Abstract
Changes in the number of chromosomes, but also variations in the copy number of chromosomal regions have been described in various pathological conditions, such as cancer and aneuploidy, but also in normal physiological condition. Our classical view of DNA replication and mitotic preservation of the chromosomal integrity is now challenged as new technologies allow us to observe such mosaic somatic changes in copy number affecting regions of chromosomes with various sizes. In order to go further in the understanding of copy number influence in normal condition we could take advantage of the novel strategy called Targeted Asymmetric Sister Chromatin Event of Recombination (TASCER) to induce recombination during the G2 phase so that we can generate deletions and duplications of regions of interest prior to mitosis. Using this approach in the mouse we could address the effects of copy number variation and segmental aneuploidy in daughter cells and allow us to explore somatic mosaics for large region of interest in the mouse.
Keywords: Chromosomal engineering, Cre/loxP, mosaic genetics, duplication, deletion.
INTRODUCTION
Our knowledge about human genetic variation has considerably evolved in the past few years with the development of genome-wide technologies unrevealing structural variations such as oligonucleotide microarray technologies and the next-generation sequencing with “paired-end” methods [1] that enable to screen the genome at a submicroscopic level. The identification of frequent copy number variants (CNVs) between individuals, including deletions and duplications of DNA sequences has definitely changed our view of genome integrity. Thousands of different CNVs have been identified (http://projects.tcag.ca/variation) overlapping a large number of genes [2, 3] and accounting for about 13% of the human genome. Estimates suggest that CNVs account for up to 24 Mb [3, 4] of the genetic difference between individuals, exceeding single nucleotide polymorphism (SNP) (2.5 Mb) [5].
In addition of being a major source of genetic diversity in the human population, CNVs are also responsible for various pathological conditions such as cancer, aneuploidies and contiguous gene syndromes (CGS). The first CNVs that were recognized were due to large chromosomal rearrangements that were visible under light microscope, causing well known genomic disorders such as Down (trisomy 21), Turner (monosomy X), Fragile X (Xq27.3) or Cri du Chat (del 5p-) syndromes [6]. These genomic disorders are often sporadic as they are de novo mutations leading to severe developmental deficits not compatible with the individual having offsprings. However, some CNVs can be inherited in a Mendelian way: Charcot-Marie-Tooth disease type 1A is a dominant neuropathy associated to a duplication of the gene for peripheral myelin protein 22 (PMP22) [7], whereas a deletion of the b-globin gene cluster is responsible for the recessive anemia-thalassemia [8]. The number of recognized genomic disorder has been drastically increasing since the 1980s by the constant discovery of submicroscopic (<5 Mb) genomic deletions and duplications associated with pathological phenotypes [9, 10]. Microdeletion 15q11.2q12 was associated with Prader-Willi syndrome in 1981 [11], a 17p13.3 deletion was found in Miller-Dieker lissencephaly syndrome [12], a 1.5 to 3.0-Mb hemizygous deletion of chromosome 22q11.2 causing haploinsufficiency of the TBX1 gene was discovered in DiGeorge syndrome [13], whereas the first recurrent microduplication was identified in 1991 in patients with Charcot-Marie-Tooth disease type 1A (CMT1A) [7]. In particular, the discovery of new CNVs has increased the diagnostic of mental retardation symptoms that affect 2-3% of the population as define new recognized syndromes by 15-20%. Moreover, CNVs are increasingly associated with complex traits such as autism [14, 15], schizophrenia [16] or epilepsy [17, 18] and susceptibility to infectious diseases such as HIV [19], Crohn disease [20, 21], psoriasis [22] or malaria [23].
A major mechanism by which rearrangements can cause a phenotype is by altering the copy number of a gene (or genes) sensitive to a dosage effect and influencing its expression [24]. But deletions and duplications can also cause gene expression perturbation through positional effect [25]. This position effect can be either the result of the physical dissociation between the transcription unit and its regulatory sequences that can sometimes lie as far as 1 Mb away from it [26, 27], or the result of the alteration of local or global regulation of chromatin structure [28-31]. These effects have been documented by some human disease states. For example, Aniridia (absence of the iris) due to haloinsufficiency of PAX6 at 11q13 has been shown to result from a chromosomal rearrangement that disrupts the region downstream of PAX6 transcription unit [32-35]. Human diseases resulting from aberrant gene expression through altered chromatin structures include FSHD (facioscapulohumeral dystrophy) (MIM 158900), a neuromuscular disorder affecting the facial and shoulder girdle muscles and one case of α-Thalassemia (MIM 141800) in which the HBA2 gene has been silenced by methylation of its promoter region by an antisense RNA derived from the truncated neighboring LUC7L gene on the opposite strand [31]. Genomic rearrangements of the genome can also trigger phenotypes by unmasking of recessive mutations or functional polymorphism of the remaining allele in the case of deletions. Hence, it was shown that, in Sotos syndrome that is due to del 5q35, the severity of the disease depends on the polymorphism of the remaining copy of the coagulation factor 12 gene [36].
CNVs often arise from meiotic recombination between highly similar (<97% sequence identity) duplicated sequences (termed low-copy repeats or LCRs) located less than 10 Mb apart. LCRs represent up to 5% of the haploid human genome [37] and can cause genomic instability as they can be at the origin of misalignment of chromosomes or chromatids and cause nonallelic homologous recombination (NAHR), resulting in unequal crossing-over [38]. But other mechanisms have been also proposed to be responsible for CNVs formation: nonhomologous end joining (NHEJ) is a mechanism that repairs DNA double strand breaks and, when defective, can lead to translocations and telomere fusion, hallmark of tumor cells [39]. Fork stalling and template switching (FoSTeS), a DNA replication error mechanism, has recently been shown to play an important role in the origin of nonrecurrent rearrangements with a complex structure leading to genomic disorders of various size [40-42]. Several studies have predicted that NHEJ or FoSTeS are at the origin of some CNVs observed in some cases of Duchenne muscular dystrophy, Smith-Magenis syndrome and Pelizaeus-Merzbacher disease [43, 44]. These mechanisms have the particularity of leading to CNV mosaicism. Occurrence of somatic mosaicism has been reported for chromosomal aberrations connected with diseases and leading to a milder phenotype of the disease [45, 46], arising somatically in cancers [47-49], or in the case the rearrangements of the immunoglobulin and T-cell-receptor genes [50]. In healthy individuals, more and more data have recently accumulated showing inter- and intra- tissues mosaicism for entire chromosome aneuploidies in germ cells, placenta, brain, skin, liver and blood [51, 52], although their occurrence remains unclear, due to the techniques that use pooling of large number of cells to study CNVs. Recent studies have started to point out at the importance of CNVs somatic mosaicism: extensive chromosomal instability and de novo recurrent CNVs have been reported in human cleavage-stage [53] and in mouse embryonic stem cell [54] respectively and somatic CNV mosaicism has been reported in different human tissues and organs [55] with studies on monozygotic twins revealing putative de novo somatic CNV events [56]. In a paper published this year, the group of Anja Weise at the Institute of Human Genetics in Magdeburg (Germany) provided the first evidence of somatic mosaicism for CNV between different cell types of one individual [57]. They used an approach that was defined to determine chromosomal parental origin in single cells based on fluorescent in situ hybridization (‘parental-origin-determination fluorescence in situ hybridization’ or pod-FISH) [58] to visualize CNVs on homologous chromosomes metaphase spreads from 10 healthy individuals and found CNV variation between different cell types but not within one cell type. They hence propose that there is early embryonic chromosome instability which results in stable mosaic pattern in human tissues.
While CNV is now recognized as a major source of genetic variability between individuals, their biological consequences are largely under investigated. Analysis of their pathological role and molecular mechanism are hard to investigate in human and require an animal model, genetically, morphologically and physiologically closer. The mouse constitutes a model organism of choice with an anatomy, physiology and genetics highly similar to humans. 80% of mouse genes have an orthologous counterpart in the human genome [59] and 99% have a sequence match. Homologous genes are found in the same order and relative orientation in large blocks of conserved syntenic regions. In addition, the ability to manipulate the mouse genome has made the mouse the primary mammalian genetic model organism. CNV can be artificially created using various chromosome engineering techniques that enable to precisely manipulate large genomic regions. Especially, the technology based on the Cre-loxP system is used to generate new chromosomes carrying deletions, duplications, inversions and translocations in targeted regions of interest and to study contiguous gene syndromes as well as normal developmental processes associated with CNV.
I. GERM LINE GENETIC ENGINEERING USING EMBRYONIC STEM (ES) CELLS
Modelling chromosomal rearrangements such as deletions, duplications, inversions or translocations in the mouse genome has been made possible by combining gene targeting in embryonic stem (ES) cells [60-63] with site-specific recombinase (SSR) systems such as the Cre-loxP [64]. Gene targeting enables to introduce small DNA sequences known as loxP sites to predefined loci by homologous recombination. Subsequent expression of the bacteriophage Cre recombinase that catalyses recombination between loxP sites [65-68] and can act in the mammalian genome without any cofactor, allow to generate large chromosomal rearrangements either in ES cells and then transmitted to the mouse by injecting the transformed ES cells to blastocysts, or directly in the mouse. The size of the loxP, small enough to be introduced very easily by genetic engineering and large enough to avoid problems associated with cryptic occurrence in eukaryotic genomes, has made the Cre-loxP system a very simple and powerful tool for mouse genomic engineering.
