Abstract
Axonal regeneration in the central nervous system is prevented, in part, by inhibitory proteins expressed by myelin, including Myelin-associated glycoprotein (MAG). Although injury to the corticospinal tract can result in permanent disability, little is known regarding the mechanisms by which MAG affects cortical neurons. Here, we demonstrate that cortical neurons plated on MAG expressing CHO cells, exhibit a striking reduction in process outgrowth. Interestingly, none of the receptors previously implicated in MAG signaling, including the p75 neurotrophin receptor or gangliosides, contributed significantly to MAG-mediated inhibition. However, blocking the small GTPase Rho or its downstream effector kinase, ROCK, partially reversed the effects of MAG on the neurons. In addition, we identified the lipid phosphatase PTEN as a mediator of MAG’s inhibitory effects on neurite outgrowth. Knockdown or gene deletion of PTEN or over expression of activated AKT in cortical neurons resulted in significant, although partial, rescue of neurite outgrowth on MAG-CHO cells. Moreover, MAG decreased the levels of phospho-Akt, suggesting that it activates PTEN in the neurons. Taken together, these results suggest a novel pathway activated by MAG in cortical neurons involving the PTEN/PI3K/AKT axis.
Keywords: MAG, PTEN, cortical, outgrowth, neuron, AKT
Introduction
The limited regrowth of axons following injury in the mammalian central nervous system (CNS) can result in debilitating and often permanent neurological deficits. Myelin, produced by oliogodendrocytes to facilitate saltatory conduction, expresses a number of proteins that inhibit axonal growth and are thought to contribute to preventing regeneration. These myelin-associated inhibitors include Nogo A, Myelin-associated glycoprotein (MAG), Oligodendrocyte myelin glycoprotein (OMgp), Ephrin-B3, and Sema4D (Filbin, 2003; Yiu and He, 2006).
MAG, one of the most extensively studied myelin-associated inhibitors, is a transmembrane glycoprotein expressed on periaxonal myelin membranes in the peripheral and central nervous systems. It plays a physiological role in maintaining myelinated axons as well as contributing to the pathology of demyelinating diseases and the inhibition of CNS regeneration (Filbin, 2003; Quarles, 2007). Several neuronal receptors have been identified for MAG, including the Nogo receptor (NgR1 and NgR2) (Domeniconi et al., 2002; Liu et al., 2002; Venkatesh et al., 2005), gangliosides GD1a and GT1b (Yang et al., 1996; Vinson et al., 2001; Vyas et al., 2002), and the more recently identified paired immunoglobulin-like receptor B (PirB) (Atwal et al., 2008).
Understanding how the various receptors contribute to the inhibition of axon outgrowth by MAG has proven remarkably complex. NgR1 is a glycosylphosphatidylinositol-linked protein that binds MAG and signals through interaction with the p75 neurotrophin receptor (p75NTR) (Wang et al., 2002; Wong et al., 2002) or a homologous protein, Taj/TROY (Park et al., 2005; Shao et al., 2005), and the novel transmembrane protein LINGO-1 (Mi et al., 2004). The NgR complex was recently reported to function in an additive manner with the PirB receptor, such that inhibiting both receptors was necessary to fully reverse the effects of myelin (Atwal et al., 2008). The effects of MAG have also been reported to depend on interaction with gangliosides, at least in some neurons (Vyas et al., 2002; Mehta et al., 2007); however, this remains controversial (Cao et al., 2007; Schnaar and Lopez, 2009). It has been suggested that MAG’s inhibitory effects involve different receptors depending on the type of neuron and the nature of the inhibition, such as acute growth cone collapse versus more long-term axon extension (Chivatakarn et al., 2007; Mehta et al., 2007; Venkatesh et al., 2007).
How these various MAG receptors transduce their signals to inhibit axon growth is also not well understood. The NgR1 complex can activate the GTP binding protein RhoA, which regulates the actin-cytoskeleton, leading to growth cone collapse and the prevention of neurite outgrowth (Hall, 1998; Niederost et al., 2002; Wang et al., 2002; Yamashita et al., 2002). However, a role for intracellular calcium (Song et al., 1998; Wong et al., 2002) and protein kinase C (PKC) (Hasegawa et al., 2004; Sivasankaran et al., 2004) has also been suggested downstream of NgR-p75NTR-LINGO. The mechanisms by which gangliosides and PirB signal have yet to be determined, although an association between PirB and the phosphatases Shp-1 and Shp-2 has been reported (Syken et al., 2006).
We set out to investigate the receptors and intracellular signaling pathways involved in MAG-mediated inhibition of neurite outgrowth, specifically in cortical neurons. One of the pathways often damaged in spinal cord injury is the corticospinal tract, resulting in debilitating clinical manifestations including paralysis (Joosten, 1997; McDonald and Sadowsky, 2002). However, very few studies have investigated how cortical neurons respond to myelin-associated inhibitors and the mechanisms underlying their inhibition.
Our results demonstrate that cortical neuron process growth is robustly inhibited by MAG, but the inhibition could not be reversed by deletion of p75NTR or by blocking gangliosides or PirB. Surprisingly, inhibition of Rho signaling only partially reversed the effect of MAG, indicating the presence of additional intracellular signals. We identify PTEN as a downstream effector of MAG-mediated inhibition of cortical neuron process growth. Together, these findings suggest a novel pathway activated by MAG in cortical neurons involving the PTEN/PI3K/AKT axis.
Results
Neurite outgrowth in cortical neurons is potently inhibited by MAG
To evaluate the effects of MAG on neurite outgrowth from cortical neurons, embryonic neurons (E15-17) isolated from mouse cortex were plated on CHO cells stably expressing MAG on the cell surface (MAG-CHO cells) or control CHO cells. After approximately 20 hours, neurons were assessed for neurite outgrowth. Neurites were detected on 43.0% of the neurons on the control CHO cells; however, only 6.7% of the neurons on MAG-CHO cells displayed neurite outgrowth (Fig 1a). Neurite lengths were also considerably diminished on MAG-CHO cells, with a 53.6% reduction in neurite length compared to control CHO cells (Fig 1a). Since the corticospinal tract does not form until after birth in rodents (Bastmeyer and O’Leary, 1996), we also decided to analyze the effects of MAG on early postnatal cortical neurons. Using cortical neurons from P1-P3 pups, we found a comparable reduction in neurite outgrowth by MAG (Fig 1a). The dramatic reduction in neurite outgrowth was not due to cell death as there was no difference in the percent of apoptotic neurons on MAG-CHO cells (13.6%) as compared to control CHO cells (14.3%) (Fig 1b).
