Abstract
Vascular disease in hypertension and diabetes is associated with increased oxidants. The oxidants arise from NADPH oxidase, xanthine oxidase, and mitochondria. Superoxide anion and hydrogen peroxide are produced by both leukocytes and vascular cells. Nitric oxide is produced in excess by inducible nitric oxide synthase, and the potent oxidant, peroxynitrite, is formed from superoxide and nitric oxide. The damage to proteins caused by oxidants is selective, affecting specific oxidant-sensitive amino acid residues. With some important vascular proteins, for example endothelial nitric oxide synthase, prostacyclin synthase, and superoxide dismutase, oxidation of a single susceptible amino acid inactivates the enzyme. The beneficial effects of antioxidants, at least in animal models of hypertension and diabetes, can in part be ascribed to protection of these and other proteins. Mutant proteins lacking their reactive constituent can recapitulate some disease phenotypes suggesting a pathogenic role of the oxidation. Thus, many of the shared functional abnormalites of hypertensive and diabetic blood vessels may be caused by oxidants. Although studies using antioxidants have failed in patients, the successful treatment of vascular disease with HMG CoA reductase inhibitors, thromboxane A2 antagonists, and polyphenols may depend upon their anti-inflammatory effects and ability to decrease production of damaging oxidants.
1. Introduction
Among all cardiovascular risk factors, diabetes mellitus (DM) and hypertension are the leading causes of cardiovascular diseases. Unfortunately, these two risk factors often co-exist, such that 60% of patients with diabetes are hypertensive, and up to 20% of subjects with hypertension are diabetic1. The worldwide morbidity of DM has increased rapidly even in developing countries, doubling the combined risk of cardiovascular events in patients with hypertension2, 3. The endothelium is the principal target of cardiovascular risk factors, including hypertension and diabetes, and is the cell most involved in the development of vascular inflammation and atherosclerosis4. Although low levels of reactive oxygen species (ROS) can play a physiological role in maintaining cardiac and vascular integrity, elevated levels of ROS play a pathophysiological role in cardiovascular dysfunction associated with hypertension and diabetes. Normally, ROS are produced in the vessel wall in a controlled and tightly regulated manner. Under physiological conditions, low concentrations of superoxide anion (O2−•) and hydrogen peroxide (H2O2) are produced in cells by mitochondria and NADPH oxidases. They are controlled by endogenous antioxidants, manganese and copper/zinc superoxide dismutase (MnSOD, Cu/Zn SOD), catalase, and glutathione peroxidases. Together with nitric oxide (•NO) these ROS function as cell signaling initiators by their ability to introduce reversible post-translational protein modifications, such as S-nitroso- and S-glutathione adducts on cysteine thiols5. For example, introducing these adducts on proteins including p21ras6, 7 and the sarcoplasmic reticulum calcium ATPase (SERCA)8, 9 can regulate vascular smooth muscle cell (VSMC) contraction and relaxation and VSMC growth. Under pathological conditions increased ROS production leads to endothelial dysfunction, impaired vascular relaxation, increased VSMC growth and hypertrophy, as well as increased deposition of extracellular matrix proteins. Although mitochondria and xanthine oxidase are implicated as sources of damaging ROS in disease, NADPH oxidases, either in inflammatory leukocytes or vascular cells, account for the bulk of the current literature on vascular pathology. In contrast to the perceived generalized nature of the damage induced by elevated ROS, it is now being recognized that ROS can have selective effects on protein constituents, and they may selectively affect important cardiovascular proteins (Figure 1).
Figure 1.