Cre-loxP recombination in ES cells can be easily generated for deletions of small regions (<100 kb) with insertion of two loxP sites in a direct orientation and cis configuration [69, 70]. However, to obtain larger deletions or duplications with loxP sites on different chromosomes the frequency of the recombination is too low and requires to be selected through the restoration of a positive selection marker [71-75] or by the deletion of a negative selection marker [76-79]. To this end, targeting vectors were designed, containing the 5’or 3’ part of the HPRT minigene with loxP located downstream and upstream respectively [71]. Use of this technique has been largely facilitated by the creation and the allocation of specific targeting vectors [80] that are now mapped on the mouse genome (Mutagenic Insertion and Chromosome Engineering Resource [MICER], http://www.sanger.ac.uk/Post-Genomics/mousegenomics/) [81], avoiding the fastidious and time-consuming task of constructing the targeting vectors. In addition, retroviral vectors can be used to target the second loxP site providing new loxP sites nested insertions extending from a few kb to several Mb around defined loci [82].
Although Cre-loxP recombination is a very powerful and convenient technique, it has to be carefully planned as its efficiency depends on many factors. Obtaining deletion, duplication or inversion depends on the position (cis or trans) of the loxP site, the relative orientation of the two loxP sites and on the cell cycle stage during which the Cre-mediated recombination occurs [83, 84]. Cre-mediated recombination efficiency depends on the recombinase activity [85], the region targeted that could induced ES cell lethality after deletion [86], the genetic distance between the loxP sites in a cis configuration (10%-0.1%) and the configuration of the loxP sites [71, 84, 87]. The size of the deletions is limited by haploinsufficiency of genes within the deleted interval that can trigger ES cell lethality and can be circumvented by making balancer deletion/duplication using trans recombination.
The possibility of manipulating large chromosomal regions has provided the scientific community with the opportunity to develop mouse models of CGS. Models developed for for del22q11.2 (DiGeorge syndrome) [88, 89] enabled to identify TBX1 as a candidate gene for the pathology [90]. Modeling Down syndrome (DS), the only viable autosomal aneuploidy in human, requires multiple chromosomal rearrangements as the around 300 human chromosome 21 ortologs are found in three syntenic regions on mouse chromosomes (Mmu) 10, 17 and 16. The phenotypic analysis of the Ts65Dn and Ts1Cje models obtained in a random manner and trisomic for part of the MMU16 [91-93], the Ts1Rhr trisomic for a ~5 Mb Down syndrome critical region (DCR) [72, 94] and the Ts1Yah model trisomic for the syntenic region on Mmu17 [95], both generated by chromosomal engineering, start to reveal the complexity of the genetic interactions at the origin of the disease. Mouse models have also been created for the Smith-Magenis [96-99] and Prader Willi [100] CGS, and to study large genes or cluster of genes such as the HoxD genes cluster [70], the Duchenne muscular dystrophy gene [75], the amyloid precursor protein gene [76] or the Nf1 gene [77]. Cre/loxP chromosome engineering in mouse ES cells was also developed to generate translocations or deletions similar to those found in cancer [86, 101-105]. Finally, in 2009, a mouse model was created for the first time to model a polygenic disease [106]. Autism, a common heterogeneous psychiatric disorder is a developmental brain disease characterized by deficit in social interaction and communication, and stereotyped repetitive behaviour. Autism has been shown to have a strong genetic basis with chromosomal anomalies accounting for 10 to 20% of the cases [107]. Among these anomalies, chromosome 15q11-13 duplication is the only recurrent cytogenetic aberration that could be associated with autism. Nakatani and collaborators (2009) generated a 6.3 Mb duplication of the conserved linkage group on Mmu7 and showed that the mouse model generated recapitulated aspects of human autism such as social abnormalities and increased anxiety and fear, providing a new tool to decipher the physiological and molecular mechanism underlying this pathology.
II. IN VIVO GERMLINE CRE-LOXP RECOMBINATION
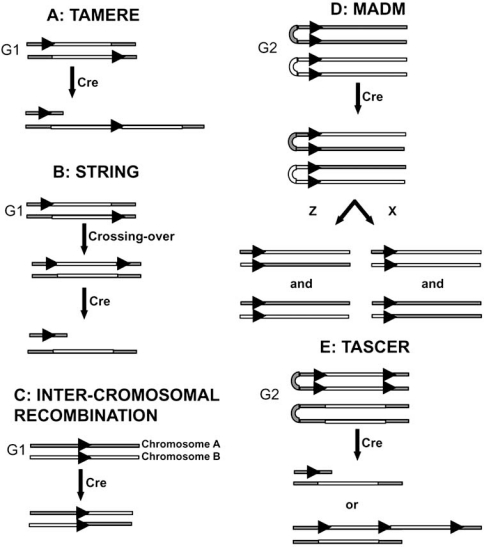
Creating chromosomal rearrangements in the whole organism offers an alternative to the in vitro protocol that is very labor-intensive and requires many rounds of ES cells genomic manipulations (Fig. 1). It requires that the loxP sites recombination occurs either in germ line cells during meiosis or ubiquitously in all of the tissues or in early embryogenesis. The rearrangements can then be transmitted to the progeny. This can be achieved by using Cre transgenic lines such as Sycp1-Cre [108], Zp3-Cre [109, 110], Protamine-Cre [111], CMV-Cre [112], R26Cre [113] or Hprt-Cre [114]. Most of the large in vivo rearrangements are deletions that are obtained from two loxP sites in the same relative orientation inserted in cis in ES cells [70, 115-117]. The frequency of recombination in cis decreases with the increasing distance between the loxP sites (0.3%-1%), but still remains high enough to be workable up to distances reaching 28 Mb [118]. For loxP sites that are originating from different mouse founder lines and hence are not in cis, deletion can still be obtained by mating the two founder lines and selecting for recombination after classical crossing-over, a technique called STRING for Sequential Targeted Recombination INduced Genomic rearrangement [118]. Similarly, inversions have been obtained in vivo with two loxP sites located in the same chromosome but with a reverse orientation [115, 119]. The different ubiquitously expressed Cre transgenic lines are not all similarly efficient in making the recombination and can lead to mosaic individuals in the first generation [73, 120, 121]. An alternative to the ubiquitous Cre transgenic lines is the Zp3-Cre in which cre expression is controlled by regulatory sequences from the mouse zona pellucida gene, expressing exclusively in the growing oocyte prior to the completion of the first meiotic division. But this transgenic Cre line was also shown to produce some mosaicism in a mouse model for DiGeorge del22q1 syndrome [122].
Fig. (1).
Schematic representation of the different in vivo strategies developed to model CNV in the mouse. (A) In the TAMERE strategy, loxP sites are inserted in a trans configuration and expression of the Cre recombinase during meiosis enables to generate cells containing a duplication with the reciprocal deletion. (B) The STRING approach takes advantage of the classical crossing-over to bring the two loxP sites into a cis configuration to generate a deletion. (C) Inter-chromosomal recombinations are obtained by introducing loxP sites at the site of the desired rearrangement and expressing the Cre recombinase. (D) The MADM strategy base on Cre-mediated inter-chromosomal recombination and G2-X segregation during mitosis, offers the possibility to introduce mosaicism in vivo. (E) The TASCER strategy takes advantages of the G2 stage of the S phase to recombine loxP sites in cis, leading to the deletion and the duplication of the sequence surrounded with the loxP.
Creating chromosomal duplications requires that the two loxP sites are on homologous chromosomes (trans) and is only possible when the two homologous chromosomes becomes physically close together. A strategy called TAMERE for TArgeting MEiotic Recombination was designed by Herault et al. (1998) [108] that makes use of a Cre under the control of the Synaptonemal Complex 1 promoter that is expressed during prophase of meiosis in male spermatocytes when chromatid pairs are closely aligned [123], enabling trans recombination [115, 124, 125]. However, for larger genomic rearrangements, while in vivo recombination is still effective for cis configurations up to 28 Mb [118], no rearrangement was obtained for a distance of 3.9 Mb [126] and hence seems to be limited to short-distance recombination. The TAMERE strategy has been used extensively to rearrange and study the HoxD complex region [108, 119, 127, 128] and has also been used to address the functional relationship between PrPc and Dpl, two genes involved in Prion disease, by making a double deletion of these two genes that are separated by 16 kb [125].
