant software (UltraLum, Claremont, CA, USA).
2.3 Critical concentration
Critical concentrations (Kcrit) were determined by inverse prediction of the abscissa intercept on a plot of the concentration dependence of tau aggregation, as described previously [24]. The accompanying standard error of the estimate(Sx) was calculated as:
| (1) |
where C.I. is the Fieller 95% confidence interval of each regression, and t0.975,n-2 is the Student’s t distribution percentage at 1- α = 0.975 and n - 2 degrees of freedom.
2.4 Dissociation kinetics
Assembled tau filaments prepared as described above were diluted 10-fold into assembly buffer containing 100 μM Thiazine red and incubated at 37°C. Aliquots were removed as a function of time up to 5 h post-dilution and assayed for total filament length. The disaggregation time course was fit to an exponential decay function:
| (2) |
where y is the filament length at time t, y0 is filament length at time zero, and kapp is the pseudo-first order rate constant for the process. After solving for kapp, the initial velocity of disaggregation (dy/dt) was determined from the first derivative of the exponential decay function [35, 36] at time t = 0:
| (3) |
Dissociation rate constant ke− was then extracted from initial velocities by converting length into tau protomer units (assuming 3.85 tau molecules/nm filament [24]) and then dividing by the number of filaments measured at time zero (i.e., it was assumed that dissociation proceeded from only one end of each filament). The association rate constant ke+ was then determined from the relationship [24]:
| (4) |
assuming a two state model (i.e., all tau was either monomeric or incorporated into filaments).
2.5 Aggregation time series
Aggregation lag times, defined as the time when the tangent to the point of maximum aggregation rate intersects the abscissa of the sigmoidal curve [37], were obtained ± SE from each time series by Gompertz regression as described in [22]. To determine the nucleation dissociation equilibrium constant, Kn, filament length data was converted to protomer concentration (cp*) assuming that all protein above the critical concentration formed filaments [24], and that the resultant filaments contained two tau protomers per β-sheet spacing [24]. Data were then fitted to the simplified homogeneous nucleation scheme of Wegner and Engel [38] assuming a dimeric nucleus [24]:
| (5) |
| (6) |
| (7) |
where ctotal, c1, and cp represent bulk tau, tau monomer, and tau filament concentrations, respectively. Parameter estimates were obtained by fitting experimentally determined values of ctotal, cp*, ke−, and ke+ to equations 5–7 in JACOBIAN™ modeling software (Numerica Technology, LLC, Cambridge, MA). The simulation yielded estimates of forward and reverse nucleation rate constants kn+ and kn−, with the ratio kn−/kn+ recorded as Kn.
2.6 Statistical analysis
The probability of differences between kinetic parameters was assessed by z-test:
| (8) |
where x1 ± Sx1 and x2 ± Sx2 are the pair of estimates ± SE being compared, and z is the 1-α point of the standard normal distribution. All statistical analyses were carried out using SigmaPlot 10.0 (Systat Software, Chicago, IL) and JMP 7.0 (SAS Institute, Cary, NC).
3. Results
3.1 Effect of pseudophosphorylation on critical concentration
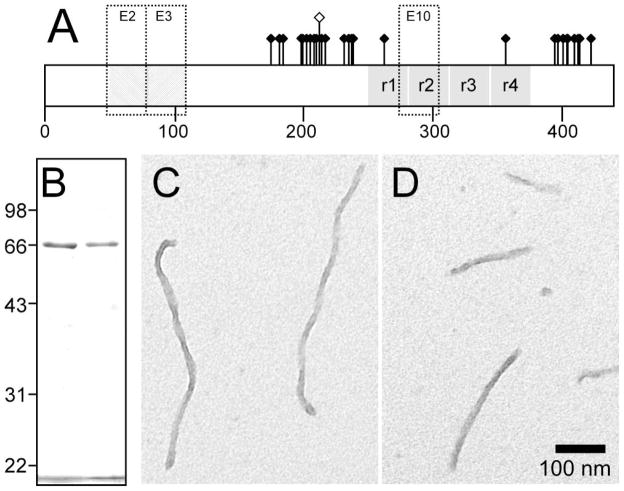
Approximately thirty phosphorylation sites have been mapped to the microtubule-binding repeat region of filamentous tau isolated from AD brain (Fig. 1a). Using recombinant tau preparations, we previously showed that incorporation of negatively charged Glu resides at some of these sites modulated aggregation propensity relative to unmodified tau in the presence of anionic surfactant inducers [21]. Among these missense mutants, T212E showed the greatest effect and so was selected for detailed analysis in the presence of Thiazine red aggregation inducer. To facilitate comparison with previous work [21], recombinant T212E was prepared in a full-length four-repeat background corresponding to the 2N4R tau isoform. After purification from a bacterial expression system, recombinant 2N4R-T212E and wild-type 2N4R tau migrated identically on SDS-polyacrylamide gel electrophoresis, confirming that they were prepared with consistent concentration and purity (Fig. 1b). When wild-type 2N4R tau (≤1 μM) was incubated with Thiazine red at near-physiological conditions of pH, ionic strength, and reducing environment, filaments with twisted ribbon morphology formed (Fig. 1c). These filaments were previously reported to have a mass-per-unit length similar to authentic brain-derived paired helical filaments [24]. When incubated under the same conditions, 2N4R-T212E produced filaments that were morphologically identical to those formed by wild-type 2N4R tau (Fig. 1d). In contrast, incubation of either tau preparation in the absence of Thiazine red for up to 24 h did not yield any detectable filaments (data not shown), suggesting that spontaneous aggregation was minimal under these conditions. These data indicate that 2N4R-T212E shares the fundamental aggregation characteristics of wild-type 2N4R tau and can be studied at physiological bulk tau concentrations in the presence of Thiazine red inducer.