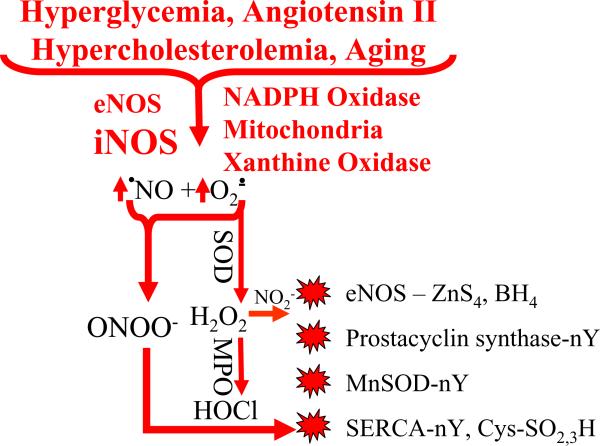
Cardiovascular risk factors increase oxidants and protein oxidation. Major cardiovascular risk factors increase vascular production of nitric oxide (•NO) and superoxide anion (O2−•) by increasing the expression and/or activity of endothelial and inducible •NO synthase (eNOS, iNOS), NADPH oxidase, xanthine oxidase, as well as increasing production of mitochondrial O2−•. •NO and O2−• react rapidly to form peroxynitrite anion (OONO−) which can increase tyrosine nitration (nY), cysteine (Cys) and zinc thiolate (ZnS4) oxidation (SO2,3H). Superoxide dismutases (SOD) form H2O2 which can also oxidize proteins, or together with leukocyte myeloperoxidase (MPO) can form hypochlorous acid (HOCl) and with nitrite (NO2−) can form nY on proteins. Important cardiovascular proteins are affected including eNOS, prostacyclin synthase, MnSOD, the sarco-endoplasmic reticulum calcium ATPase (SERCA).
2. Reactive oxygen and nitrogen species
The principal ROS made by cells is O2−•, an anion radical produced by reduction of oxygen. H2O2 is produced from O2−• either by spontaneous dismutation or enzymatic dismutation by the three isoforms of SOD - Cu/Zn, Mn, and extracellular Cu/Zn SOD. In normal tissues, most H2O2 is converted to water by catalase. •NO produced in vascular cells from constitutive endothelial nitric oxide synthase (eNOS) or inducible NOS, induced by inflammatory cytokines, reacts rapidly with O2−• to form the short-lived peroxynitrite (OONO−). OONO− is termed a reactive nitrogen species (RNS) because of its high reactivity with protein, DNA, and lipids. Leukocyte myeloperoxidase (MPO), H2O2, and chloride anion can also produce hypochlorous acid (HOCl). When O2−• is kept low, •NO remains functionally active. When O2−• is elevated it not only destroys •NO, but the ROS and RNS mentioned here also oxidize protein targets of •NO (Figure 1).
3. NADPH oxidase as a source for increased ROS production
NADPH oxidase is the primary source of ROS in the vasculature and is functionally active in all cells within the vessel wall, including endothelial cells, VSMCs, fibroblasts and monocytes/macrophages10, 11. NADPH oxidase is a multi-component enzyme that is comprised of membrane components p22phox and gp91phox (termed Nox2, or its homologues Nox1, 3–5), and cytoplasmic components p47phox, p67phox and the small G protein, rac1, which plays a role in activating NADPH oxidase. Unlike leukocytes which express Nox2, rat VSMCs express mainly the Nox4 isoform, which together with p22phox are the major components of the active Nox4-based NADPH oxidase complex12, 13. Unlike the leukocyte oxidase which produces O2−• in a burst during activation, there is a continuous low-level of Nox4-derived ROS production in vascular cells, the activation of which does not require rac1, p67phox or p47phox14–16. Also, unlike the leukocytic NADPH oxidase whose NADPH oxidase releases O2−• into the extracellular space, the vascular NADPH oxidases release O2− intracellularly, where it and the ROS and RNS produced from it can act as intracellular signaling molecules or, when produced in excess, can cause damage.
4. Angiotensin II-induced Hypertension and ROS
Oxidative stress caused by increased ROS and RNS plays an important pathophysiological role in hypertension. Treatment with antioxidants or agents to inhibit NADPH oxidase decrease ROS production, prevent target-organ cellular damage, and decrease blood pressure in animal models and in human hypertension (reviewed in ref. 17). The renin-angiotensin-aldosterone system is a major activator of NADPH oxidase and ROS production in hypertension18–20. Angiotensin II (Ang II) stimulates NADPH oxidase both by increasing expression of NADPH oxidase subunits as well as by increasing ROS production in VSMCs, endothelial cells, adventitial fibroblasts21, 22, and in intact arteries23–26. Many of these effects are mediated by Ang II type-1 (AT1) receptors and are blocked by losartan27, 28, except apparently in cultured adventitial fibroblasts21. Some of the therapeutic blood pressure-lowering effects of AT1 receptor blockers in human patients may be attributed to reduction of oxidative stress and increase of plasma antioxidant capacity29.