Beside the Cre-loxP system described here, the Flp-FRT system is another tyrosine site-specific recombinase (SSR) system that has been used as tool for DNA and genome engineering. However, FLP recombinase was found to be less efficient than Cre, partly due to an unsuitable optimum temperature for mammalian cells [129]. Recently, a new Cre-like recombinase, called Dre, was identified from P1-related phage D6 by Sauer and McDermott (2004) [130]. Dre recombinase has DNA specificity for a 32 bp DNA site (rox) distinct from the loxP and shows no cross-recombination with the Cre-loxP system. Like the Cre, it catalyzes both integrative and excisive recombination and requires no co-factor. The Dre-rox system was validated in the mouse by Anastassiadis and collaborators (2009) [131] who tested the efficiency of Dre-rox recombination in vivo by developing ubiquitous mouse lines based on the CAGGs promoter [132] and the ROSA26 locus. This new SSR provides a new useful toolkit for mouse genome engineering. Finally, recent applications for large serine SRRs such as phiC31 and phiBT1 [133] in eukaryotic genome engineering have emerged as new additional tools.
Association of the Cre-loxP system with transposon-based approaches should considerably speed up the generation of large-scale rearrangements in vivo. Transposons are mobile genetic elements that function as a bipartite system with a transposase to move around to different positions within the genome, potentially perturbating gene function. They have been extensively used for random mutagenesis and have been shown to be efficient both in vitro in ES cells and in vivo in the germline or somatically [134-141]. The most widely studied transposon/transposase system for mammalian mutagenesis is the Sleeping Beauty transposon of the Tc1-like mariner family [142]. Transposons have the advantage that they are active in vivo and only require breeding of transgenic mice, their insertion sites can be defined thanks to the transposon serving as a tag. More recently, an alternative transposon called Piggybac, derived from the cabbage looper moth (trichoplusia ni) has been developed, that can carry larger payloads between its transposon repeats [138]. PiggyBac transposons offer the possibility to easily distribute loxP sites throughout the mouse genome and, associated with the Cre-loxP technology, permit to generate rapidly and easily genome-wide chromosome rearrangements [121].
III. SOMATIC GENETIC ENGINEERING
Mosaicism in copy number variation has been observed in human [143]. It is a direct consequence of the missegregation of a chromosome during mitosis: while the two copies of one homologous chromosome migrate in one daughter cell that will be trisomic, the second will have only one copy being now monosomic.
The percentage of aneuploid mosaicism depends on the developmental stage, the lineage of the cell that is affected and its viability, and the type of chromosomal rearrangement produced. If the rearrangement occurs early during embryogenesis, the mosaicism can be generalized to all cell lineages and can result in major phenotypic changes that are often lethal. For example, in Turner syndrome more than 98% of conceptuses that are 45, X do not survive to term and only mosaic are thought to be compatible with life [144].
Mosaic aneuploidy can be registered in all somatic cell populations contributing to normal inter individual variability, with a frequency of 1.25–1.45% per chromosome and a percentage of aneuploid cells above 30% reported in blood cells [145] and brain [146-148]. Reasons for the presence of such aneuploid cells and its consequence remain for the moment speculative.
Consequences of somatic aneuploid mosaicism can be analysed in the mouse by generating segmental aneuploid mosaicism in mouse tissues using the Cre-loxP system during the G2 phase of mitosis. Two different strategies were developed using this system. Zong et al. (2005) [149] developed a technique utilizing Cre-mediated inter-chromosomal recombination prior to cell division (mitotic recombination) and took advantage of the G2-X segregation during mitosis to induce recombination events between homologous chromosomes in somatic cells and to mark the resulting daughter cells with different genotypes. They called this technique Mosaic Analysis with Double Markers (MADM). In addition to create and study sporadic loss of heterozygosity, this technique can also be used with a pre-introduced deletion or duplication on one chromosome to create CNV mosaics composed of wild-type daughter cells bearing the normal allele or mutant cells containing the allele with the deletion or the duplication. Another strategy takes advantages of the property of the Cre recombinase to react on G2 phase of mitosis to directly generate different types of cells with either the duplication or the deletion of one chromosomal region targeted with the loxP sites [150]. The TASCER (Targeted Asymmetric Sister Chromatid Recombination) takes advantage of the presence of loxP sites on sister chromatids after replication of DNA in G2 phase during mitosis in order to generate in vivo cells harbouring microdeletions and microduplications. LoxP sites were inserted on the same chromosome in a cis configuration separated by up to 2.2 Mb. Upon mating Cis/+ mice with a general Cre deleter line, mosaic animals were obtained with cells containing either the deletion or the duplication. Indeed, if loxP recombination occurs after DNA replication, in addition to the expected deletion, the corresponding duplication was also obtained corresponding to the intermediate reaction product containing three loxP sites. The duplication with presence of three loxP sites represents the intermediate product between the double cis configuration with four loxP sites of the G2 stage cells and the deletion end product. This is the first report of an in vivo generated duplication coming from a cis configuration, presumably because, in most reports, loxP sites were not as distant, resulting in a better efficiency avoiding the presence of the intermediate genomic duplication configuration. Hence, the increased distance tends to decrease the Cre-mediated recombination frequency [87] somehow preserving the chromosome with a large duplication. Using TASCER on two chromosomal regions homologous to the telomeric part of human chromosome 21, cells with partial monosomy and trisomy were efficiently recovered at a relatively constant level in numerous different tissues. The TASCER mechanism necessitates that the Cre is particularly active during the G2 phase during cell proliferation. This phenomenon has been described in vitro in embryonic stem cells [84, 151] but with much less efficiency than in vivo. The reason for this discrepancy might be that expression of the Cre is more stable in vivo and that the in vivo environment is more favourable to the viability of the aneuploid cells. Change in the chromatin structure, with complexes such as the cohexin one inducing physical connection of sister chromatids, can also facilitate the mitotic recombination [152, 153].
Efficiency of the TASCER is also depending of the type of Cre transgenic line used, as these do not express the Cre recombinase with the same efficacy. Hence, Duchon et al. (2008) [150] were able to induce up to 2.2 Mb deletions and duplications with a set of three different Cre lines, but could not obtain a larger modification of 9.2 Mb that might require a Cre line with a stronger promoter such as the CAG promoter inserted in the Hprt locus of the Hprt1tm1(Cre)Mnn mouse line, which has been used successfully in a recent study [114]. The fact that cells bearing the duplication was found in many different organs despite the less stable presence of three loxP sites [154] indicates that the trisomic state might be beneficial in some tissues and point out at the necessity to be very careful when using the Cre-loxP system in vivo to generate deletions.
Whereas chromosomal mosaicism has been observed in pathological conditions, such as Turner syndrome [155] or in cancer [156], the TASCER publication did not report any dramatic changes in the survival of trisomic and monosomic cells produced. In some tissues such as the muscle, this can be explained by compensatory effects due to their organization in a plurinuclear syncitia. However, in other non syncitial tissues, other mechanisms such as non-cell autonomous effect should be proposed to explain this phenomenon.
Although mosaic aneuploidy and CNVs have been reported in nonpathological conditions in different cell populations such as neurons [51, 52, 146-148, 157-159], their role and biological consequence to the organ structure and function in health and disease remains under explored. In the brain, mosaic DNA rearrangements have been suggested to contribute to the mechanism for neuronal cell diversification [160]. However, these are only suppositions and there is to date little evidence supporting a functional consequence for mosaic CNV in nonpathological conditions. To answer those questions, future studies need to examine the effect of somatic CNV on cell physiology. TASCER that results into substantial segmental aneuploid mosaicism, offers the possibility to explore consequences of such a phenomenon in a model organism. It further can be used to to induce partial aneuploid conditions such as those detected in cancer cells and to study further the consequence of loss or gain of copy number for chromosomal regions during tumorigenesis [156], avoiding the fastidious need of gene-by-gene inactivation or ES-cell MICER recombination system to study such phenomenon. TASCER hence offers new perspective for the generation and analysis of CNV variation in health and disease.
ACKNOWLEDGEMENTS
We thank members of the laboratory for helpful discussion, and members of the AnEUploidy consortium (www.aneuploidy.org). This work was funded by grants from the National Centre for Scientific Research (CNRS), the Fondation Jerome Lejeune, and the AnEUploidy project (LSHG-CT-2006-037627) supported by the European commission under FP6.