Fig. 1. Comparison of 2N4R and 2N4R-T212E tau preparations.
(A) Distribution of ~30 hydroxyamino acid residues affected by phosphorylation depicted on isoform 2N4R. This isoform contains alternatively spliced exons 2 and 3 (E2 and E3), each of which encodes an acidic 29-residue segment, and exon 10 (E10), which encodes an additional microtubule binding repeat. Pseudophosphorylation mutant T212E is distinguished graphically by a raised hollow symbol. (B) Comparison of recombinant 2N4R (left lane) and 2N4R-T212E (right lane) protein preparations by SDS-polyacrylamide gel electrophoresis (10% acrylamide) and Coomassie blue staining. The bands migrating at ~66 kDa correspond to these tau proteins [33], whereas the band below 22 kDa corresponds to the dye front. The preparations were consistent with respect to protein concentration and purity. (C,D) Morphology of synthetic tau filaments. Full-length wild-type 2N4R tau (C) and mutant 2N4R-T212E (D) were incubated (1 μM concentration) without agitation in the presence of 100 μM Thiazine red (24 h at 37°C) and viewed by transmission electron microscopy. 2N4R-T212E produced unbranched filaments ~16 nm in diameter with no obvious differences in morphology or length distribution from wild-type 2N4R. Scale bar = 100 nm.
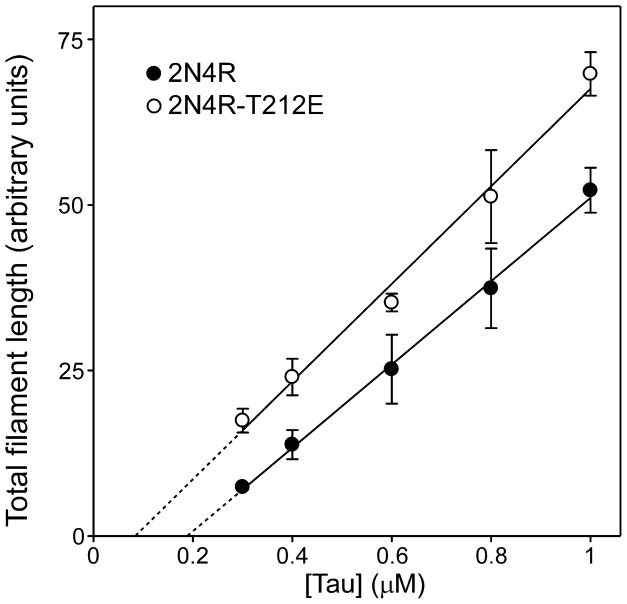
Characterization of aggregation propensity began with estimation of the minimal tau concentration required to support aggregation of 2N4R and 2N4R-T212E. In nucleation-dependent reactions, the minimal concentration is termed the critical concentration (Kcrit), and approximates the dissociation equilibrium constant for elongation (Ke) [24]. The minimal concentration of 2N4R estimated from the abscissa intercept of a plot of aggregation versus tau concentration was 186 ± 25 nM (Fig. 2; Table 1). In contrast, the minimal concentration of 2N4R-T212E was 84 ± 28 nM (Fig. 2; Table 1), differing from wild-type tau by 2.2 ± 0.4 fold (p < 0.01).
Fig. 2. Pseudophosphorylation lowers critical concentration.
Wild-type 2N4R tau (●) and mutant 2N4R-T212E (○) were incubated at varying bulk concentrations in the presence of Thiazine red inducer for 24 h at 37°C, then assayed for filament formation by electron microscopy. Each data point represents total filament length as a function of bulk protein concentration (triplicate determination), whereas the solid lines represent best fit of the data points to linear regression. The abscissa intercept, which was obtained by extrapolation (dotted lines), was used to estimate critical concentration (Kcrit; Table 1). 2N4R-T212E aggregated with a lower Kcrit than did wild-type 2N4R.
Table 1.