Figure 1.
Cortical neuron outgrowth is dramatically inhibited by cell surface expressed MAG. A, Embryonic (E15-17) or postnatal (P1-3) cortical neurons were plated on control CHO or MAG-CHO cells for ~20 hrs then fixed and stained for TuJ1. Neurite outgrowth was measured as the percent of neurons with neurites or length of the longest neurite per neuron. Representative images are shown of embryonic cortical neuron cultures and the quantified data depicted below. Data represents mean ± SEM. For % neurons with neurites, n=8 for embryonic neurons and n=2 for postnatal neurons with 2-3 replicates per experiment. For length analysis, n=3 with 2 replicates per experiment. B, The percent of apoptotic neurons on control CHO and MAG-CHO cells was determined for embryonic cortical neurons. There was no significant difference between conditions based on a Student’s t-test. Data represent mean ± SEM (n=4). C, Neurite outgrowth of CGNs on control CHO and MAG-CHO cells was quantified. Data represents mean ± SEM (n=6 for % neurons with neurites, n=3 for neurite length). (*, p<0.01, **, p<0.0003 based on a Student’s t-test.)
We performed similar neurite outgrowth experiments with CGNs, a neuron type often used to study inhibition of axonal growth by myelin proteins such as MAG. As expected, neurite outgrowth was significantly reduced (Fig 1c); however, the response of cortical neurons to MAG was substantially greater than that of CGNs. We observed an 86.0% reduction in the percent of embryonic cortical neurons with neurites, a 71.1% reduction in outgrowth of postnatal cortical neurons, but only a 34.1% reduction in outgrowth of CGNs. Similarly, we detected a 34.5% reduction in neurite length in CGNs on MAG-CHO cells relative to control CHO cells, compared to 53.6% for cortical neurons. These results suggest that cortical neurons are particularly sensitive to inhibition by MAG, raising the question as to the underlying mechanisms.
The effect of MAG on cortical neuron outgrowth is independent of its known receptors
MAG has been shown to signal through a complex of the NgR1, p75NTR and LINGO in several types of neurons. In order to characterize the receptors playing a role in cortical neurite outgrowth inhibition by MAG, we first analyzed the expression of p75NTR and TROY in embryonic mouse cortex and cortical cultures, since these are the signal transducers in the tripartite complex. P75NTR was detected in cortical tissue as well as cortical neuron cultures, but there was no detectable signal for TROY (Supplemental Figure 1a). Therefore, we investigated whether p75NTR was necessary for mediating the effects of MAG in cortical neurons. When plated on MAG expressing CHO cells, neurite outgrowth from both p75ntr+/+ and p75ntr−/− neurons was similarly inhibited (Fig 2a), indicating that this receptor is not required for the response to surface bound MAG. As a comparison, we analyzed outgrowth of p75ntr+/+ and p75ntr−/− CGNs on MAG-CHO cells and found a similar response in both genotypes (Fig 2a). These results suggest that membrane bound MAG does not signal through the p75NTR-NgR-LINGO complex in cortical neurons or CGNs.
Figure 2.
Inhibition of neurite outgrowth in cortical neurons by MAG is not mediated by p75NTR, gangliosides or PirB. A, p75ntr+/+ or p75ntr−/− postnatal cortical neurons or CGNs were plated on control CHO or MAG-CHO cells for ~20 hrs, at which point the cells were fixed and stained for TuJ1. Neurite outgrowth was measured as the percent of neurons with neurites. Data represent mean ± SEM (n=2, 2-4 replicates per experiment). B, Cortical neurons or CGNs were plated on control CHO or MAG-CHO cells in the presence of neurominidase (40 mU/ml). Significant difference in the inhibition of neurite outgrowth was detected for CGNs, but not for cortical neurons. Data represents mean ± SEM (n=2, 2 replicate wells per experiment). C, Cortical neurons or CGNs were plated on control CHO or MAG-CHO cells in the presence of 50μg/ml of control antibody (human IgG) or PirB antibody. In CGNs, anti-PirB significantly reduced the inhibitory effects of MAG (n=3). However, there was no significant difference in the inhibition of neurite outgrowth by MAG in cortical neurons treated with anti-PirB (n=4). Data represents mean ± SEM. (*, p<0.05, **, p<0.01, ***, p<0.001 by two way ANOVA).
Gangliosides have also been proposed to contribute to the inhibitory effect of MAG on neurite outgrowth, particularly in CGNs and hippocampal neurons (Mehta et al., 2007). Since GT1b and GD1a are expressed in cortical neurons (Lee et al., 2007), we investigated whether gangliosides mediate the MAG effect in cortical neurons. Neurons plated on MAG-CHO or control CHO cells, were treated with neurominidase to cleave off sialic acid moieties, which are required for MAG binding to GT1b and GD1a (Vyas et al., 2002). In agreement with previous studies (DeBellard et al., 1996; Venkatesh et al., 2007), we observed significant reversal of CGN neurite outgrowth on MAG-CHO cells in the presence of neurominidase. However, even with concentrations as high as 40 mU/ml there was no significant increase in outgrowth of cortical neurons on MAG-CHO cells (Fig 2b), indicating that membrane bound MAG signals independent of gangliosides in these neurons.
Recently, Atwal et al (2008) identified PirB as a functional receptor for the myelin-associated inhibitors, including MAG. In agreement with previous findings, (Syken et al., 2006), we detected PirB in cortical neurons (Supplemental Figure 1b); therefore we investigated its role in mediating the effects of MAG on neurite outgrowth. Cortical neurons were plated on control CHO or MAG-CHO cells in the presence of a PirB function-blocking antibody previously shown to reverse the inhibitory effects of myelin proteins in dorsal root ganglia (DRG) neurons and CGNs (Atwal et al., 2008). However, blocking PirB did not significantly attenuate the inhibition of neurite outgrowth by membrane bound MAG (Fig 2c). Increasing the concentration of the antibody 4-fold did not provide any further increase in neurite growth (data not shown). In contrast, there was a significant, although only partial, rescue of CGN neurite outgrowth on MAG-CHO cells in the presence of the PirB antibody (Fig 2c) in agreement with Atwal et al (2008). Taken together, these results demonstrate that membrane bound MAG prevents axonal growth in cortical neurons independent of the known receptors, suggesting there is another receptor system yet to be identified.