REFERENCES
- 1.Tuzun E, Sharp AJ, Bailey JA, Kaul R, Morrison VA, Pertz LM, Haugen E, Hayden H, Albertson D, Pinkel D, Olson MV, Eichler EE. Fine-scale structural variation of the human genome. Nat. Genet. 2005;37:727–732. doi: 10.1038/ng1562. [DOI] [PubMed] [Google Scholar]
- 2.Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen WW, Cho EK, Dallaire S, Freeman JL, Gonzalez JR, Gratacos M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang FT, Zhang JJ, Zerjal T, Zhang J, Armengol L, Conrad DF, Estivill X, Tyler-Smith C, Carter NP, Aburatani H, Lee C, Jones KW, Scherer SW, Hurles ME. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang YJ, Aerts J, Andrews TD, Barnes C, Campbell P, Fitzgerald T, Hu M, Ihm CH, Kristiansson K, MacArthur DG, MacDonald JR, Onyiah I, Pang AWC, Robson S, Stirrups K, Valsesia A, Walter K, Wei J, Tyler-Smith C, Carter NP, Lee C, Scherer SW, Hurles ME. Origins and functional impact of copy number variation in the human genome. Nature. 2010;464:704–712. doi: 10.1038/nature08516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichler EE, Nickerson DA, Altshuler D, Bowcock AM, Brooks LD, Carter NP, Church DM, Felsenfeld A, Guyer M, Lee C, Lupski JR, Mullikin JC, Pritchard JK, Sebat J, Sherry ST, Smith D, Valle D, Waterston RH Human Genome Structural Variation Working Group. Completing the map of human genetic variation. Nature. 2007;447:161–165. doi: 10.1038/447161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altshuler D, Pollara VJ, Cowles CR, Van Etten WJ, Baldwin J, Linton L, Lander ES. An SNP map of the human genome generated by reduced representation shotgun sequencing. Nature. 2000;407:513–516. doi: 10.1038/35035083. [DOI] [PubMed] [Google Scholar]
- 6.Lupski JR. Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet. 1998;14:417–422. doi: 10.1016/s0168-9525(98)01555-8. [DOI] [PubMed] [Google Scholar]
- 7.Lupski J, de Oca-Luna R, Slaugenhaupt S, Pentao L, Guzzetta V, Trask B, Saucedo-Cardenas O, Barker D, Killian J, Garcia C, Chakravarti A, Patel P. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219–32. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- 8.Higgs DR, Vickers MA, Wilkie AOM, Pretorius IM, Jarman AP, Weatherall DJ. A review of the molecular genetics of the human alpha-globine gene cluster. Blood. 1989;73:1081–1104. [PubMed] [Google Scholar]
- 9.Schmickel RD. Contiguous gene syndromes - a component of recognizable syndromes. J. Pediatr. 1986;109:231–241. doi: 10.1016/s0022-3476(86)80377-8. [DOI] [PubMed] [Google Scholar]
- 10.Schluth-Bolard C, Till M, Edery P, Sanlaville D. New chromosomal syndromes. Pathol. Biol. 2008;56:380–387. doi: 10.1016/j.patbio.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Ledbetter DH, Riccardi VM, Airhart SD, Strobel RJ, Keenan BS, Crawford JD. Deletions of chromosome -15 as a cause of the Prader-Willi Syndrome. N. Engl. J. Med. 1981;304:325–329. doi: 10.1056/NEJM198102053040604. [DOI] [PubMed] [Google Scholar]
- 12.Dobyns WB, Reiner O, Carrozzo R, Ledbetter DH. A Human brain malformation associated with the deletion of the Lis1 gene located at chromosome -17P13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- 13.Edelmann L, Pandita RK, Spiteri E, Funke B, Goldberg R, Palanisamy N, Chaganti RSK, Magenis E, Shprintzen RJ, Morrow BE. A common molecular basis for rearrangement disorders on chromosome 22q11. Human Mol. Genet. 1999;8:1157–1167. doi: 10.1093/hmg/8.7.1157. [DOI] [PubMed] [Google Scholar]
- 14.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature Rev. Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefansson H, Rujescu D, Cichon S, Pietilainen OPH, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Moller HJ, Hartmann A, Shianna KV, Ge DL, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Muhleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nothen MM, Peltonen L, Collier DA, St Clair D, Stefansson K. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–U61. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suls A, Claeys KG, Goossens D, Harding B, Van Luijk R, Scheers S, Deprez L, Audenaert D, Van Dyck T, Beeckmans S, Smouts I, Ceulemans B, Lagae L, Buyse G, Barisic N, Misson JP, Wauters J, Del-Favero J, De Jonghe P, Claes LRE. Microdeletions involving the SCN1A gene may be common in SCN1A-mutation-negative SMEI patients. Hum. Mutat. 2006;27:914–920. doi: 10.1002/humu.20350. [DOI] [PubMed] [Google Scholar]
- 18.Erez A, Patel AJ, Wang XQ, Xia ZL, Bhatt SS, Craigen W, Cheung SW, Lewis RA, Fang P, Davenport SLH, Stankiewicz P, Lalani SR. Alu-specific microhomology-mediated deletions in CDKL5 in females with early-onset seizure disorder. Neurogenetics. 2009;10:363–369. doi: 10.1007/s10048-009-0195-z. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, Nibbs RJ, Freedman BI, Quinones MP, Bamshad MJ, Murthy KK, Rovin BH, Bradley W, Clark RA, Anderson SA, O'Connell RJ, Agan BK, Ahuja SS, Bologna R, Sen L, Dolan MJ, Ahuja SK. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 20.Fellermann K, Stange DE, Schaeffeler E, Schmalzl H, Wehkamp J, Bevins CL, Reinisch W, Teml A, Schwab M, Lichter P, Radlwimmer B, Stange EF. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am. J. Hum. Genet. 2006;79:439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarroll SA, Huett A, Kuballa P, Chilewski SD, Landry A, Goyette P, Zody MC, Hall JL, Brant SR, Cho JH, Duerr RH, Silverberg MS, Taylor KD, Rioux JD, Altshuler D, Daly MJ, Xavier RJ. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn's disease. Nat. Genet. 2008;40:1107–1112. doi: 10.1038/ng.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollox EJ, Huffmeier U, Zeeuwen P, Palla R, Lascorz J, Rodijk-Olthuis D, van de Kerkhof PCM, Traupe H, de Jongh G, den Heijer M, Reis A, Armour JAL, Schalkwijk J. Psoriasis is associated with increased beta-defensin genomic copy number. Nat. Genet. 2008;40:23–25. doi: 10.1038/ng.2007.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flint J, Hill AVS, Bowden DK, Oppenheimer SJ, Sill PR, Serjeantson SW, Banakoiri J, Bhatia K, Alpers MP, Boyce AJ, Weatherall DJ, Clegg JB. High-frequencies of alpha-thalassemia are the result of natural-selection by malaria. Nature. 1986;321:744–750. doi: 10.1038/321744a0. [DOI] [PubMed] [Google Scholar]
- 24.Lupski JR, Wise CA, Kuwano A, Pentao L, Parke JT, Glaze DG, Ledbetter DH, Greenberg F, Patel PI. Gene dosage is a mechanism for Charcot-Marie-Tooth disease type 1A. Nat. Genet. 1992;1:29–33. doi: 10.1038/ng0492-29. [DOI] [PubMed] [Google Scholar]
- 25.Kleinjan DA, van Heyningen V. Long-range control of gene expression: Emerging mechanisms and disruption in disease. Am. J. Hum. Genet. 2005;76:8–32. doi: 10.1086/426833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeifer D, Kist R, Dewar K, Devon K, Lander ES, Birren B, Korniszewski L, Back E, Scherer G. Campomelic dysplasia translocation breakpoints are scattered over 1 Mb proximal to SOX9: Evidence for an extended control region. Am. J. Hum. Genet. 1999;65:111–124. doi: 10.1086/302455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimura-Yoshida C, Kitajima K, Oda-Ishii I, Tian E, Suzuki M, Yamamoto M, Suzuki T, Kobayashi M, Aizawa S, Matsuo I. Characterization of the pufferfish Otx2 cis-regulators reveals evolutionarily conserved genetic mechanisms for vertebrate head specification. Development. 2004;131:57–71. doi: 10.1242/dev.00877. [DOI] [PubMed] [Google Scholar]
- 28.Kleinjan DJ, van Heyningen V. Position effect in human genetic disease. Hum. Mol. Genet. 1998;7:1611–1618. doi: 10.1093/hmg/7.10.1611. [DOI] [PubMed] [Google Scholar]
- 29.Jiang GC, Yang F, van Overveld PGM, Vedanarayanan V, van der Maarel S, Ehrlich M. Testing the position-effect variegation hypothesis for facioscapulohumeral muscular dystrophy by analysis of histone modification and gene expression in subtelomeric 4q. Hum. Mol. Genet. 2003;12:2909–2921. doi: 10.1093/hmg/ddg323. [DOI] [PubMed] [Google Scholar]
- 30.Saveliev A, Everett C, Sharpe T, Webster Z, Festenstein R. DNA triplet repeats mediate heterochromatin-protein-1-sensitive variegated gene silencing. Nature. 2003;422:909–913. doi: 10.1038/nature01596. [DOI] [PubMed] [Google Scholar]