Summary of aggregation parameters
| Protein | aKcrit (nM) | ake− (s−1) | ake+ (mM−1s−1) | Lag Time (h) | Kn (mM) |
|---|---|---|---|---|---|
| 2N4R | 186 ± 25 | 0.020 ± 0.001 | 105 ± 15 | 0.81 ± 0.06 | 21.7 |
| T212E | 84 ± 28** | 0.011 ± 0.001** | 133 ± 46 | 0.28 ± 0.05** | 5.1 |
Overall constants reflecting events at both filament ends
p < 0.01 versus 2N4R tau
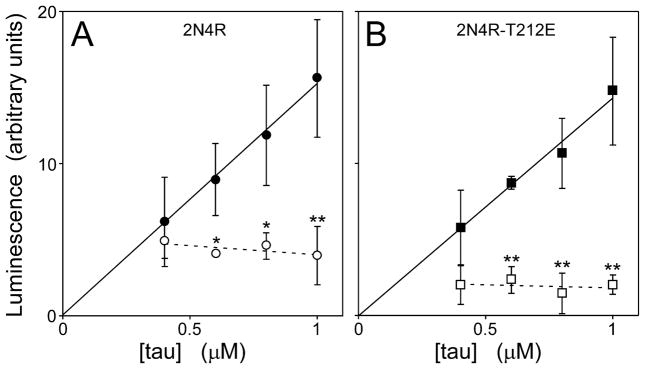
Critical behavior also is reflected in the levels of protein monomer at reaction plateau, which remain constant as bulk concentrations rise above Kcrit [39]. To confirm that tau aggregation in the presence of Thiazine red displayed critical behavior, the levels of soluble tau at reaction plateau was estimated after ultracentrifugation(200,000 g for 1 h). In the absence of Thiazine red, soluble 2N4R and 2N4R-T212E increased linearly with bulk tau concentration up to at least 1 μM (Fig. 3), consistent with the absence of detectable filament formation in these samples. In contrast, the presence of Thiazine red aggregation inducer yielded a nearly constant amount of 2N4R and 2N4R-T212E in the supernatant regardless of bulk tau concentration (Fig. 3). Under these conditions, average plateau levels of soluble 2N4R were 2.1 ± 0.2 fold higher (p < 0.01) than soluble 2N4R-T212E (Fig. 3). These data were consistent with critical behavior, and indicated that phosphorylation mimicry at residue 212 increased aggregation propensity by depressing the minimum concentration of tau needed to support fibril formation.
Fig. 3. Pseudophosphorylation lowers tau solubility.
(A) wild-type 2N4R and (B) 2N4R-T212E were incubated at varying bulk concentrations in the presence (○,□) or absence (●,■) of Thiazine red inducer until reaction plateau (24 h at 37°C), then subjected to centrifugation (200,000g for 1 h). The amount of tau remaining in the soluble fraction was then quantified on immuno-dot blots using monoclonal antibody Tau5 and enhanced chemiluminescence detection. Each data point represents tau immunoreactivity as a function of bulk protein concentration (triplicate determination), whereas the lines represent best fit of the data points to linear regression (solid line regressions were constrained to pass through the origin). In the absence of Thiazine red, soluble 2N4R or 2N4R-T212E increased linearly with bulk tau concentration (solid lines). In contrast, the presence of Thiazine red greatly reduced solubility of both 2N4R-T212E and wild-type 2N4R, both of which remained constant over the 0.4 – 1 μM bulk tau concentration range (dashed lines). Averaged over this range, levels of soluble 2N4R were 2.1 ± 0.2 fold higher than soluble 2N4R-T212E. *, p < 0.05; **, p < 0.01, presence compared to absence of Thiazine red inducer.
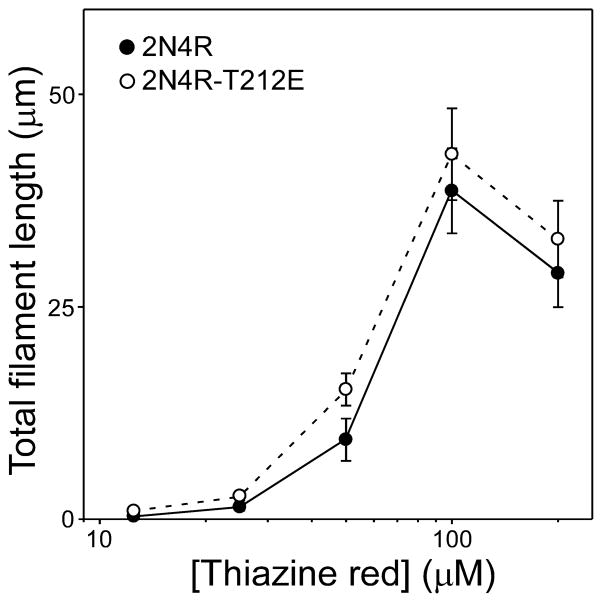
To confirm that the decrease in Kcrit was a result of increased ability to aggregate and not a change in sensitivity to Thiazine red induction, 2N4R and 2N4R-T212E were incubated with different concentrations of Thiazine red at constant tau supersaturation (i.e. at a constant tau concentration above Kcrit) (Fig. 4). The concentration effect relationship was similar for both tau forms with maximal efficacy near 100 μM, indicating that the response of 2N4R-T212E to Thiazine red was not perturbed. Together these data indicate that the depression of Kcrit observed with pseudophosphorylation reflects differences in aggregation propensity, and not differential sensitivity to Thiazine red inducer.