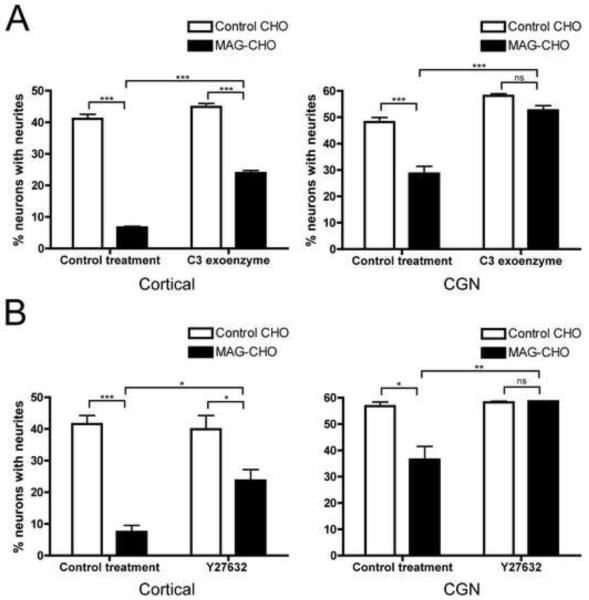
Inhibiting Rho or ROCK partially rescues cortical neuron outgrowth on a MAG substrate
Previous studies demonstrated that the GTPase RhoA and its effector Rho kinase (ROCK) are key mediators of the inhibitory effects of MAG in CGNs and DRG neurons (Lehmann et al., 1999; Niederost et al., 2002; Fournier et al., 2003; Alabed et al., 2006) and the NgR1-p75-LINGO complex can activate this pathway (Wang et al., 2002; Yamashita et al., 2002; Yamashita and Tohyama, 2003). Therefore, to determine whether this cascade also contributes to the inhibition of neurite outgrowth by MAG in cortical neurons we treated the cells with C3 exoenzyme (C3), a Rho inhibitor, or Y27632, a ROCK inhibitor. As a positive control, we confirmed that both compounds could fully reverse the inhibition of neurite outgrowth by MAG in CGNs (Fig 3a and 3b, respectively). Surprisingly, C3 or Y27632 only partially reversed the inhibition of neurite outgrowth in cortical neurons (Fig 3a and 3b). Both compounds rescued process extension to an equivalent extent, consistent with Rho and ROCK acting in the same pathway. Thus, although Rho and ROCK play a significant role in mediating the effects of MAG in cortical neurons, there must be other signaling pathways involved.
Figure 3.
Inhibition of neurite outgrowth in cortical neurons by MAG is partially reversed by blocking Rho signaling. A, Cortical neurons (left panel) were harvested and plated on control CHO or MAG-CHO cells in the presence of vehicle or cell permeable C3 exoenzyme (1μg/ml). 20 hrs after plating, neurons were fixed and immunostained for TuJ1. Neurite outgrowth was measured as the percent of neurons with neurites. Data represents mean ± SEM (n=4). Similar experiments were conducted with CGNs (right panel) (n=2). B, Cortical neurons (left panel) or CGNs (right panel) were plated on control CHO or MAG-CHO cells in the presence of vehicle or the ROCK inhibitor Y27632 (100μM). 20 hrs after plating the neurons were fixed and immunostained for TuJ1. Neurite outgrowth was measured as the percent of neurons with neurites. Data represent mean ± SEM (n=2). (*, p<0.05, **, p<0.01, ***, p<0.001 by two way ANOVA).
MAG inhibits neurite outgrowth in cortical neurons through a PTEN dependent mechanism
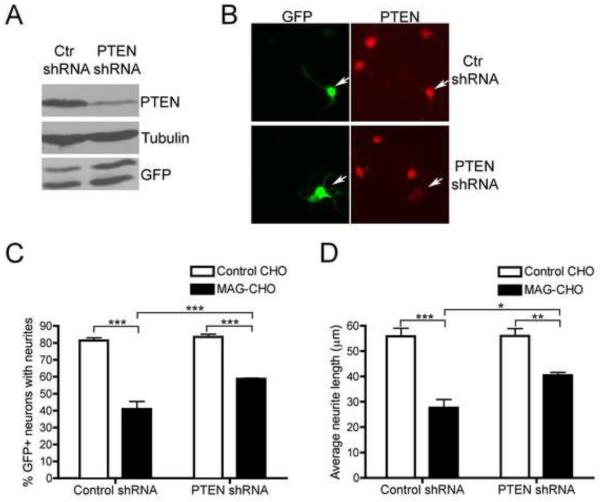
Numerous signal transduction pathways have been implicated in the regulation of neurite outgrowth (Hou et al., 2008). We tested inhibitors of several of these pathways for their ability to reverse the effects of MAG: SP600125, a c-Jun N-terminal kinase inhibitor, BIO/Inhibitor IX, an inhibitor of GSK3, Roscovitine, which targets cdk5, and the Src-family kinase inhibitor PP2. However, we did not observe any notable rescue (data not shown). We were also interested in evaluating a possible role for PTEN (Phosphatase and tensin homologue deleted on chromosome 10), since it was recently reported that deletion of this phosphatase resulted in considerable regeneration of retinal ganglion cell axons following optic nerve injury (Park et al., 2008). To determine whether MAG signals through PTEN to negatively regulate neurite outgrowth we first evaluated the effect of PTEN knockdown. Cortical neurons were co-transfected with GFP and a control shRNA or PTEN shRNA and plated on laminin for 72 hours to allow for efficient PTEN knockdown, at which point they were replated onto MAG-CHO cells or control CHO cells. PTEN knockdown was confirmed by Western blot analysis of neurons collected after 72 hours (Fig 4a) and immunostaining of replated neurons (Fig 4b). Approximately 98% of GFP+ neurons co-transfected with PTEN shRNA had decreased PTEN staining. Interestingly, knocking down PTEN significantly restored neurite outgrowth in neurons plated on the MAG-CHO cells (a 20.2% increase in the percent of neurons with neurites and a 22.7% increase in the average neurite length relative to neurons on control CHO cells), although there was not a complete recovery to control levels (Fig 4c and Fig 4d). It is important to note that reducing PTEN did not affect neurite outgrowth when the neurons were plated on control CHO cells (Fig 4c and Fig 4d). These results suggested that PTEN contributes to the growth inhibitory signal elicited by MAG.
Figure 4.
PTEN knockdown in cortical neurons partially reverses neurite outgrowth inhibition by MAG. Cortical neurons were electroporated with GFP and control shRNA or PTEN shRNA and plated on laminin-coated dishes for 72 hrs to allow for optimal PTEN knockdown. A, At 72 hrs, neurons were lysed and analyzed by Western blot or, B, replated and 20 hrs later fixed and immunostained for PTEN to verify PTEN knockdown or, C and D, replated on control CHO or MAG-CHO cells for neurite outgrowth analysis. The cells were fixed 20 hrs after plating and immunostained for Tuj1. Neurite outgrowth was analyzed by counting the percentage of GFP+ neurons with neurites (C) (n=4) or measuring neurite lengths of GFP+ neurons (D) (n=3). Data represent mean ± SEM (*, p<0.05, **, p<0.01, ***, p<0.001 by two way ANOVA).