- 31.Tufarelli C, Stanley JAS, Garrick D, Sharpe JA, Ayyub H, Wood WG, Higgs DR. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat. Genet. 2003;34:157–165. doi: 10.1038/ng1157. [DOI] [PubMed] [Google Scholar]
- 32.Fantes J, Redeker B, Breen M, Boyle S, Brown J, Fletcher J, Jones S, Bickmore W, Fukushima Y, Mannens M, Danes S, Vanheyningen V, Hanson I. Aniridia associated cyogenetic rearrangements suggest that a position effect may cause the mutant phenotype. Hum. Mol. Genet. 1995;4:415–422. doi: 10.1093/hmg/4.3.415. [DOI] [PubMed] [Google Scholar]
- 33.Lauderdale J, Wilensky JS, Oliver ER, Walton DS, Glaser T. 3 ' deletions cause aniridia by preventing PAX6 gene expression. Proc. Nat. Acad. Sci.USA. 2000;97:13755–13759. doi: 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinjan DA, Seawright A, Schedl A, Quinlan RA, Danes S, van Heyningen V. Aniridia-associated translocations, DNase hypersensitivity, sequence comparison and transgenic analysis redefine the functional domain of PAX6. Hum. Mol. Genet. 2001;10:2049–2059. doi: 10.1093/hmg/10.19.2049. [DOI] [PubMed] [Google Scholar]
- 35.Crolla JA, van Heyningen V. Frequent chromosome aberrations revealed by molecular cytogenetic studies in patients with aniridia. Am. J. Hum. Genet. 2002;71:1138–1149. doi: 10.1086/344396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurotaki N, Shen JJ, Touyama M, Kondoh T, Visser R, Ozaki T, Nishimoto J, Shiihara T, Uetake K, Makita Y, Harada N, Raskin S, Brown CW, Hoglund P, Okamoto N, Lupski JR. Phenotypic consequences of genetic variation at hemizygous alleles: Sotos syndrome is a contiguous gene syndrome incorporating coagulation factor twelve (FXII) deficiency. Genet. Med. 2005;7:479–483. doi: 10.1097/01.gim.0000177419.43309.37. [DOI] [PubMed] [Google Scholar]
- 37.Bailey JA, Yavor AM, Massa HF, Trask BJ, Eichler EE. Segmental duplications: Organization and impact within the current Human Genome Project assembly. Genome Res. 2001;11:1005–1017. doi: 10.1101/gr.187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stankiewicz P, Lupski JR. Genome architecture, rearrangements and genomic disorders. Trends in Genet. 2002;18:74–82. doi: 10.1016/s0168-9525(02)02592-1. [DOI] [PubMed] [Google Scholar]
- 39.Espejel S, Franco S, Rodriguez-Perales S, Bouffler SD, Cigudosa JC, Blasco MA. Mammalian Ku86 mediates chromosomal fusions and apoptosis caused by critically short telomeres. EMBO J. 2002;21:2207–2219. doi: 10.1093/emboj/21.9.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JA, Carvalho CMB, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;131:1235–1247. doi: 10.1016/j.cell.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 41.Zhang F, Khajavi M, Connolly AM, Towne CF, Batish SD, Lupski JR. The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat. Genet. 2009;41:849–U115. doi: 10.1038/ng.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cocquempot O, Brault V, Babinet C, Herault Y. Fork Stalling and Template Switching As a Mechanism for Polyalanine Tract Expansion Affecting the DYC Mutant of HOXD13, a New Murine Model of Synpolydactyly. Genetics. 2009;183:23–30. doi: 10.1534/genetics.109.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korbel JO, Urban AE, Affourtit JP, Godwin B, Grubert F, Simons JF, Kim PM, Palejev D, Carriero NJ, Du L, Taillon BE, Chen ZT, Tanzer A, Saunders ACE, Chi JX, Yang FT, Carter NP, Hurles ME, Weissman SM, Harkins TT, Gerstein MB, Egholm M, Snyder M. Paired-end mapping reveals extensive structural variation in the human genome. Science. 2007;318:420–426. doi: 10.1126/science.1149504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arlt MF, Mulle JG, Schaibley VM, Ragland RL, Durkin SG, Warren ST, Glover TW. Replication Stress Induces Genome-wide Copy Number Changes in Human Cells that Resemble Polymorphic and Pathogenic Variants. Am. J. Hum. Genet. 2009;84:339–350. doi: 10.1016/j.ajhg.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gratacos M, Nadal M, Martin-Santos R, Pujana MA, Gago J, Peral B, Armengol L, Ponsa I, Miro R, Bulbena A, Estivill X. A polymorphic genomic duplication on human chromosome 15 is a susceptibility factor for panic and phobic disorders. Cell. 2001;106:367–379. doi: 10.1016/s0092-8674(01)00447-0. [DOI] [PubMed] [Google Scholar]
- 46.Youssoufian H, Pyeritz RE. Mechanisms and consequences of somatic mosaicism in humans. Nat. Rev. Genet. 2002;3:748–758. doi: 10.1038/nrg906. [DOI] [PubMed] [Google Scholar]
- 47.Yang JJ, Bhojwani D, Yang WJ, Cai XJ, Stocco G, Crews K, Wang JH, Morrison D, Devidas M, Hunger SP, Willman CL, Raetz EA, Pui CH, Evans WE, Relling MV, Carroll WL. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood. 2008;112:4178–4183. doi: 10.1182/blood-2008-06-165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leary RJ, Lin JC, Cummins J, Boca S, Wood LD, Parsons DW, Jones S, Sjoblom T, Park BH, Parsons R, Willis J, Dawson D, Willson JKV, Nikolskaya T, Nikolsky Y, Kopelovich L, Papadopoulos N, Pennacchio LA, Wang TL, Markowitz SD, Parmigiani G, Kinzler KW, Vogelstein B, Velculescu VE. Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc. Natl. Acad. Sci. USA. 2008;105:16224–16229. doi: 10.1073/pnas.0808041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Natrajan R, Warren W, Messahel B, Reis-Filho JS, Brundler MA, Dome JS, Grundy PE, Vujanic G, Pritchard-Jones K, Jones C. Complex patterns of chromosome 9 alterations including the p16(INK4a) locus in Wilms tumours. J. Clin. Pathol. 2008;61:95–102. doi: 10.1136/jcp.2007.047159. [DOI] [PubMed] [Google Scholar]
- 50.Bergman Y. Allelic exclusion in B and T lymphopoiesis. Semin. Immunol. 1999;11:319–328. doi: 10.1006/smim.1999.0188. [DOI] [PubMed] [Google Scholar]
- 51.Iourov IY, Vorsanova SG, Yurov YB. Chromosomal variation in mammalian neuronal cells: Known facts and attractive hypotheses. Int. Rev. Cytol. 2006;249:143–91. doi: 10.1016/S0074-7696(06)49003-3. [DOI] [PubMed] [Google Scholar]
- 52.Yurov YB, Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Kutsev SI, Pellestor F, Beresheva AK, Demidova IA, Kravets VS, Monakhov VV, Soloviev IV. Aneuploidy and confined chromosomal mosaicism in the developing human brain. Plos One. 2007;6:e558. doi: 10.1371/journal.pone.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanneste E, Voet T, Le Caignec C, Ampe M, Konings P, Melotte C, Debrock S, Amyere M, Vikkula M, Schuit F, Fryns JP, Verbeke G, D'Hooghe T, Moreau Y, Vermeesch JR. Chromosome instability is common in human cleavage-stage embryos. Nat. Med. 2009;15:577–583. doi: 10.1038/nm.1924. [DOI] [PubMed] [Google Scholar]
- 54.Liang Q, Conte N, Skarnes WC, Bradley A. Extensive genomic copy number variation in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2008;105:17453–17456. doi: 10.1073/pnas.0805638105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piotrowski A, Bruder CEG, Andersson R, de Stahl TD, Menzel U, Sandgren J, Poplawski A, von Tell D, Crasto C, Bogdan A, Bartoszewski R, Bebok Z, Krzyzanowski M, Jankowski Z, Partridge EC, Komorowski J, Dumanski JP. Somatic mosaicism for copy number variation in differentiated human tissues. Hum. Mutat. 2008;29:1118–1124. doi: 10.1002/humu.20815. [DOI] [PubMed] [Google Scholar]
- 56.Bruder CEG, Piotrowski A, Gijsbers A, Andersson R, Erickson S, de Stahl TD, Menzel U, Sandgren J, von Tell D, Poplawski A, Crowley M, Crasto C, Partridge EC, Tiwari H, Allison DB, Kornorowski J, van Ommen GJB, Boomsma DI, Pedersen NL, den Dunnen JT, Wirdefeldt K, Dumanski JP. Phenotypically concordant and discordant monozygotic twins display different DNA copy-number-variation profiles. Am. J. Med. Genet. 2008;82:763–771. doi: 10.1016/j.ajhg.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mkrtchyan H, Gross M, Hinreiner S, Polytiko A, Manvelyan M, Mrasek K, Kosyakova N, Ewers E, Nelle H, Liehr T, Volleth M, Weise A. Early embryonic chromosome instability results in stable mosaic pattern in human tissues. Plos One. 2010;5:e9591. doi: 10.1371/journal.pone.0009591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weise A, Gross M, Mrasek K, Mkrtchyan H, Horsthemke B, Jonsrud C, Von Eggeling F, Hinreiner S, Witthuhn V, Claussen U, Liehr T. Parental-origin-determination fluorescence in situ hybridization distinguishes homologous human chromosomes on a single-cell level. Int. J. Mol. Med. 2008;21:189–200. doi: 10.3892/ijmm.21.2.189. [DOI] [PubMed] [Google Scholar]