Fig. 4. Tau mutants share a common sensitivity to aggregation inducer Thiazine red.
Wild-type 2N4R (●) and 2N4R-T212E (○) were incubated (24 h at 37°C) at constant supersaturation (i.e., 0.5 μM above Kcrit) in the presence of varying concentrations of Thiazine red and then assayed for filament formation by electron microscopy. Each data point represents total filament length per field ± SD from triplicate determinations. Under these conditions, the concentration effect relationship for Thiazine red was similar for both tau species.
3.2 Effect of pseudophosphorylation on the extension reaction
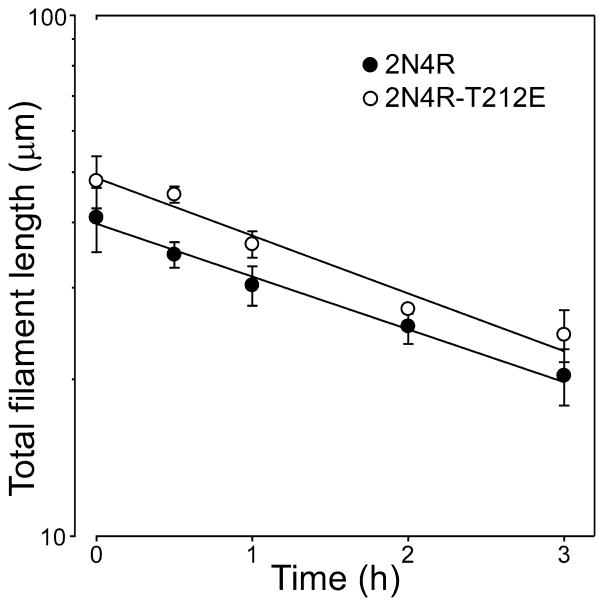
From Equation 4, Kcrit approximates the ratio of the rate of monomer dissociation from filament ends (ke−) to the rate of monomer addition to filament ends (ke+). A decrease in Kcrit could result from a stabilization of filaments (i.e., a decrease in ke−), enhanced monomer addition (an increase in ke+), or a combination of both. To distinguish between these possibilities, ke− was estimated from the disaggregation rate of preassembled filaments composed of 2N4R and 2N4R-T212E tau. Rate constant ke+ was then calculated from estimates of ke− and Kcrit for each mutant through Equation 4. Results show that disaggregation followed first order kinetics as predicted for a Poisson-like distribution of filament lengths undergoing endwise depolymerization [35] (Fig. 5), and that ke− for 2N4R-T212E is decreased nearly two-fold relative to wild-type 2N4R tau (Table 1). In contrast, there was no significant difference in calculated values of ke+ (Table 1). These data indicate that filaments formed by 2N4R-T212E are inherently more stable and less prone to disaggregate than filaments formed by wild-type 2N4R tau. Furthermore, these observations were consistent for two different filament morphologies formed from two distinct inducers (Thiazine red, herein; octadecyl sulfate, [21, 22]).
Fig. 5. Dissociation rate constants for filament extension.
Tau filaments prepared from wild-type 2N4R (●) and 2N4R-T212E (○) in the presence of 100 μM Thiazine red at 37°C were diluted 10-fold into assembly buffer containing Thiazine red, and the resultant disaggregation was followed as a function of time by electron microscopy. Each data point represents total filament length per field ± SD (triplicate observations), whereas the solid lines represent best fit of data points to linear regression. The first-order decay constant kapp was estimated from each regression, and used in conjunction with filament length (shown in the figure) and number (2N4R = 127 ± 18; 2N4R-T212E = 298 ± 30) at time t = 0 to calculate dissociation rate constant ke− (see Table 1). Despite similar decay constants for 2N4R and 2N4R-T212E filaments in this experiment, estimates of ke− differed owing to the differences in numbers of filaments at time t = 0.