To further establish a role for PTEN in MAG mediated inhibition of neurite outgrowth, we evaluated the effect of genetically deleting pten in cortical neurons. Mice expressing Cre recombinase under an EMX1 promoter (Gorski et al., 2002) were crossed with pten floxed mice (Lesche et al., 2002). PTEN knockout was confirmed in cortical neurons by Western blot analysis of cultured neurons (Fig 5a). Embryonic EMX1-Cre;pten+/+, WT;ptenfl/fl or EMX1-Cre; ptenfl/fl cortical neurons were plated on control CHO or MAG-CHO cells for 20 hours. A significant increase in the percent of neurons with neurites and neurite length was observed for pten null neurons on MAG-CHO cells compared to EMX1-Cre;pten+/+ or WT;ptenfl/fl neurons (Fig 5b and 5c). Interestingly, when pten null cortical neurons were treated with C3 exoenzyme, neurite outgrowth on MAG-CHO cells was not significantly different from that on control CHO cells (Fig 5b and Fig 5c). The additive effect of PTEN knockout and Rho inactivation suggests that both molecules contribute to inhibition of cortical neurite outgrowth by MAG, likely acting through different pathways.
Figure 5.
PTEN knockout cortical neurons are less susceptible to inhibition by MAG. A, Lack of PTEN expression in EMX1-Cre;ptenfl/fl neurons was confirmed by Western blot analysis of cortical neuron culture lysates. B and C, EMX1-Cre;pten+/+, wildtype;ptenfl/fl or EMX1-Cre;ptenfl/fl (pten−/−) embryonic neurons (E17) were plated on control CHO or MAG-CHO cells in the presence or absence of C3 exoenzyme (1μg/ml) and subsequently fixed and immunostained for Tuj1. No difference was observed between the EMX1-Cre;pten+/+ and the wildtype;ptenfl/flneurons; therefore the data were combined and are labeled as pten+/+. Neurite outgrowth was evaluated by determining the percent of neurons with neurites (B) or measuring neurite lengths (C). Data represent mean ± SEM (n=3, *, p<0.05, **, p<0.01, ***, p<0.001 by two way ANOVA).
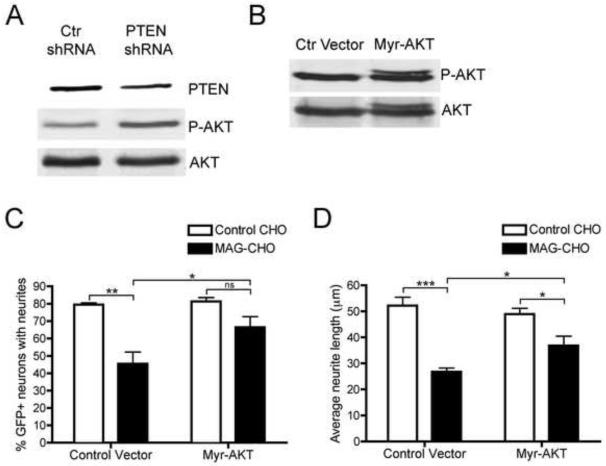
One of the primary biological functions of PTEN is to counteract phosphatidylinositol 3-kinase (PI3K) by de-phosphorylating phosphatidylinositol 3,4,5-trisphosphate (PIP3), thereby inhibiting activation of the downstream kinase AKT. As expected, we observed an increase in phosphorylated AKT in neurons transfected with PTEN shRNA (Fig 6a). To further establish a role for PTEN downstream of MAG, we assessed the effect of expressing an activated form of AKT, by-passing the effects of PTEN. A myristoylated form of AKT (myr-AKT) was transfected into cortical neurons and neurite outgrowth on MAG-CHO cells was compared to that on control CHO cells. The activation of AKT by the expression of myr-AKT was confirmed by an increase in the phosphorylated form of the enzyme (Fig 6b). Cortical neurons were co-transfected with GFP and myr-AKT or a control vector and 72 hours later, the neurons were replated on control CHO or MAG-CHO cells for twenty hours. When transfected with myr-AKT, there was a significant, 24.4% increase in the percent of neurons with neurites on MAG-CHO cells relative to neurons transfected with a control vector (Fig 6c). A similar 23.8% increase in average neurite length was observed for neurons expressing myr-AKT on MAG-CHO cells compared to control transfected cells (Fig 6d). These results indicate that preventing activation of PTEN by expressing active AKT can reverse the inhibition of neurite outgrowth by MAG.
Figure 6.
Expression of constitutively active AKT in cortical neurons reverses the inhibition of neurite outgrowth by MAG. A, Cortical neurons were electroporated with control shRNA or PTEN shRNA, cultured for 72 hrs, then subjected to Western blot analysis for levels of PTEN, phospho-AKT and AKT. B, Cortical neurons were electroporated with control vector or myr-AKT and 72 hrs later lysed and the level of phospho-AKT and total AKT analyzed by Western blot. C and D, Cortical neurons were electroporated with GFP and control vector or myr-AKT and plated on laminin-coated dishes for 72 hrs at which point they were replated on control CHO or MAG-CHO cells. The neurons were fixed 20 hr after plating, immunostained for Tuj1 and neurite outgrowth was analyzed by counting the percentage of GFP+ neurons with neurites (C) or determining neurite lengths of GFP+ neurons (D). Data represents mean ± SEM (n=3, *, p < 0.05, **, p<0.01, ***, p<0.001 by two way ANOVA).
Finally, to demonstrate that PTEN is regulated by MAG, we assessed the activity of AKT in neurons plated on MAG-CHO cells. If MAG activates PTEN, then the level of phospho-AKT should be reduced. We were specifically interested in the response of the neurons to membrane-bound MAG; therefore, the neurons were plated on MAG-CHO cells or control CHO cells for 2 - 4 hours, then isolated by fluorescence-activated cell sorting (FACS) and the level of phospho-AKT measured by Western blot. To separate neurons from CHO cells, the neurons were labeled with calcein dye prior to plating. Reanalysis of collected cells confirmed that only calcein positive cells were collected (Fig 7a). Sorted cells were lysed and subjected to Western blot analysis for TuJ1 expression and levels of phosphorylated AKT. To verify that this method reliably detected changes in AKT phosphorylation and that the phosphorylation was not reversed during the sorting protocol, the neurons on control CHO cells were treated with BDNF, which is known to increase AKT phosphorylation in cortical neurons (Hetman et al., 1999). We consistently detected an increase in phosphorylated AKT following BDNF treatment (Fig 7b and 7c). Importantly, we also found that neurons plated on MAG-CHO cells had significantly reduced levels of phosphorylated AKT compared to those plated on control CHO cells (Fig 7b and 7c). Taken together, these results reveal a novel mechanism by which MAG inhibits cortical neurite outgrowth through inhibition of the PI3K/AKT pathway.