- 59.Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigó R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 60.Evans MJ, Kaufman MH. Estabishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 61.Gordon JW, Ruddle FH. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science. 1981;214:1244–1246. doi: 10.1126/science.6272397. [DOI] [PubMed] [Google Scholar]
- 62.Doetschman T, Gregg RG, Maeda N, Hooper ML, Melton DW, Thompson S, Smithies O. Targeted correction of a mutant Hprt gene in mouse embryonic stem-cells. Nature. 1987;330:576–578. doi: 10.1038/330576a0. [DOI] [PubMed] [Google Scholar]
- 63.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem-cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 64.Sauer B, Henderson N. Site-specific DNA recombination in mammalian-cells by the Cre recombinase of bacteriophage-P1. Proc. Natl. Acad. Sci. USA. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sauer B, Henderson N. Cre-stimulated recombination at loxP-containing DNA sequences placed into the mammalian genome. Nucleic Acids Res. 1989;17:147–161. doi: 10.1093/nar/17.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sauer B. Manipulation of transgenes by site-specific recombination - use of Cre recombinase. Guide to Techniques in Mouse Development. 1993;225:890–900. doi: 10.1016/0076-6879(93)25056-8. [DOI] [PubMed] [Google Scholar]
- 67.Sternberg N, Hamilton D. Bacteriophage-P1 site-specific recombination. 1. recombination between loxP sites. J. Mol. Biol. 1981;150:467–486. doi: 10.1016/0022-2836(81)90375-2. [DOI] [PubMed] [Google Scholar]
- 68.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA-polymerase-beta gene segment in T-cells using cell-type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 69.Spitz F, Gonzalez F, Peichel C, Vogt TF, Duboule D, Zakany J. Large scale transgenic and cluster deletion analysis of the HoxD complex separate an ancestral regulatory module from evolutionary innovations. Genes Dev. 2001;15:2209–2214. doi: 10.1101/gad.205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zakany J, Duboule D. Synpolydactyly in mice with a targeted deficiency in the HoxD complex. Nature. 1996;384:69–71. doi: 10.1038/384069a0. [DOI] [PubMed] [Google Scholar]
- 71.Ramirezsolis R, Liu PT, Bradley A. Chromosome engineering in mice. Nature. 1995;378:720–724. doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- 72.Olson LE, Richtsmeier JT, Leszl J, Reeves RH. A chromosome 21 critical region does not cause specific down syndrome phenotypes. Science. 2004;306:687–690. doi: 10.1126/science.1098992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Puech A, Saint-Jore B, Merscher S, Russell RG, Cherif D, Sirotkin H, Xu H, Factor S, Kucherlapati R, Skoultchi AI. Normal cardiovascular development in mice deficient for 16 genes in 550 kb of the velocardiofacial/DiGeorge syndrome region. Proc. Natl. Acad. Sci. USA. 2000;97:10090–10095. doi: 10.1073/pnas.97.18.10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suemori H, Noguchi S. Hox C cluster genes are dispensable for overall body plan of mouse embryonic development. Dev. Biol. 2000;220:333–342. doi: 10.1006/dbio.2000.9651. [DOI] [PubMed] [Google Scholar]
- 75.Kudoh H, Ikeda H, Kakitani M, Ueda A, Hayasaka M, Tomizuka K, Hanaoka K. A new model mouse for Duchenne muscular dystrophy produced by 2.4 Mb deletion of dystrophin gene using Cre-loxP recombination system. Biochem. Biophys. Res. Commun. 2005;328:507–516. doi: 10.1016/j.bbrc.2004.12.191. [DOI] [PubMed] [Google Scholar]
- 76.Li ZW, Stark G, Gotz J, Rulicke T, Gschwind M, Huber G, Muller U, Weissmann C. Generation of mice with a 200-kb amyloid precursor protein gene deletion by Cre recombinase-mediated site-specific recombination in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1996;93:12052–12052. doi: 10.1073/pnas.93.12.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlake T, Schupp I, Kutsche K, Mincheva A, Lichter P, Boehm T. Predetermined chromosomal deletion encompassing the Nf-1 gene. Oncogene. 1999;18:6078–6082. doi: 10.1038/sj.onc.1203021. [DOI] [PubMed] [Google Scholar]
- 78.Zhu YW, Jong MC, Frazer KA, Gong E, Krauss RM, Cheng JF, Boffelli D, Rubin EM. Genomic interval engineering of mice identifies a novel modulator of triglyceride production. Proc. Natl. Acad. Sci. USA. 2000;97:1137–1142. doi: 10.1073/pnas.97.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nóbrega M, Zhu Y, Plajzer-Frick I, Afzal V, Rubin E. Megabase deletions of gene deserts result in viable mice. Nature. 2004;431:988–93. doi: 10.1038/nature03022. [DOI] [PubMed] [Google Scholar]
- 80.Zheng BH, Mills AA, Bradley A. A system for rapid generation of coat color-tagged knockouts and defined chromosomal rearrangements in mice. Nucleic Acids Res. 1999;27:2354–2360. doi: 10.1093/nar/27.11.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adams DJ, Biggs PJ, Cox T, Davies R, van der Weyden L, Jonkers J, Smith J, Plumb B, Taylor R, Nishijima I, Yu YJ, Rogers J, Bradley A. Mutagenic insertion and chromosome engineering resource (MICER) Nat. Genet. 2004;36:867–871. doi: 10.1038/ng1388. [DOI] [PubMed] [Google Scholar]
- 82.Su H, Wang XH, Bradley A. Nested chromosomal deletions induced with retroviral vectors in mice. Nat. Genet. 2000;24:92–95. doi: 10.1038/71756. [DOI] [PubMed] [Google Scholar]
- 83.Zheng BH, Mills AA, Bradley A. Introducing defined chromosomal rearrangements into the mouse genome. Methods. 2001;24:81–94. doi: 10.1006/meth.2001.1160. [DOI] [PubMed] [Google Scholar]
- 84.Yu YJ, Bradley A. Engineering chromosomal rearrangements in mice. Nat. Rev. Genet. 2001;2:780–790. doi: 10.1038/35093564. [DOI] [PubMed] [Google Scholar]
- 85.Baubonis W, Sauer B. Genomic targeting with purified cre recombinase. Nucleic Acids Res. 1993;21:2025–2029. doi: 10.1093/nar/21.9.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu PT, Zhang HJ, McLellan A, Vogel H, Bradley A. Embryonic lethality and tumorigenesis caused by segmental aneuploidy on mouse chromosome 11. Genetics. 1998;150:1155–1168. doi: 10.1093/genetics/150.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng BH, Sage M, Sheppeard EA, Jurecic V, Bradley A. Engineering mouse chromosomes with Cre-loxP: Range, efficiency, and somatic applications. Mol. Cell. Biol. 2000;20:648–655. doi: 10.1128/mcb.20.2.648-655.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindsay EA, Botta A, Jurecic V, Carattini-Rivera S, Cheah YC, Rosenblatt HM, Bradley A, Baldini A. Congenital heart disease in mice deficient for the DiGeorge syndrome region. Nature. 1999;401:379–383. doi: 10.1038/43900. [DOI] [PubMed] [Google Scholar]
- 89.Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficiency in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- 90.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 91.Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome-16 - a new model system for studying down-syndrome. Prog. Clin. Biol. Res. 1990;360:263–280. [PubMed] [Google Scholar]
- 92.Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for down-syndrome exhibits learning and behavior deficits. Nat. Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 93.Sago H, Carlson EJ, Smith DJ, Kilbridge J, Rubin EM, Mobley WC, Epstein CJ, Huang TT. Ts1Cje, a partial trisomy 16 mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proc. Natl. Acad. Sci. USA. 1998;95:6256–6261. doi: 10.1073/pnas.95.11.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olson LE, Roper RJ, Sengstaken CL, Peterson EA, Aquino V, Galdzicki Z, Siarey R, Pletnikov M, Moran TH, Reeves RH. Trisomy for the Down syndrome 'critical region' is necessary but not sufficient for brain phenotypes of trisomic mice. Hum. Mol. Genet. 2007;16:774–782. doi: 10.1093/hmg/ddm022. [DOI] [PubMed] [Google Scholar]