Several NADPH oxidases may play a role in blood pressure control. Overexpression on Nox1 in mouse VSMC causes a marked increase in systolic blood pressure and hypertrophy in response to Ang II30. In addition, in Nox1 knockout mice, the basal blood pressure is lower, and there was complete protection against Ang II-induced increase in blood pressure and medial hypertrophy. This was attributed to the elimination of O2−• production and improved •NO function31, 32. Nox2 appears to be equally important for the sequelae of Ang II-induced hypertension. Nox2 knockout mice had lower basal blood pressure compared to their controls33, but blood pressure increased similarly in the two strains when Ang II was infused. Despite this, Ang II-induced vascular hypertrophy was entirely prevented in the Nox2 knockout mice and the elevated oxidants were eliminated. This indicates that while the pressor response to Ang II itself does not depend on Nox2 or O2−•, the vascular oxidants and smooth muscle hypertrophy do33. In a model of renovascular hypertension Nox2-derived O2−• decreased •NO function, and there was marked protection from hypertension in the Nox2 knockout mice34. In low renin salt-sensitive hypertension, a tat-peptide inhibitor of Nox2 normalized ROS generation and endothelium-dependent vascular relaxation35. The dual roles of Nox1 and Nox2 in Ang II-induced hypertension in the mouse may be due to the fact that Nox1 is localized in VSMC, but Nox2 is primarily located in endothelial cells and adventitial fibroblasts in normal mice or those infused with Ang II33. Interpreting the roles of the different oxidases is also made more difficult by the potential paracrine roles played by diffusible •NO, O2−•, and H2O225, 36. Ang II increases leukocyte infiltration into the adventitia and intima, accounting for additional contribution to ROS production by leukocyte Nox2. The importance in Ang II-induced hypertension of p47phox, another component of NADPH oxidase involved in its activation, was demonstrated in p47phox knockout mice which failed to develop hypertension in response to Ang II infusion37.
5. NADPH oxidase and Diabetes
Both acute and chronic hyperglycemia is associated with endothelial dysfunction38, 39. The deleterious effects of hyperglycemia in type 2 diabetes are often amplified by coexisting conditions associated with insulin resistance, including hyperlipidemia and hypertension. Activation of NAD(P)H oxidase is implicated in oxidative stress associated with hyperglycemia. Treatment of human umbilical vein endothelial cells with high glucose increases NADPH oxidase expression, levels of oxidative stress markers, and apoptosis40. Moreover, ROS production and expression of p22phox and p47phox are increased in mouse microvascular ECs treated with high glucose41. p47phox siRNA decreases glucose-stimulated O2− production in SMC, implicating involvement of p47phox upregulation and/or phosphorylation in ROS generation in response to hyperglycemia42.
Accumulating evidence suggests that sustained NAD(P)H oxidase ROS generation contributes to endothelial dysfunction in diabetes43, 44. Antioxidants protect against the deleterious effects of high glucose on vascular endothelial cells45. The rac1-regulated NADPH oxidase subunits, Nox1 and Nox2, have been implicated in the abnormal endothelial vasodilatorfunction in diabetes46. Consistent with a role for these Nox isoforms, adenoviral vectors expressing DN rac-1 decrease O2−• production and significantly improve vascular relaxation47. The renin-angiotensin system is activated in diabetes, so maybe an important activator of NADPH oxidase. Ang II acts through the AT1 receptor to inhibit many of the actions of insulin in the vasculature, including vasodilation. The increased AT1 receptor/NAD(P)H oxidase activation appears to contribute to vascular insulin resistance, endothelial dysfunction, apoptosis, and inflammation48.
6. Other Sources of Oxidants in Hypertension and Diabetes
Endothelial nitric oxide synthase (eNOS) consists of both oxidase and reductase domains. When the two enzymatic activities are uncoupled by lack of arginine substrate or tetrahydrobiopterin (BH4) cofactor, eNOS produces O2−• in addition to •NO, making the enzyme a generator of OONO−. OONO− resulting from uncoupling of eNOS has been implicated in Ang II-induced hypertension and diabetes49, 50. Xanthine oxidase is also a source of ROS in atherosclerosis and has been implicated in hypertension and diabetes51, 52. Some of the therapeutic effects of allopurinol used to control uric acid levels in many patients with hypertension and diabetes may result from inhibiting this enzyme. Excess mitochondrial production of ROS is implicated in the setting of hyperglycemia and hyperlipidemia in diabetes53, but in part because mitochondrial inhibitors are so toxic and no deficient mouse models are available, the role of excess ROS from mitochondria during in vivo pathologies is poorly understood.