3.3 Effect of pseudophosphorylation on the nucleation reaction
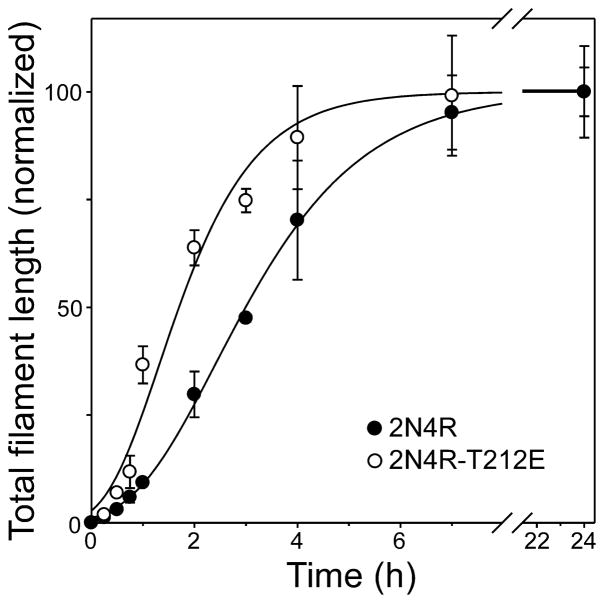
In the presence of Thiazine red inducer, the aggregation reaction of tau is driven by the rapid equilibration of assembly competent monomers with a thermodynamic nucleus, defined as the least stable species reversibly interconverted with monomer [40]. Since elongation can proceed efficiently only after the nucleus has formed, the rate of aggregation depends on nucleation rate as well as protein concentration and the rate of elongation. To determine whether pseudophosphorylation affected nucleation rate, tau aggregation time course was quantified for both wild type 2N4R and 2N4R-T212E at constant supersaturation in the presence of Thiazine red. Under these conditions, differences in reaction rates primarily reflect differences in rates of nucleation and of protein concentrations [41]. Both reaction progress curves displayed lag, exponential growth, and equilibrium phases (Fig. 6). However, fitting each curve to a 3-parameter Gompertz growth function revealed that 2N4R-T212E aggregated with a significantly shorter lag time than wild-type 2N4R tau despite being present at lower bulk concentrations (Table 1). These data suggest that pseudophosphorylation accelerated the nucleation phase of the tau aggregation reaction.
Fig. 6. Effects of tau mutations on aggregation time course.
Wild-type 2N4R (●) and 2N4R-T212E (○) were incubated (37°C) at constant supersaturation (i.e., 0.2 μM above Kcrit) in the presence of 100 μM Thiazine red, and then assayed for filament formation as a function of time. Each data point represents mean total filament lengths/field (expressed as % plateau length) calculated from triplicate electron microscopy images whereas each normalized curve represents best fit of the data points to a three parameter Gompertz growth function. The fits were used to calculate lag time (Table 1). 2N4R-T212E aggregated significantly faster than wild-type 2N4R when compared at constant supersaturation.
To test this hypothesis, each time series was fit to an explicit nucleation-extension model using equations 5– 7 and experimental values for rate constants ke+ and ke− as constraints. The nucleation dissociation equilibrium constant, Kn, was then estimated from the model. The calculations revealed that pseuphosphorylation mutant 2N4R-T212E increased the efficiency of nucleation by decreasing Kn~4 fold (Table 1). Together these data indicate that incorporation of negative charge at residue 212 directly increases aggregation propensity, and that it does so at the nucleation step by decreasing Kn and at the extension step by decreasing ke−.
4. Discussion
These results confirm that incorporation of negative charge into tau protein can directly modulate aggregation propensity irrespective of reported indirect effects on tau turnover [42] or proline isomerization [43]. Two mechanisms have been proposed to account for direct effects. The first posits that charge neutralization decreases the isoelectric point of tau protein resulting in lower solubility at physiological pH [44, 45]. The second mechanism predicts that conformational changes induced by phosphorylation promote or stabilize self-association [16]. These mechanisms need not be mutually exclusive. In fact, phosphorylation sites on tau may exert their effects in tandem, with some modifications enhancing fibrillization ([17, 21, 46–48] and herein) and others having neutral or inhibitory effects[49, 50].
Previously we reported that pseudophosphorylation mutants, including 2N4R-T212E, did not modulate filament nucleation rate [21]. The discrepancy results in part from the use of sodium octadecyl sulfate (ODS) as inducer in the previous report. ODS is a surfactant that functions in micellar form by presenting a negatively-charged surface for filament nucleation [23]. As a result, the rate of filament induction by ODS is limited by its rate of micellization, which is only 80% complete after ~45 min [22]. Because lag times for 2N4R and 2N4R-T212E ranged from 42 – 59 min [21], the rate of filament nucleation cannot be distinguished from the rate of ODS micellization, and potential differences among tau species are masked. In contrast, the depressing effect of pseudophosphorylation on minimal concentration is consistent between arachidonic acid, ODS, and Thiazine red inducers ([21] and herein). Absolute values of minimal concentration vary among these inducers, however, in part because non-fibrillar tau binds and coats the surface of anionic inducers, adding a third component to the equilibrium between filaments and monomer [23]. As a result, minimum concentrations for filament formation are low micromolar in the presence of anionic surfactants, but submicromolar in the presence of Thiazine red. A second source of discrepancy pertains to filament morphology. Filaments induced under reducing conditions by anionic surfactants such as arachidonic acid contain ~1 tau molecule per β-sheet spacing [51], whereas those induced by Thiazine red contain ~2 tau molecules per β-sheet spacing [52]. Aggregation kinetics may vary with filament structure.