- 95.Pereira PL, Magnol L, Sahun I, Brault V, Duchon A, Prandini P, Gruart A, Bizot JC, Chadefaux-Vekemans B, Deutsch S, Trovero F, Delgado-Garcia JM, Antonarakis SE, Dierssen M, Herault Y. A new mouse model for the trisomy of the Abcg1-U2af1 region reveals the complexity of the combinatorial genetic code of down syndrome. Hum. Mol. Genet. 2009;18:4756–4769. doi: 10.1093/hmg/ddp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walz K, Caratini-Rivera S, Bi WM, Fonseca P, Mansouri DL, Lynch J, Vogel H, Noebels JL, Bradley A, Lupski JR. Modeling del(17)(p11.2p11.2) and dup(17)(p11.2p11.2) contiguous gene syndromes by chromosome engineering in mice: Phenotypic consequences of gene dosage imbalance. Mol. Cell. Biol. 2003;23:3646–3655. doi: 10.1128/MCB.23.10.3646-3655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walz K, Spencer C, Kaasik K, Lee CC, Lupski JR, Paylor R. Behavioral characterization of mouse models for Smith-Magenis syndrome and dup(17)(p11.2p11.2) Hum. Mol. Genet. 2004;13:367–378. doi: 10.1093/hmg/ddh044. [DOI] [PubMed] [Google Scholar]
- 98.Yan J, Keener VW, Bi WM, Walz K, Bradley A, Justice MJ, Lupski JR. Reduced penetrance of craniofacial anomalies as a function of deletion size and genetic background in a chromosome engineered partial mouse model for Smith-Magenis syndrome. Hum. Mol. Genet. 2004;13:2613–2624. doi: 10.1093/hmg/ddh288. [DOI] [PubMed] [Google Scholar]
- 99.Bi WM, Ohyama T, Nakamura H, Yan J, Visvanathan J, Justice MJ, Lupski JR. Inactivation of Rai1 in mice recapitulates phenotypes observed in chromosome engineered mouse models for Smith-Magenis syndrome. Hum. Mol. Genet. 2005;14:983–995. doi: 10.1093/hmg/ddi085. [DOI] [PubMed] [Google Scholar]
- 100.Tsai TF, Jiang YH, Bressler J, Armstrong D, Beaudet AL. Paternal deletion from Snrpn to Ube3a in the mouse causes hypotonia, growth retardation and partial lethality and provides evidence for a gene contributing to Prader-Willi syndrome. Hum. Mol. Genet. 1999;8:1357–1364. doi: 10.1093/hmg/8.8.1357. [DOI] [PubMed] [Google Scholar]
- 101.Smith AJH, Desousa MA, Kwabiaddo B, Heppellparton A, Impey H, Rabbitts P. A site-directed chromosomal translocation induced in embryonic stem-cells by cre-loxp recombination. Nat. Genet. 1995;9:376–385. doi: 10.1038/ng0495-376. [DOI] [PubMed] [Google Scholar]
- 102.Smith AJH, Xian J, Richardson M, Johnstone KA, Rabbitts PH. Cre-loxP chromosome engineering of a targeted deletion in the mouse corresponding to the 3p21.3 region of homozygous loss in human tumours. Oncogene. 2002;21:4521–4529. doi: 10.1038/sj.onc.1205530. [DOI] [PubMed] [Google Scholar]
- 103.Vandeursen J, Fornerod M, Vanrees B, Grosveld G. Cre-mediated site-specific translocation between nonhomologous mouse chromosomes. Proc. Natl. Acad. Sci. USA. 1995;92:7376–7380. doi: 10.1073/pnas.92.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koike H, Horie K, Fukuyama H, Kondoh G, Nagata S, Takeda J. Efficient biallelic mutagenesis with Cre/loxP-mediated inter-chromosomal recombination. EMBO Rep. 2002;3:433–437. doi: 10.1093/embo-reports/kvf097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Biggs PJ, Vogel H, Sage M, Martin LA, Donehower LA, Bradley A. Allelic phasing of a mouse chromosome 11 deficiency influences p53 tumorigenicity. Oncogene. 2003;22:3288–3296. doi: 10.1038/sj.onc.1206384. [DOI] [PubMed] [Google Scholar]
- 106.Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, Tomonaga S, Watanabe Y, Chung YJ, Banerjee R, Iwamoto K, Kato T, Okazawa M, Yamauchi K, Tanda K, Takao K, Miyakawa T, Bradley A, Takumi T. Abnormal Behavior in a Chromosome-Engineered Mouse Model for Human 15q11-13 Duplication Seen in Autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beaudet AL. Autism: highly heritable but not inherited. Nat. Med. 2007;13:534–536. doi: 10.1038/nm0507-534. [DOI] [PubMed] [Google Scholar]
- 108.Herault Y, Rassoulzadegan M, Cuzin F, Duboule D. Engineering chromosomes in mice through targeted meiotic recombination (TAMERE) Nat. Genet. 1998;20:381–384. doi: 10.1038/3861. [DOI] [PubMed] [Google Scholar]
- 109.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- 110.de Vries WN, Binns LT, Fancher KS, Dean J, Moore R, Kemler R, Knowles BB. Expression of Cre recombinase in mouse oocytes: A means to study maternal effect genes. Genesis. 2000;26:110–112. [PubMed] [Google Scholar]
- 111.O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc. Natl. Acad. Sci. USA. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dupe V, Davenne M, Brocard J, Dolle P, Mark M, Dierich A, Chambon P, Rijli FM. In vivo functional analysis of the Hoxa-1 3' retinoic acid response element (3'RARE) Development. 1997;124:399–410. doi: 10.1242/dev.124.2.399. [DOI] [PubMed] [Google Scholar]
- 113.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 114.Tang SHE, Silva FJ, Tsark WMK, Mann JR. A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ. Genesis. 2002;32:199–202. doi: 10.1002/gene.10030. [DOI] [PubMed] [Google Scholar]
- 115.Zakany J, Kmita M, Duboule D. A dual role for Hox genes in limb anterior-posterior asymmetry. Science. 2004;304:1669–1672. doi: 10.1126/science.1096049. [DOI] [PubMed] [Google Scholar]
- 116.Kmita M, Kondo T, Duboule D. Targeted inversion of a polar silencer within the HoxD complex re-allocates domains of enhancer sharing. Nat. Genet. 2000;26:451–454. doi: 10.1038/82593. [DOI] [PubMed] [Google Scholar]
- 117.Stemmler MP, Hecht A, Kemler R. E-cadherin intron 2 contains cis-regulatory elements essential for gene expression. Development. 2005;132:965–976. doi: 10.1242/dev.01662. [DOI] [PubMed] [Google Scholar]
- 118.Spitz F, Herkenne C, Morris MA, Duboule D. Inversion-induced disruption of the Hoxd cluster leads to the partition of regulatory landscapes. Nat. Genet. 2005;37:889–893. doi: 10.1038/ng1597. [DOI] [PubMed] [Google Scholar]
- 119.Kmita M, van der Hoeven F, Zakany J, Krumlauf R, Duboule D. Mechanisms of Hox gene colinearity: transposition of the anterior Hoxb1 gene into the posterior HoxD complex. Genes Dev. 2000;14:198–211. [PMC free article] [PubMed] [Google Scholar]
- 120.Besson V, Brault V, Duchon A, Togbe D, Bizot JC, Quesniaux VFJ, Ryffel B, Herault Y. Modeling the monosomy for the telomeric part of human chromosome 21 reveals haploinsufficient genes modulating the inflammatory and airway responses. Hum. Mol. Genet. 2007;16:2040–2052. doi: 10.1093/hmg/ddm152. [DOI] [PubMed] [Google Scholar]
- 121.Wu S, Ying G, Wu Q, Capecchi MR. Toward simpler and faster genome-wide mutagenesis in mice. Nat. Genet. 2007;39:922–930. doi: 10.1038/ng2060. [DOI] [PubMed] [Google Scholar]
- 122.Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore BS, Lopez M, Pandita RK, Lia M, Carrion D, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 123.Vidal F, Sage J, Cuzin F, Rassoulzadegan M. Cre expression in primary spermatocytes: a tool for genetic engineering of the germ line. Mol. Reprod. Dev. 1998;51:274–280. doi: 10.1002/(SICI)1098-2795(199811)51:3<274::AID-MRD6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 124.Herault Y, Kmita M, Sawaya CC, Duboule D. A nested deletion approach to generate Cre deleter mice with progressive Hox profiles. Int. J. Dev. Biol. 2002;46:185–191. [PubMed] [Google Scholar]
- 125.Genoud N, Behrens A, Miele G, Robay D, Heppner FL, Freigang S, Aguzzi A. Disruption of Doppel prevents neurodegeneration in mice with extensive Prnp deletions. Proc. Natl. Acad. Sci. USA. 2004;101:4198–4203. doi: 10.1073/pnas.0400131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olson LE, Tien J, South S, Reeves RH. Long-range chromosomal engineering is more efficient in vitro than in vivo. Transgenic Res. 2005;14:325–332. doi: 10.1007/s11248-005-0389-6. [DOI] [PubMed] [Google Scholar]
- 127.Kmita M, Fraudeau N, Herault Y, Duboule D. Serial deletions and duplications suggest a mechanism for the collinearity of Hoxd genes in limbs. Nature. 2002;420:145–150. doi: 10.1038/nature01189. [DOI] [PubMed] [Google Scholar]