Figure 7.
MAG reduces AKT phosphorylation in cortical neurons. Cortical neurons were labeled with calcein, plated on control or MAG expressing CHO cells for 2-4 hr, and then subjected to FACS to isolate the neurons from the CHO cells. A, The isolation of neurons from CHO cells using calcein labeling was confirmed by reanalysis of the sorted cells. The left panel depicts unlabeled neurons (green) plated on CHO cells (orange), which can be roughly differentiated from each other based on side scatter and forward scatter differences. The central panel shows calcein-labeled neurons plated on CHO cells. The small peak for CHO cells at higher fluorescence intensity in the central panel is likely neuron doublets. The right panel depicts fluorescent cells that were collected by FACS, then reanalyzed to verify that fluorescent negative cells were excluded. B, Following FACS, neurons that had been plated on control CHO or MAG-CHO were lysed and analyzed by Western blot for phospho-AKT, total AKT, and TuJ1 expression (to further confirm neuronal isolation and as a loading control). BDNF treated or untreated neurons plated on control CHO cells and isolated by FACS served as a control for the experimental method. C, Quantitative analysis of the ratio of phospho-AKT to total AKT. Values were normalized to untreated neurons plated on control CHO cells. Data represents mean ± SEM (n=5, * p = 0.0005, based on Student’s t-test).
Discussion
Cortical neurons are frequently damaged in spinal cord injury and fail to regenerate, in part, due to the presence of growth inhibitory proteins at the site of injury. Furthermore, recent findings suggest that inhibitors expressed by myelin may normally function in the refinement of cortical circuitry during development (McGee et al., 2005). However, very little is known about how cortical neurons respond to specific, endogenous inhibitors of axon growth. Here, we investigated the response of cortical neurons to MAG, a myelin protein known to prevent axonal outgrowth in other neurons. Our results indicate that both embryonic and postnatal cortical neurons are particularly sensitive to inhibition of neurite outgrowth by MAG. Interestingly, the effects of MAG were not mediated by any of its known receptors, but involved both the Rho/ROCK pathway and a novel pathway involving the lipid phosphatase PTEN.
MAG is a multifunctional, transmembrane protein expressed on the periaxonal surface of myelinating cells in both the peripheral and central nervous system. It is important for long-term axon-myelin stability and for proper structuring of nodes of Ranvier (Quarles, 2007; Schnaar and Lopez, 2009). In addition, MAG regulates axonal growth, being able to promote it or inhibit it depending on the type of neuron as well as the developmental stage. MAG enhances neurite outgrowth from a number of embryonic neurons, including retinal ganglion cells (Cai et al., 2001), spinal neurons (Turnley and Bartlett, 1998; Cai et al., 2001), and dorsal root ganglia (DRG) neurons (Johnson et al., 1989; Mukhopadhyay et al., 1994; DeBellard et al., 1996; Cai et al., 2001), whereas postnatal axon outgrowth of these neurons is inhibited. In cortical neurons, we observed dramatic inhibition of neurite outgrowth by MAG at both embryonic (E15 to E17) and postnatal (P1-3) ages.
The mechanisms by which MAG mediates these various signals are poorly understood. Due to the relevance for spinal cord injury, much attention has focused on elucidating the signals responsible for inhibiting axonal growth. The best characterized receptor complex mediating the effects of MAG is the one formed by the gpi-linked Nogo receptor, p75NTR or TROY and LINGO. However, we found that p75NTR was not involved in preventing neurite outgrowth in cortical neurons and no expression of TROY was detectable (Fig. 2). These results are in agreement with those of Chivatakarn et al (2007) who found that cortical neurons from mice with NgR1 deleted were as responsive to MAG-CHO cells as wild-type neurons. Since MAG can also bind NgR2 (Venkatesh et al., 2005), it is possible their results could be explained by NgR2 substituting for NgR1. However, taken together with our findings, the more likely conclusion is that the NgR-p75NTR-LINGO complex is not required for the inhibition of neurite out growth by membrane-bound MAG in cortical neurons.
The role of the NgR-p75NTR-LINGO complex in mediating the effects of MAG appears to depend on the type of neuron and the form of MAG used. For example, Venkatesh et al reported that wild-type and p75ntr null retinal ganglion cells (RGCs) and CGNs were equally inhibited by MAG-CHO cells; however, p75ntr−/− DRG neurons were significantly less inhibited than wild-type neurons, suggesting that membrane-bound MAG signals through p75NTR in DRGs but not RGCs or CGNs (Venkatesh et al., 2007). In contrast, numerous reports have demonstrated a requirement for p75NTR in mediating the response of CGNs to a soluble fragment of MAG containing the extracellular domain (Wang et al., 2002; Yamashita et al., 2002; Yamashita and Tohyama, 2003). A soluble form of MAG has been detected following incubation of spinal cord extracts in vitro (Tang et al., 2001), but whether this cleaved product of MAG is generated after neuronal injury is not known. Ultimately, a major objective of studies on MAG’s ability to inhibit neurite outgrowth is to understand how regeneration could be facilitated following axonal damage in vivo. However, p75ntr−/− mice do not exhibit any significant improvement in regeneration of the CST following spinal cord injury, indicating that this receptor does not have a major role in preventing axonal growth from cortical neurons in vivo (Song et al., 2004; Zheng et al., 2005).
Two other receptors have been reported for MAG, the gangliosides GT1b and GD1a, and PirB, a major histocompatibility complex class 1 receptor. However, we found that neither of these molecules significantly contributed to the inhibitory effects of MAG in cortical neurons. Of course we cannot rule out the possibility that the concentration of PirB antibody used was not high enough to disrupt the interaction of MAG with cortical neurons. Although not statistically significant, there was a trend toward a partial reversal of the inhibition by MAG with anti-PirB. However, taken together, these results suggest that there exists an additional, yet to be identified receptor for membrane-bound MAG that transduces a signal preventing neurite outgrowth in cortical neurons.