The results presented herein also disagree with equilibrium aggregation measurements made with pseudophosphorylation mutants including T212E and heparin inducer [26]. The discrepancy could result from differences in protein concentration, which were submicromolar range in the present study and ~60 μM in the heparin-induced study. In nucleation-dependent reactions, the amount of aggregation at equilibrium is proportional to the net concentration of protein above the critical concentration rather than bulk protein concentration. Thus, when bulk concentrations are high relative to critical concentrations, net concentrations may not differ substantially among tau constructs, thereby masking potential differences in aggregation propensity. Heparin-mediated tau aggregation reportedly is nucleation dependent under non-reducing conditions [53].
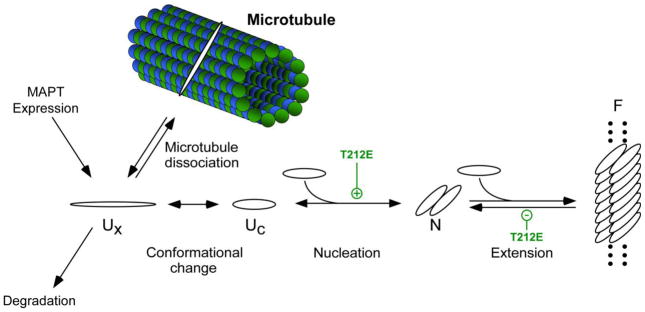
4.1 Implications of tau aggregation mechanism
On the basis of tau aggregation kinetics in the presence of exogenous inducer Thiazine red [24], we have proposed that four key steps must be overcome for tau to aggregate in disease (Fig. 7). First, the concentration of free tau in the cytoplasm must be sufficient to support filament formation. This can be accomplished through increased MAPT expression, decreased tau degradation, or decreases in tau-microtubule binding affinity. The role of tau phosphorylation in modulating tau-microtubule affinity is well established [8–10]. For example, T212E reportedly has diminished ability to promote microtubule assembly [26], consistent with an impaired ability to bind tubulin. In addition, tau phosphorylation has been reported to decrease proteasome-mediated tau turnover in a neuronal cell model [54]. Thus, occupancy of certain tau phosphorylation sites may raise free cytoplasmic tau concentrations through multiple mechanisms.
Fig. 7. Effect of T212E mutation on the tau fibrillization pathway.
Normal tau binds tightly to microtubules but dissociates upon phosphorylation to form free tau, which exists as a natively disordered, assembly incompetent monomer (Ux). A conformational change to an assembly competent state accelerates polymerization (Uc). Once assembly competent species form, the rate-limiting step in tau fibrillization is formation of dimer, which represents the thermodynamic nucleus (N). Following nucleation, extension occurs through further addition of assembly competent monomers to the filament (F) ends. Introduction of negative charge at residue T212 in the form of pseudophosphorylation, and potentially phosphorylation, affects multiple points in the pathway. See text for details.
The second step involves the transition of dissociated tau monomers to an assembly-competent conformation (Fig. 7). This step is proposed to be a barrier to aggregation because high concentrations (i.e., up to 100 μM) of free tau alone are insufficient to support aggregation or seeding reactions in vitro [55]. Phosphorylation of tau at multiple sites within the proline-rich region of tau (including T212) can induce local polyproline II helix conformation [16]. Adoption of such conformations, which are associated with protein-protein interfaces [56], may help overcome the resistance of monomeric unmodified tau proteins to aggregation.
Once aggregation-competent conformations are adopted, the rate-limiting step in filament formation becomes dimerization [24], which is energetically disfavored at physiological tau concentrations, and therefore a third key point of control (Fig. 7). In the case of 2N4R-T212E, increased aggregation propensity included acceleration of filament nucleation rate. Consistent with this observation, tau dimerization can be promoted in vitro by NCLK/cdk5 [57], which includes T212 as a target [31].
The final step in fibrillization is mediated by an extension reaction. Although not rate limiting, equilibria at filament ends dictate the minimal concentration of tau required to support aggregation. Pseudophosphorylation at T212 enhanced filament elongation by decreasing the rate at which monomers dissociated from filament ends. These effects are not unique to the twisted ribbon morphology induced by Thiazine red, also having been observed with the filamentous morphologies induced by anionic surfactants [36]. These results indicate that the effects of 2N4R-T212E on filament stability are not inducer specific. However, its effects on filament elongation differ from that of certain frontotemporal dementia linked missense mutations, some of which act to increase the rate of monomer addition to filament ends without affecting filament stability [58].
4.2 Conclusions
Together, these data suggest that occupancy of specific tau phosphorylation sites could potentially modulate key rate-limiting steps along the fibrillization pathway. This reinforces the contribution of tau hyperphosphorylation to neurological disease and provides further support for hyperphosphorylation as a target for pharmacological efforts in treatment of tauopathies.
Acknowledgments
This work was supported by the National Institutes of Health grantAG14452.