7. Oxidants and Vascular Function in Hypertension and Diabetes
Both in animal models and patients with diabetes and hypertension endothelium-dependent vasodilator responses to acetylcholine may be attenuated. Studies of diabetic and hypertensive rodent arteries have shown that resting arteries can contract in response to acetylcholine, suggesting that an endothelium-derived contractile agent is produced. In isolated aortic rings from diabetic rabbits, or rings from normal rabbits incubated in high glucose, both the impaired relaxations and endothelium-dependent contractions are prevented by cyclooxygenase inhibitors and thromboxane receptor antagonists, but not by thromboxane synthase inhibitors, suggesting that an eicosanoid, such as prostaglandin endoperoxide (PGH2) or hydroxyeicosatetraenoic acids (HETE's) are produced (Figure 2)54. Antioxidant enzymes, SOD and catalase, and antioxidant compounds, including allopurinol, prevent or restore normal function, indicating a role of ROS in the responses. Although the eicosanoids and the ROS involved have yet to be precisely defined, observations in other rodent models of diabetes and hypertension show similar findings55–61. ROS can both disrupt eicosanoid metabolism as well as produce isoprostanes by direct oxidation of arachidonic acid to account for thromboxane A2 receptor (TP) activation. Indeed, many of the beneficial therapeutic effects of TP antagonists in preventing vascular dysfunction, atherosclerosis, hypertension, and nephropathy in rodent models of hypertension and diabetes are mimicked by antioxidants. Furthermore, TP receptor activation markedly enhances inflammatory signaling in vascular cells62, and a TP antagonist markedly decreased vascular inflammation and tissue oxidants in atherosclerotic diabetic mice63, 64, suggesting that TP receptor activation can be implicated in oxidant generation. S 18886, a TP receptor antagonist improves endothelium-dependent vasodilation in patients with coronary artery disease, suggesting that TP receptor agonists contribute to human vascular dysfunction.
Figure 2.
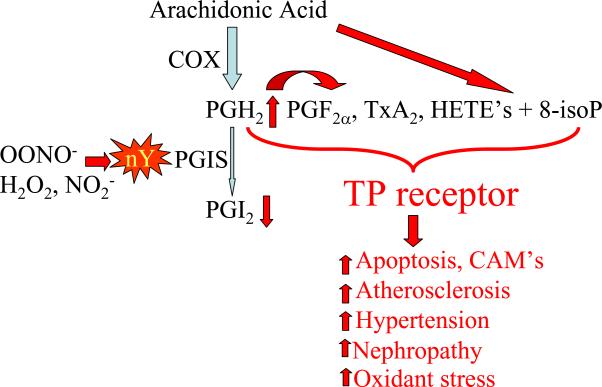
Tyrosine nitration (nY) of prostacyclin synthase (PGIS) increases stimulation of thromboxane A2 (TP) receptors. Nitration of PGIS at tyrosine-430 inactivates the enzyme resulting in shunting of arachidonic acid metabolites to products that stimulate the TP receptor. Cyclooxygenase produces prostaglandin endoperoxide (PGH2) which produces more prostaglandin (F2α) and thromboxane (Tx) A2. More arachidonic acid derived hydroxyeicosatetraenoic acids (HETE's) also are produced. Furthermore, oxidants generate more 8-isoprostanes (isoP) directly from arachidonic acid which also stimulates TP receptors. These products can all be implicated in apoptotic and inflammatory cell responses, increased atherosclerosis, hypertension, and nephropathy. TP receptor stimulation also further augments the generation of reactive oxygen species.