Down stream of many signals inhibiting process outgrowth and extension in cells are the small GTPase Rho and its effector kinase ROCK. Increased Rho activity has been detected in neurons following spinal cord injury (Dubreuil et al., 2003; Madura et al., 2004) and application of the Rho inhibitor C3 or the ROCK inhibitor Y27632 at the site of injury resulted in significant, although limited, regeneration of corticospinal tract fibers as well as functional improvement in locomotion and coordination (Dergham et al., 2002; Fournier et al., 2003). Similarly, genetic deletion of ROCKII revealed that this kinase plays a role in restricting axon outgrowth following spinal cord injury (Duffy et al., 2009). MAG has been reported to increase Rho activity in neurons (Lehmann et al., 1999; Niederost et al., 2002; Yamashita et al., 2002; Fournier et al., 2003) and, not unexpectedly, we found that inhibiting Rho or ROCK significantly reversed the effect of membrane-bound MAG on cortical neurons. However, we were surprised to find that blocking Rho or ROCK was only partially effective in the cortical neurons; significant inhibition of neurite outgrowth remained in the neurons plated on MAG-CHO cells when C3 or Y27632 was added. This result suggested that membrane-bound MAG was activating additional growth inhibitory signals.
We identified PTEN as an essential component of MAG’s inhibitory effects on neurite outgrowth in cortical neurons. PTEN is a lipid phosphatase that indirectly inactivates the kinase Akt by reducing the levels of phosphatidylinositol 3,4,5 trisphosphate, which is required for Akt activation. A previous study suggested that the PI3K/AKT/mTOR pathway promotes axon extension and demonstrated that deletion of PTEN or the mTOR repressor, Tuberous sclerosis complex 1 (TSC1), significantly improved regeneration in the optic nerve following a crush injury (Park et al., 2008). Here, we demonstrated that membrane-bound MAG actively reduced Akt phosphorylation, thereby implicating activation of PTEN by this inhibitor. Suppression of Akt activity would decrease activation of Rac, a GTP binding protein that promotes neurite extension through regulation of actin polymerization, and increase the activation of Glycogen synthase kinase 3 (GSK3), a kinase that regulates microtubule dynamics (Park et al.). Similar to our findings, PTEN was recently identified as a target of Sema3A in triggering growth cone collapse of sensory neurons (Chadborn et al., 2006). Thus, PTEN appears to be a key component in another signal transduction pathway, like the Rho/ROCK cascade, that is activated by inhibitors of axon growth.
The mechanisms by which PTEN is regulated are not well understood. It can be serine/threonine phosphorylated by casein kinase 2 (CK2) and GSK3 and evidence exists suggesting that this modification inhibits its activity, reduces membrane localization and/or causes destabilization of the protein (Leslie et al., 2008). Recently, NGF was reported to increase PTEN phosphorylation by CK2 in hippocampal neurons, thereby promoting axonal growth (Arevalo and Rodriguez-Tebar, 2006). Reactive oxygen species generated in response to various growth factors can also suppress PTEN activity by causing the formation of disulfide bonds (Lee et al., 2002; Leslie et al., 2003; Kwon et al., 2004; Seo et al., 2005). In contrast, the recruitment to membranes by increasing the local concentration of acidic lipids, such as phosphatidylinositol 3,4 bisphosphate, can activate PTEN (Campbell et al., 2003; Iijima et al., 2004; Redfern et al., 2008). How MAG or Sema3A modulate PTEN activity is not known. Chadborn et al (2006) suggested that Sema3A induces a local accumulation of PTEN at the growth cone, leading to a depletion of phosphatidylinositol 3,4,5 trisphosphate. Determining the mechanisms by which MAG activates PTEN will be an interesting topic for future studies.
Gaining a more complete understanding of the different mechanisms employed by myelin-associated inhibitors in specific neuron types could help in developing therapeutics that will be effective for specific fiber tracts and brain regions following injury. Our results with cortical neurons suggest that in addition to Rho/ROCK, PTEN is another therapeutic target that may enhance regeneration of these neurons. Furthermore, the identification of PTEN as a downstream effector of MAG in preventing neurite outgrowth may have implications for other processes mediated by MAG such as axon-myelin stability and the refinement of cortical circuitry.
Materials and Methods
Primary neuron cultures
Cerebellar granule neuron (CGN) cultures were prepared from postnatal day 4-7 mice. Cerebella were isolated, dissociated in 0.125% trypsin (Worthington) at 37°C for 15 min, washed in PBS, triturated and subsequently plated in Neurobasal media (Gibco) containing B27 supplement (Gibco), 25mM KCl, 33mM dextrose, 2mM glutamine, and 100U/ml penicillin / 100μg/ml streptomycin (Gibco). Cortical neurons were isolated at E15-E17 or postnatal day 1-3, dissociated in 0.06% trypsin (Worthington) for 30 minutes at room temperature, triturated and plated in Neurobasal media with B27 supplement on dishes coated with 2μg/ml laminin (Invitrogen) or on CHO cells for neurite outgrowth assays.
Immunostaining
Cells were fixed in 3.7% formalin, blocked with 10% normal goat serum in PBS containing 0.2% triton X-100, incubated with primary antibody overnight and visualized with fluorescently labeled secondary antibodies and mounted with medium containing DAPI (Vectashield, Vector Labs). Primary antibodies used: anti-PTEN (1:100, Cell Signaling), anti-TuJ1 (1:500, Covance), anti-PirB (A-20, 1:50, Santa Cruz) and chicken anti-GFP for enhancement of GFP fluorescence (1:500, Ambion). Secondary antibodies used: Rhodamine conjugated donkey anti-rabbit (1:300, Jackson ImmunoResearch), goat anti-mouse Alexa 488 (1:500, Invitrogen), Rhodamine conjugated donkey anti-mouse (1:400, Jackson ImmunoResearch), donkey anti-goat Alexa 488 (1:500, Invitrogen) and Cy2 conjugated donkey anti-chicken (1:200, Jackson ImmunoResearch).