Abbreviations
- ODS
sodium octadecyl sulfate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 2.Ksiezak-Reding H, Liu WK, Yen SH. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992;597:209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- 3.Kopke E, Tung YC, Shaikh S, Alonso AC, Iqbal K, Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 4.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Watanabe A, Titani K, Ihara Y. Hyperphosphorylation of tau in PHF. Neurobiol Aging. 1995;16:365–371. doi: 10.1016/0197-4580(95)00027-c. [DOI] [PubMed] [Google Scholar]
- 5.Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer’s disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanger DP, Byers HL, Wray S, Leung KY, Saxton MJ, Seereeram A, Reynolds CH, Ward MA, Anderton BH. Novel phosphorylation sites in tau from Alzheimer brain support a role for casein kinase 1 in disease pathogenesis. The Journal of biological chemistry. 2007;282:23645–23654. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 7.Lindwall G, Cole RD. The purification of tau protein and the occurrence of two phosphorylation states of tau in brain. J Biol Chem. 1984;259:12241–12245. [PubMed] [Google Scholar]
- 8.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM. Abnormal tau phosphorylation at Ser396 in Alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- 9.Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- 10.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Yin H, Kuret J. Casein kinase 1 delta phosphorylates tau and disrupts its binding to microtubules. The Journal of biological chemistry. 2004;279:15938–15945. doi: 10.1074/jbc.M314116200. [DOI] [PubMed] [Google Scholar]
- 12.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau’s amino-terminal projection domain. J Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eidenmuller J, Fath T, Maas T, Pool M, Sontag E, Brandt R. Phosphorylation-mimicking glutamate clusters in the proline-rich region are sufficient to simulate the functional deficiencies of hyperphosphorylated tau protein. Biochem J. 2001;357:759–767. doi: 10.1042/0264-6021:3570759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bielska AA, Zondlo NJ. Hyperphosphorylation of tau induces local polyproline II helix. Biochemistry. 2006;45:5527–5537. doi: 10.1021/bi052662c. [DOI] [PubMed] [Google Scholar]
- 17.Haase C, Stieler JT, Arendt T, Holzer M. Pseudophosphorylation of tau protein alters its ability for self-aggregation. J Neurochem. 2004;88:1509–1520. doi: 10.1046/j.1471-4159.2003.02287.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuret J, Congdon EE, Li G, Yin H, Yu X, Zhong Q. Evaluating triggers and enhancers of tau fibrillization. Microsc Res Tech. 2005;67:141–155. doi: 10.1002/jemt.20187. [DOI] [PubMed] [Google Scholar]
- 19.Friedhoff P, Schneider A, Mandelkow EM, Mandelkow E. Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry. 1998;37:10223–10230. doi: 10.1021/bi980537d. [DOI] [PubMed] [Google Scholar]
- 20.Chirita CN, Necula M, Kuret J. Anionic Micelles and Vesicles Induce tau Fibrillization in vitro. J Biol Chem. 2003;278:25644–25650. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- 21.Necula M, Kuret J. Pseudophosphorylation and glycation of tau protein enhance but do not trigger fibrillization in vitro. J Biol Chem. 2004;279:49694–49703. doi: 10.1074/jbc.M405527200. [DOI] [PubMed] [Google Scholar]
- 22.Necula M, Kuret J. A static laser light scattering assay for surfactant-induced tau fibrillization. Anal Biochem. 2004;333:205–215. doi: 10.1016/j.ab.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 23.Chirita CN, Congdon EE, Yin H, Kuret J. Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry. 2005;44:5862–5872. doi: 10.1021/bi0500123. [DOI] [PubMed] [Google Scholar]
- 24.Congdon EE, Kim S, Bonchak J, Songrug T, Matzavinos A, Kuret J. Nucleation-dependent tau filament formation: the importance of dimerization and an estimation of elementary rate constants. The Journal of biological chemistry. 2008;283:13806–13816. doi: 10.1074/jbc.M800247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann R, Lee VM, Leight S, Varga I, Otvos L., Jr Unique Alzheimer’s disease paired helical filament specific epitopes involve double phosphorylation at specific sites. Biochemistry. 1997;36:8114–8124. doi: 10.1021/bi970380+. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38delta or JNK2 in the presence of heparin generates the AT100 epitope. Journal of neurochemistry. 2006;99:154–164. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- 27.Wille H, Drewes G, Biernat J, Mandelkow EM, Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992;118:573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barghorn S, Zheng-Fischhofer Q, Ackmann M, Biernat J, von Bergen M, Mandelkow EM, Mandelkow E. Structure, microtubule interactions, and paired helical filament aggregation by tau mutants of frontotemporal dementias. Biochemistry. 2000;39:11714–11721. doi: 10.1021/bi000850r. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. Journal of neurochemistry. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- 30.Perrin RJ, Woods WS, Clayton DF, George JM. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- 31.Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer HE, Mandelkow EM, Mandelkow E. Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo ES, Shin RW, Billingsley ML, Van deVoorde A, O’Connor M, Trojanowski JQ, Lee VM. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament tau. Neuron. 1994;13:989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 33.Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 34.LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci USA. 1995;92:10369–10373. doi: 10.1073/pnas.92.22.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristofferson D, Karr TL, Purich DL. Dynamics of linear protein polymer disassembly. J Biol Chem. 1980;255:8567–8572. [PubMed] [Google Scholar]