8. Antioxidant defenses in hypertension and diabetes
A number of antioxidants are involved in maintaining defenses against oxidative stress. These mechanisms vary in different intracellular and extracellular compartments and comprise enzymatic and non-enzymatic types. The major vascular enzymatic antioxidants are SOD, catalase, and glutathione peroxidase. Non-enzymatic antioxidants include endogenous ascorbic acid (Vitamin C), α-tocopherol (vitamin E), glutathione, and exogenous carotenoids and flavonoids. Under normal conditions, there is a balance between both the activities and the intracellular levels of these antioxidants. Overproduction of ROS and RNS depletes both enzymatic and non-enzymatic antioxidants leading to additional ROS/RNS accumulation and cellular damage.
Low antioxidant bioavailability promotes cellular oxidative stress and oxidative damage associated with hypertension65. In hypertensive patients, the ratio of oxidized to reduced glutathione was significantly higher, and the activities of SOD, catalase, and glutathione peroxidase were significantly lower in whole blood and peripheral mononuclear cells when compared with normal subjects66. In spontaneously hypertensive rats (SHR) and stroke-prone SHR (SHRSP), the levels of 8-hydroxy-2'-deoxyguanosine, a marker for oxidative stress-induced DNA damage, and protein carbonylation, a marker for protein oxidation, were enhanced in aorta, heart, and kidney, while the expression of thioredoxin was markedly suppressed in those tissues compared with Wistar-Kyoto rats (WKY)67.
Increased oxidative stress and impaired antioxidant defense mechanisms are believed to be the important factors contributing to the pathogenesis and progression of diabetes mellitus. In alloxan-induced diabetic rabbits, the concentration of glutathione and the activities of Cu,Zn-SOD, catalase, and glutathione peroxidase in aortic endothelial cells were significantly decreased compared with controls68. The levels of endogenous antioxidants metallothionein I and II were significantly decreased in skeletal muscle and plasma of type 2 diabetic patients compared with control subjects69.
9. Vascular Oxidant Targets in Hypertension and Diabetes
Rather than indiscriminately affecting cellular constituents, ROS and RNS may affect proteins and their amino acid residues selectively. Sites within proteins that participate in enzyme catalysis and regulation may be particularly susceptible. OONO− in particular is highly reactive with tyrosine – particularly tyrosyl radicals, with reactive cysteine thiols, and with methionine and tryptophan amino acids. Because some of these residues are essential for enzyme function, “one hit” by an oxidant can inactivate a protein, requiring its resynthesis. Oxidants may therefore produce targeted disruption of vascular function.
9.1. Prostacyclin Synthase
Prostacyclin (PGI2) is a potent vasodilator which has anti-platelet, anti-inflammatory, and antioxidant actions within the vasculature. A tyrosyl radical on tyrosine-430 of PGI2 synthase is essential for its catalytic activity. The enzyme is extremely sensitive to OONO− which causes tyrosine nitration at the site and enzymatic inactivation at concentrations as low as 50 nanomolar70, 71. To the extent that PGI2 is a major metabolite of arachidonic acid, the degree to which PGI2 synthase is inactivated, eicosanoid metabolism can be redirected towards vasoconstrictor products including PGH2, PGF2α, TxA2, and HETE's, all of which can be implicated in causing vascular contraction, inflammation, and thrombosis by stimulating TP receptors (Figure 2).
9.2. eNOS
As mentioned above, eNOS can generate OONO− when it is uncoupled. eNOS normally exists as a dimer joined by a zinc atom coordinated by four sulfur atoms, termed a zinc thiolate center (ZnS4). As the sulfur atoms in this center are highly reactive to OONO−, and when oxidized lead to uncoupling of enzymatic activity49. Thus, OONO− produced by eNOS can decrease •NO production and further increase OONO−. The OONO− thus formed can also affect nearby proteins as demonstrated by the fact that in endothelium exposed to high glucose, not only is the eNOS ZnS4 oxidized and the enzyme uncoupled, but PGI2 synthase is tyrosine-nitrated and inactivated. Inhibiting eNOS activity prevents the PGI2 synthase inactivation72, indicating that eNOS uncoupling accounts for the decrease in PGI2.