Neurite outgrowth assays
Neurite outgrowth assays on MAG transfected CHO cells have been previously described (Mukhopadhyay et al., 1994). Cortical neurons or CGNs (40,000/well) were plated on confluent monolayers of MAG-CHO or control CHO cells in 8 well tissue culture slides (Lab-Tek, Nalge Nunc International). After incubation for ~20 hours, co-cultures were fixed and immunostained with anti-TuJ1, as described above. Where indicated, compounds were added at the time of neuronal plating: cell permeable C3 exoenzyme (1μg/ml, Cytoskeleton, CT04), Y27632 (100μM, Sigma), neurominidase (40mU/ml, MP Biomedicals), function blocking PirB antibody (50μg/ml, kindly provided by M. Tessier-Levine, Genentech, CA). The percent of neurons with neurites was quantified in a blinded manner using a Zeiss fluorescence microscope at 40x magnification. A neurite was defined as a process at least twice the length of the cell body. For each experiment, 2-4 wells were used for each condition and at least 250 neurons were counted per condition. Neurite lengths were determined using ImageJ software analysis of images taken with a Zeiss fluorescence microscope at 40x magnification. The longest neurite ≥1 cell body was measured for each neuron, with at least 145 neurites evaluated for each experimental condition.
Mice
CD1 mice (Charles River), C57BL/6 mice with the p75 gene deleted (Lee et al., 1992), EMX1-Cre mice (Gorski et al., 2002) or PTEN floxed mice (Lesche et al., 2002) (Jackson Laboratory) were used, as indicated. All experiments with animals were approved by the Animal Care and Use Committee at Vanderbilt University.
HEK293 cell transfections
HEK293 cells, maintained in DMEM with 10% fetal bovine serum, were transfected with constructs expressing p75 (in pcDNA3) or TROY (in pFLAG-CMV-1) using Lipofectamine (Invitrogen) according to the manufacturer’s protocol. The TROY plasmid was generously provided by Zhigang He (Harvard Medical School, Boston, MA) (Park et al., 2005).
Neuronal transfections
Cortical neurons were co-transfected with GFP (pEGFP, Clontech) (1 μg) and control shRNA (5 μg) or PTEN shRNA (5 μg; in pSUPER.retro.puro; generously provided by C. Arteaga, Vanderbilt University, Nashville, TN (Miller et al., 2009)), or with GFP and myristoylated Akt (Myr-AKT; in pUSE; kindly provided by W. Pao, Vanderbilt University, Nashville, TN) or control vector by electroporation with an Amaxa Nucleofactor device according to the manufacturer’s protocol. Cells were plated on laminin-coated dishes and re-fed at 24 hr post-transfection. The neurons were incubated 48 hr to allow for PTEN knockdown or Myr-Akt expression, then they were incubated in trypsin and dispase and gently lifted with a cell lifter. Neurons were spun down and resuspended in Neurobasal/B27. 10,000 neurons/well were plated on 8-well slides containing confluent monolayers of CHO or MAG-CHO cells in Neurobasal/B27. Twenty hours after plating, cells were fixed and immunostained for TuJ1 and GFP and analyzed for neurite outgrowth. The remaining neurons were collected for Western blot analysis or replated on a laminin coated 8-well slide for immunostaining to verify PTEN knockdown. Myr-Akt expression was confirmed by Western blot. For analysis of the percent of neurons with neurites, 3-4 wells were counted per condition in a blinded manner for each experiment and a minimum of 800 neurons total were counted per experimental condition. Replicate wells, with at least 145 total neurites measured per experimental condition, were evaluated in a blinded fashion for neurite length.
FACS isolation of neurons
Cortical neurons isolated from E15 to E17 mice were labeled with calcein (2nM PBS) (Invitrogen, Molecular Probes) for 30 min with gentle rotation at room temperature, washed with PBS and incubated in PBS at room temperature for an additional 30 minutes. The labeled neurons (5 × 106) were resuspended in Neurobasal/B27 and plated on a confluent monolayer of MAG-CHO or control CHO cells in 6 cm dishes. After 2-4 hrs, the cells were washed twice with ice cold PBS to remove non-adhered neurons and then harvested by gentle scraping. The cells were re-suspended in PBS and passed through a cell strainer (BD Falcon, 40μm nylon) to remove cell clumps. The calcein labeled neurons were collected by FACS using a BD FACS Aria cell sorter. Reanalysis of sorted cells verified that only calcein labeled cells were collected. The sorted neurons were lysed and subjected to Western blot analysis as indicated. For control experiments involving BDNF treatment, the cells were treated with 100ng/ml BDNF for 10 minutes prior to harvesting for FACS analysis. Recombinant BDNF was generously provided by Regeneron, Inc.
Western blot analysis
Cells were lysed in NP40 lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 10% glycerol) containing a protease inhibitor cocktail (Complete Mini, Roche). For experiments analyzing phosphorylated proteins, 1mM sodium orthovanadate, 1mM sodium fluoride, and 1mM β-glycerophosphate were added to the lysis buffer. Cell lysates were subjected to SDS-PAGE and Western blotting using anti-AKT (1:1000, Cell Signaling), anti-phospho-AKT (Ser483) (1:1000, Cell Signaling), anti-PTEN (1:1000, Cell Signaling), anti-TuJ1 (1:1000, Covance), anti-p75NTR (1:1000, generated from a GST fusion protein with the p75NTR intracellular domain), anti-TROY (1:500, E19, Santa Cruz), mouse anti-GFP (1:1000, Roche), or anti-α-tubulin (1:1000, Calbiochem). Secondary antibodies conjugated to horse radish peroxidase included anti-rabbit (1:3000, Thermo Scientific) and anti-mouse (1:3000, Promega). For FACS collected neurons, the Odyssey infrared imaging system (Licor) was used for Western blot analysis and quantification according to the manufacturer’s protocol. Secondary fluorescent antibodies used for Odyssey were IRDye goat anti-mouse 800 and goat anti-rabbit 680 (Licor).
Apoptosis analysis
Cortical neurons were plated on control CHO or MAG-CHO cells. ~20 hrs following initial plating, neurons were fixed and stained with TuJ1 antibody and mounted with DAPI containing medium. Apoptotic cells were analyzed by counting condensed, pyknotic nuclei and the ratio of pyknotic nuclei to total neurons counted per field are represented as % apoptotic nuclei.
Supplementary Material
Supplemental Figure1: A, Lysates from E15 cortex, P6 cerebellum or cortical neuron cultures were analyzed by Western blot with antibodies to p75NTR or TROY. HEK 293 cells overexpressing p75NTR or TROY served as positive controls. B, Embryonic cortical neuron cultures were immunostained with an antibody to PirB. Staining in which primary antibody was omitted is included as a control.