- 36.Necula M, Kuret J. Site-specific pseudophosphorylation modulates the rate of tau filament dissociation. FEBS letters. 2005;579:1453–1457. doi: 10.1016/j.febslet.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 37.Evans KC, Berger EP, Cho CG, Weisgraber KH, Lansbury PT., Jr Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:763–767. doi: 10.1073/pnas.92.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wegner A, Engel J. Kinetics of the cooperative association of actin to actin filaments. Biophysicalchemistry. 1975;3:215–225. doi: 10.1016/0301-4622(75)80013-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhao D, Moore JS. Nucleation-elongation: a mechanism for cooperative supramolecular polymerization. Org Biomol Chem. 2003;1:3471–3491. doi: 10.1039/b308788c. [DOI] [PubMed] [Google Scholar]
- 40.Ferrone F. Analysis of protein aggregation kinetics. Methods Enzymol. 1999;309:256–274. doi: 10.1016/s0076-6879(99)09019-9. [DOI] [PubMed] [Google Scholar]
- 41.Fesce R, Benfenati F, Greengard P, Valtorta F. Effects of the neuronal phosphoprotein synapsin I on actin polymerization. II. Analytical interpretation of kinetic curves. The Journal of biological chemistry. 1992;267:11289–11299. [PubMed] [Google Scholar]
- 42.Johnson GV. Tau phosphorylation and proteolysis: insights and perspectives. J Alzheimers Dis. 2006;9:243–250. doi: 10.3233/jad-2006-9s326. [DOI] [PubMed] [Google Scholar]
- 43.Balastik M, Lim J, Pastorino L, Lu KP. Pin1 in Alzheimer’s disease: multiple substrates, one regulatory mechanism? Biochim Biophys Acta. 2007;1772:422–429. doi: 10.1016/j.bbadis.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruben GC, Ciardelli TL, Grundke-Iqbal I, Iqbal K. Alzheimer disease hyperphosphorylated tau aggregates hydrophobically. Synapse. 1997;27:208–229. doi: 10.1002/(SICI)1098-2396(199711)27:3<208::AID-SYN7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 45.Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. The Journal of biological chemistry. 2004;279:34873–34881. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 46.Ding H, Matthews TA, Johnson GV. Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J Biol Chem. 2006;281:19107–19114. doi: 10.1074/jbc.M511697200. [DOI] [PubMed] [Google Scholar]
- 47.Gohar M, Yang W, Strong W, Volkening K, Leystra-Lantz C, Strong MJ. Tau phosphorylation at threonine-175 leads to fibril formation and enhanced cell death: implications for amyotrophic lateral sclerosis with cognitive impairment (ALSci) J Neurochem. 2008;108:634–643. doi: 10.1111/j.1471-4159.2008.05791.x. [DOI] [PubMed] [Google Scholar]
- 48.Kuhla B, Haase C, Flach K, Luth HJ, Arendt T, Munch G. Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J Biol Chem. 2007;282:6984–6991. doi: 10.1074/jbc.M609521200. [DOI] [PubMed] [Google Scholar]
- 49.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 50.Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX. Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. The European journal of neuroscience. 2007;26:3429–3436. doi: 10.1111/j.1460-9568.2007.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King ME, Ghoshal N, Wall JS, Binder LI, Ksiezak-Reding H. Structural analysis of Pick’s disease-derived and in vitro-assembled tau filaments. Am J Pathol. 2001;158:1481–1490. doi: 10.1016/S0002-9440(10)64099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Congdon EE, Kim S, Bonchak J, Songrug T, Matzavinos A, Kuret J. Nucleation-dependent tau filament formation: the importance of dimerization and an estimation of elementary rate constants. J Biol Chem. 2008;283:13806–13816. doi: 10.1074/jbc.M800247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedhoff P, von Bergen M, Mandelkow EM, Davies P, Mandelkow E. A nucleated assembly mechanism of Alzheimer paired helical filaments. Proc Natl Acad Sci USA. 1998;95:15712–15717. doi: 10.1073/pnas.95.26.15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poppek D, Keck S, Ermak G, Jung T, Stolzing A, Ullrich O, Davies KJ, Grune T. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. The Biochemical journal. 2006;400:511–520. doi: 10.1042/BJ20060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko LW, DeTure M, Sahara N, Chihab R, Yen SH. Cellular models for tau filament assembly. J Mol Neurosci. 2002;19:311–316. doi: 10.1385/jmn:19:3:309. [DOI] [PubMed] [Google Scholar]
- 56.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- 57.Paudel HK. The regulatory Ser262 of microtubule-associated protein tau is phosphorylated by phosphorylase kinase. J Biol Chem. 1997;272:1777–1785. [PubMed] [Google Scholar]
- 58.Chang E, Kim S, Yin H, Nagaraja HN, Kuret J. Pathogenic missense MAPT mutations differentially modulate tau aggregation propensity at nucleation and extension steps. J Neurochem. 2008;107:1113–1123. doi: 10.1111/j.1471-4159.2008.05692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]