9.3. MnSOD
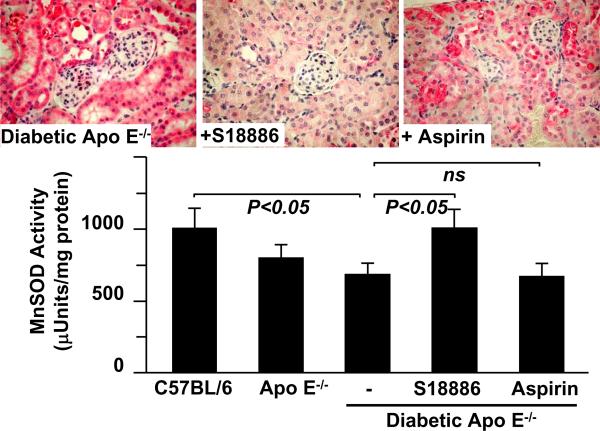
MnSOD, the SOD2 isoform, is located in mitochondria where it is thought to be needed to protect cellular constituents from O2−• derived from the electron transport chain. Like PGI2 synthase, MnSOD has a tyrosyl radical which is essential for its enzymatic activity on tyrosine-34. As much as 20% of all tyrosine nitration in kidneys of Ang II-mediated hypertensive rat and mouse kidney is accounted for by MnSOD tyrosine nitration64, 73. An antibody that specifically recognizes the nitrotyrosine in MnSOD stains a variety of diseased tissues including blood vessels and heart from hypertensive and diabetic animals and patients indicating that the enzyme is attacked by oxidants in disease74 (Figure 3). As nitration of MnSOD inactivates the enzyme, loss of its scavenging activity can be implicated also in further increases in O2−• levels. Realization that this positive reinforcing of oxidant stress could contribute to pathology has led to development of several MnSOD mimetics for therapeutic use75. Also, treatment with a TP antagonist protects against tyrosine nitration of MnSOD, loss of its enzymatic activity, and proteinuria in diabetic atherosclerotic mice64.
Figure 3.
Tyrosine nitration and inactivation of MnSOD in diabetic apoliprotein E deficient mice is prevented by TP antagonist. Upper panels show examples of immunohistochemical staining of kidneys of atherosclerotic, hyperlipidemic apolipoprotein E deficient mice that were given type-1 diabetes by administration of streptozotocin. The red staining was achieved with a sequence-specific antibody that detects nitration of tyrosine-34 of MnSOD74. Treatment of the mice with the TP antagonist, S18886 restored staining to a level indistinguishable from that in non-diabetic control mice, whereas aspirin had no significant effect. The bar graph shows that the renal MnSOD enzymatic activity was significantly decreased from control in the diabetic mice, and that treatment with S18886, but not aspirin prevented the decrease. Data from reference 64.
9.4. SERCA
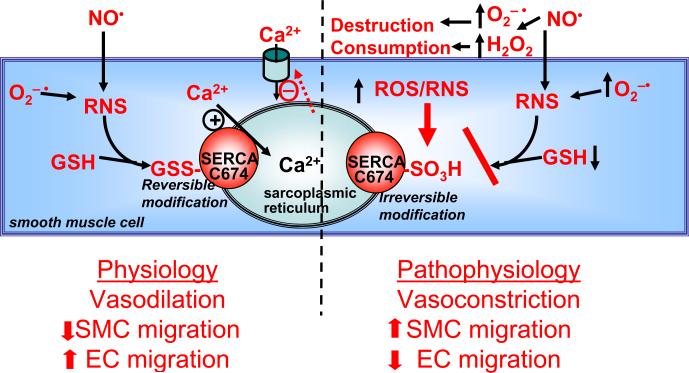
SERCA is a 110 kDa protein which accumulates Ca2+ into the sarcoplasmic/endoplasmic reticulum of all cells. As more than 99% of total cell Ca2+ resides in the stores and the levels there inversely regulate Ca2+ influx from the extracellular space into the cell, SERCA is a major regulator of intracellular free Ca2+ which is responsible for regulating many cell functions. SERCA is also responsible for rapid uptake of Ca2+ into cardiac myocytes, accounting for diastolic relaxation of the heart. SERCA has a reactive thiolate anion on cysteine-674 which when adducted with glutathione adducts increases Ca2+ uptake activity (Figure 4). The same reactive thiol is quantitatively and irreversibly oxidized by concentrations of OONO− in the 100–400 micromolar range. Despite the requirement for this high concentration in vitro, chronically elevated oxidants oxidize SERCA in diseased tissues. Iodoacetamide labeling of the free reactive cysteine thiol on cysteine-674 indicates that more than 50% of the thiol is oxidized in atherosclerotic rabbit aorta, accounting for the impaired ability of •NO to relax the blood vessel8. An antibody recognizing irreversible sulfonic acid oxidation of cysteine-674 reveals widespread oxidation of this cysteine in blood vessels and heart from hypertensive, diabetic, and atherosclerotic, blood vessels in rodents and patients76 (Figure 5). To the extent that redox regulation of SERCA is important for vascular and cardiac relaxation, irreversible oxidation of cysteine-674 is an indicator of physiological impairment. In the aorta of chronically diabetic pigs, SERCA with oxidized cysteine-674 was found in a 70 kDa form, consistent with irreversible oxidation of the protein resulting in its degradation76.