Acknowledgements
The authors are grateful to Dr. Marc Tessier-Lavigne and Genentech for generously providing the antibody to PirB and helpful discussions. The Vanderbilt Medical Center Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). This work was supported by NIH grants RO1NS048249 and RO1NS038220 and the Christopher and Dana Reeve Foundation (B.D.C), F30 NS061403 and T32 GM07347 (A.L.P), and NIH grants RO1NS037060 and U54NS041073 (M.T.F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh SH, Fournier AE. Neuronal responses to myelin are mediated by rho kinase. J Neurochem. 2006;96:1616–1625. doi: 10.1111/j.1471-4159.2006.03670.x. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Rodriguez-Tebar A. Activation of casein kinase II and inhibition of phosphatase and tensin homologue deleted on chromosome 10 phosphatase by nerve growth factor/p75NTR inhibit glycogen synthase kinase-3beta and stimulate axonal growth. Mol Biol Cell. 2006;17:3369–3377. doi: 10.1091/mbc.E05-12-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Bastmeyer M, O’Leary DD. Dynamics of target recognition by interstitial axon branching along developing cortical axons. J Neurosci. 1996;16:1450–1459. doi: 10.1523/JNEUROSCI.16-04-01450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RB, Liu F, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:33617–33620. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- Cao Z, Qiu J, Domeniconi M, Hou J, Bryson JB, Mellado W, Filbin MT. The inhibition site on myelin-associated glycoprotein is within Ig-domain 5 and is distinct from the sialic acid binding site. J Neurosci. 2007;27:9146–9154. doi: 10.1523/JNEUROSCI.2404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadborn NH, Ahmed AI, Holt MR, Prinjha R, Dunn GA, Jones GE, Eickholt BJ. PTEN couples Sema3A signalling to growth cone collapse. J Cell Sci. 2006;119:951–957. doi: 10.1242/jcs.02801. [DOI] [PubMed] [Google Scholar]
- Chivatakarn O, Kaneko S, He Z, Tessier-Lavigne M, Giger RJ. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 2007;27:7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBellard ME, Tang S, Mukhopadhyay G, Shen YJ, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci. 1996;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, Kimura N, Cai H, Deng K, Gao Y, He Z, Filbin M. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol. 2003;162:233–243. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy P, Schmandke A, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci. 2009;29:15266–15276. doi: 10.1523/JNEUROSCI.4650-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Fujitani M, Hata K, Tohyama M, Yamagishi S, Yamashita T. Promotion of axon regeneration by myelin-associated glycoprotein and Nogo through divergent signals downstream of Gi/G. J Neurosci. 2004;24:6826–6832. doi: 10.1523/JNEUROSCI.1856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol. 2008;267:125–181. doi: 10.1016/S1937-6448(08)00603-5. [DOI] [PubMed] [Google Scholar]
- Iijima M, Huang YE, Luo HR, Vazquez F, Devreotes PN. Novel mechanism of PTEN regulation by its phosphatidylinositol 4,5-bisphosphate binding motif is critical for chemotaxis. J Biol Chem. 2004;279:16606–16613. doi: 10.1074/jbc.M312098200. [DOI] [PubMed] [Google Scholar]
- Johnson PW, Abramow-Newerly W, Seilheimer B, Sadoul R, Tropak MB, Arquint M, Dunn RJ, Schachner M, Roder JC. Recombinant myelin-associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron. 1989;3:377–385. doi: 10.1016/0896-6273(89)90262-6. [DOI] [PubMed] [Google Scholar]
- Joosten EA. Corticospinal tract regrowth. Prog Neurobiol. 1997;53:1–25. doi: 10.1016/s0301-0082(97)00024-5. [DOI] [PubMed] [Google Scholar]
- Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Koo DB, Ko K, Ko K, Kim SM, Jung JU, Ryu JS, Jin JW, Yang HJ, Do SI, Jung KY, Choo YK. Effects of daunorubicin on ganglioside expression and neuronal differentiation of mouse embryonic stem cells. Biochem Biophys Res Commun. 2007;362:313–318. doi: 10.1016/j.bbrc.2007.07.142. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27:5464–5476. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. Embo J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- Madura T, Yamashita T, Kubo T, Fujitani M, Hosokawa K, Tohyama M. Activation of Rho in the injured axons following spinal cord injury. EMBO Rep. 2004;5:412–417. doi: 10.1038/sj.embor.7400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359:417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NR, Lopez PH, Vyas AA, Schnaar RL. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells. J Biol Chem. 2007;282:27875–27886. doi: 10.1074/jbc.M704055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G, Gonzalez-Angulo AM, Hennessy BT, Mills GB, Kennedy JP, Lindsley CW, Arteaga CL. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69:4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Yiu G, Kaneko S, Wang J, Chang J, He XL, Garcia KC, He Z. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- Redfern RE, Redfern D, Furgason ML, Munson M, Ross AH, Gericke A. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry. 2008;47:2162–2171. doi: 10.1021/bi702114w. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Lopez PH. Myelin-associated glycoprotein and its axonal receptors. J Neurosci Res. 2009;87:3267–3276. doi: 10.1002/jnr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell. 2005;16:348–357. doi: 10.1091/mbc.E04-05-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song XY, Zhong JH, Wang X, Zhou XF. Suppression of p75NTR does not promote regeneration of injured spinal cord in mice. J Neurosci. 2004;24:542–546. doi: 10.1523/JNEUROSCI.4281-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Tang S, Qiu J, Nikulina E, Filbin MT. Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration. Mol Cell Neurosci. 2001;18:259–269. doi: 10.1006/mcne.2001.1020. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Bartlett PF. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport. 1998;9:1987–1990. doi: 10.1097/00001756-199806220-00013. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Sheu SS, Giger RJ. Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J Cell Biol. 2007;177:393–399. doi: 10.1083/jcb.200702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. The Nogo-66 receptor homolog NgR2 is a sialic acid-dependent receptor selective for myelin-associated glycoprotein. J Neurosci. 2005;25:808–822. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson M, Strijbos PJ, Rowles A, Facci L, Moore SE, Simmons DL, Walsh FS. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J Biol Chem. 2001;276:20280–20285. doi: 10.1074/jbc.M100345200. [DOI] [PubMed] [Google Scholar]
- Vyas AA, Patel HV, Fromholt SE, Heffer-Lauc M, Vyas KA, Dang J, Schachner M, Schnaar RL. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci U S A. 2002;99:8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LJ, Zeller CB, Shaper NL, Kiso M, Hasegawa A, Shapiro RE, Schnaar RL. Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc Natl Acad Sci U S A. 1996;93:814–818. doi: 10.1073/pnas.93.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci U S A. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure1: A, Lysates from E15 cortex, P6 cerebellum or cortical neuron cultures were analyzed by Western blot with antibodies to p75NTR or TROY. HEK 293 cells overexpressing p75NTR or TROY served as positive controls. B, Embryonic cortical neuron cultures were immunostained with an antibody to PirB. Staining in which primary antibody was omitted is included as a control.