- 128.Kmita M, Tarchini B, Duboule D, Herault Y. Evolutionary conserved sequences are required for the insulation of the vertebrate Hoxd complex in neural cells. Development. 2002;129:5521–5528. doi: 10.1242/dev.00151. [DOI] [PubMed] [Google Scholar]
- 129.Buchholz F, Ringrose L, Angrand PO, Rossi F, Stewart AF. Different thermostabilities of FLP and Cre recombinases: implications for applied site-specific recombination. Nucleic Acids Res. 1996;24:4256–4262. doi: 10.1093/nar/24.21.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sauer B, McDermott J. DNA recombination with a heterospecific Cre homolog identified from comparison of the pac-c1 regions of P1-related phages. Nucleic Acids Res. 2004;32:6086–6095. doi: 10.1093/nar/gkh941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anastassiadis K, Fu J, Patsch C, Hu SB, Weidlich S, Duerschke K, Buchholz F, Edenhofer F, Stewart AF. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis. Model. Mech. 2009;2:508–515. doi: 10.1242/dmm.003087. [DOI] [PubMed] [Google Scholar]
- 132.Schaft J, Ashery-Padan R, van der Hoeven F, Gruss P, Stewart AF. Efficient FLP recombination in mouse ES cells and oocytes. Genesis. 2001;31:6–10. doi: 10.1002/gene.1076. [DOI] [PubMed] [Google Scholar]
- 133.Xu Z, Lee NCO, Dafhnis-Calas F, Malla S, Smith MCM, Brown WRA. Site-specific recombination in Schizosaccharomyces pombe and systematic assembly of a 400kb transgene array in mammalian cells using the integrase of Streptomyces phage phiBT1. Nucleic Acids Res. 2008;36:e9. doi: 10.1093/nar/gkm1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Horie K, Kuroiwa A, Ikawa M, Okabe M, Kondoh G, Matsuda Y, Takeda J. Efficient chromosomal transposition of a Tc1/mariner- like transposon Sleeping Beauty in mice. Proc. Natl. Acad. Sci. USA. 2001;98:9191–9196. doi: 10.1073/pnas.161071798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Horie K, Yusa K, Yae K, Odajima J, Fischer SEJ, Keng VW, Hayakawa T, Mizuno S, Kondoh G, Ijiri T, Matsuda Y, Plasterk RHA, Takeda J. Characterization of Sleeping Beauty transposition and its application to genetic screening in mice. Mol. Cell. Biol. 2003;23:9189–9207. doi: 10.1128/MCB.23.24.9189-9207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carlson CM, Dupuy A, Fritz S, Roberg-Perez K, Fletcher CF, Largaespada DA. Transposon mutagenesis of the mouse germline. Genetics. 2003;165:243–256. doi: 10.1093/genetics/165.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature. 2005;436:272–276. doi: 10.1038/nature03681. [DOI] [PubMed] [Google Scholar]
- 138.Ding S, Wu XH, Li G, Han M, Zhuang Y, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 139.Dupuy AJ, Fritz S, Largaespada DA. Transposition and gene disruption in the male germline of the mouse. Genesis. 2001;30:82–88. doi: 10.1002/gene.1037. [DOI] [PubMed] [Google Scholar]
- 140.Keng VW, Yae K, Hayakawa T, Mizuno S, Uno Y, Yusa K, Kokubu C, Kinoshita T, Akagi K, Jenkins NA, Copeland NG, Horie K, Takeda J. Region-specific saturation germline mutagenesis in mice using the Sleeping Beauty transposon system. Nat. Methods. 2005;2:763–769. doi: 10.1038/nmeth795. [DOI] [PubMed] [Google Scholar]
- 141.Geurts AM, Collier LS, Geurts JL, Oseth LL, Bell ML, Mu D, Lucito R, Godbout SA, Green LE, Lowe SW, Hirsch BA, Leinwand LA, Largaespada DA. Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers. Plos Genet. 2006;2:1413–1423. doi: 10.1371/journal.pgen.0020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ivics Z, Hackett PB, Plasterk RH, Izsvak Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 143.Hahnemann JM, Vejerslev LO. European collaborative research on mosaicism in CVS (EUCROMIC)--fetal and extrafetal cell lineages in 192 gestations with CVS mosaicism involving single autosomal trisomy. Am. J. Med. Genet. 1997;70:179–187. doi: 10.1002/(sici)1096-8628(19970516)70:2<179::aid-ajmg15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 144.Allanson JE, Ohara P, Farkas LG, Nair RC. Anthropometric craniofacial pattern profiles in Down syndrome. Am. J. Med. Genet. 1993;47:748–752. doi: 10.1002/ajmg.1320470530. [DOI] [PubMed] [Google Scholar]
- 145.Cheung SW, Shaw CA, Scott DA, Patel A, Sahoo T, Bacino CA, Pursley A, Li J, Erickson R, Gropman AL, Miller DT, Seashore MR, Summers AM, Stankiewicz P, Chinault AC, Lupski JR, Beaudet AL, Sutton VR. Microarray-based CGH detects chromosomal mosaicism not revealed by conventional cytogenetics. Am. J. Med. Genet. A. 2007;143A:1679–1686. doi: 10.1002/ajmg.a.31740. [DOI] [PubMed] [Google Scholar]
- 146.Kingsbury MA, Yung YC, Peterson SE, Westra JW, Chun J. Aneuploidy in the normal and diseased brain. Cell. Mol. Life Sci. 2006;63:2626–2641. doi: 10.1007/s00018-006-6169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rehen SK, Yung YC, McCreight MP, Kaushal D, Yang AH, Almeida BSV, Kingsbury MA, Cabral KMS, McConnell MJ, Anliker B, Fontanoz M, Chun J. Constitutional aneuploidy in the normal human brain. J. Neurosci. 2005;25:2176–2180. doi: 10.1523/JNEUROSCI.4560-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yurov YB, Iourov IY, Vorsanova SG, Demidova IA, Kravetz VS, Beresheva AK, Kolotii AD, Monakchov VV, Uranova NA, Vostrikov VA, Soloviev IV, Liehr T. The schizophrenia brain exhibits low-level aneuploidy involving chromosome 1. Schizophr. Res. 2008;98:139–147. doi: 10.1016/j.schres.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 149.Zong H, Espinosa S, Su HH, Muzumdar MD, Luo LQ. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 150.Duchon A, Besson V, Pereira PL, Magnol L, Herault Y. Inducing segmental aneuploid mosaicism in the mouse through targeted asymmetric sister chromatid event of recombination. Genetics. 2008;180:51–59. doi: 10.1534/genetics.108.092312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu PT, Jenkins NA, Copeland NG. Efficient Cre-loxP-induced mitotic recombination in mouse embryonic stem cells. Nat. Genet. 2002;30:66–72. doi: 10.1038/ng788. [DOI] [PubMed] [Google Scholar]
- 152.Losada A, Hirano M, Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 2002;16:3004–3016. doi: 10.1101/gad.249202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takata H, Matsunaga S, Morimoto A, Ma N, Kurihara D, Ono-Maniwa R, Nakagawa M, Azuma T, Uchiyama S, Fukui K. PHB2 protects sister-chromatid cohesion in mitosis. Curr. Biol. 2007;17:1356–1361. doi: 10.1016/j.cub.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 154.Leneuve P, Colnot S, Hamard G, Francis F, Niwa-Kawakita M, Giovannini M, Holzenberger M. Cre-mediated germline mosaicism: a new transgenic mouse for the selective removal of residual markers from tri-lox conditional alleles. Nucleic Acids Res. 2003;31:e21. doi: 10.1093/nar/gng021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Modi D, Bhartiya D. Y chromosome mosaicism and occurrence of gonadoblastoma in cases of Turner syndrome and amenorrhoea. Reprod. Biomed. Online. 2007;15:547–553. doi: 10.1016/s1472-6483(10)60387-2. [DOI] [PubMed] [Google Scholar]
- 156.Weaver BAA, Cleveland DW. Aneuploidy: instigator and inhibitor of tumorigenesis. Cancer Res. 2007;67:10103–10105. doi: 10.1158/0008-5472.CAN-07-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arnoldus EPJ, Peters ACB, Bots G, Vanderploeg M. Somatic pairing of chromosome 1 centromeres in interphase nuclei of human cerebellum. Hum. Genet. 1989;83:231–234. doi: 10.1007/BF00285162. [DOI] [PubMed] [Google Scholar]
- 158.Yurov YB, Lourov IY, Monakhov VV, Kolotii AD, Demidova EA, Beresheva AK, Kravets VS, Kutsev SI, Vorsanova SG. The variation of aneuploidy frequency and somatic chromosome pairing in fetal human brain. Chromosome Res. 2005;13:53–54. [Google Scholar]
- 159.Westra JW, Peterson SE, Yung YC, Mutoh T, Barral S, Chun J. Aneuploid mosaicism in the developing and adult cerebellar cortex. J. Comp. Neurol. 2008;507:1944–1951. doi: 10.1002/cne.21648. [DOI] [PubMed] [Google Scholar]
- 160.Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441:1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]