Figure 4.
Redox regulation of SERCA by reactive oxygen/nitrogen species. RNS produced by •NO and O2−• increase glutathione adducts (GSS-) of cysteine(C)-674 of SERCA. This increases Ca2+ uptake into sarcoplasmic reticulum stores, inhibiting store-dependent Ca2+ influx, and decreasing cytosolic Ca2+ which causes vasodilation, inhibits smooth muscle cell (SMC) migration, and increases endothelial cell (EC) migration. Under pathophysiological conditions higher levels of ROS increase the destruction and consumption of •NO, producing RNS which can oxidize the SERCA C674 thiol (−SO3H), preventing its reversible S-glutathiolation and blocking the stimulation of SERCA by •NO. Thus, the redox status of C674 can determine physiological and pathophysiological changes in vascular tone and cell migration. From reference 8.
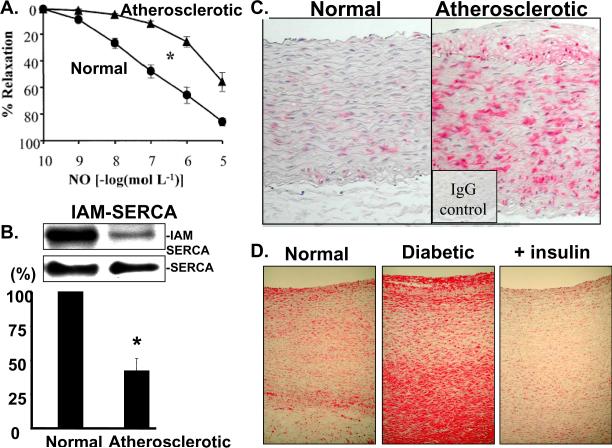
Figure 5.
Oxidation of SERCA in atherosclerotic and diabetic aorta. A. Impaired aortic relaxations to •NO in rabbits made atherosclerotic by feeding a high cholesterol diet for 10 weeks. B. The decreased response can be explained in part by decreased labeling of the free thiol on cysteine-674 with biotin-tagged IAM (upper blot) summarized in bar graph. There is no change in total SERCA expression (lower blot). C and D. Immunohistochemical staining of oxidized SERCA cysteine-674 by a sequence specific antibody that recognizes the sulfonic acid thiol. Increased staining is seen in atherosclerotic rabbit aorta (C) obtained from the same study as data in panels A and B, as well as in the aorta of a pig maintained diabetic and hypercholesterolemic for 1 year, but not in a diabetic pig treated with insulin for 1 year. Data from references 8 and76.
9.5. Antioxidant Therapy
In rodents, antioxidant agents such as Tempol, apocynin, butylated hydroxytoluene, and vitamin E and C show remarkable effects in preventing both oxidation of proteins and hypertensive and diabetic cardiovascular disease77–83. The same can be said of transgenic mouse models in which NADPH oxidase components are genetically eliminated34, 84. Despite the ability to prevent disease, the evidence that antioxidants can reverse the effects of disease either in rodents and human patients is limited. Acute administration of antioxidants may restore vascular function, suggesting that elevated oxidants do play a role85–88. However, treatment of patients with vitamin C and E have not revealed any significant long term benefit29, 78, 89–91. The lack of efficiency may be attributed to the low doses used, to the failure to demonstrate antioxidant efficacy in vivo, or potentially to interference with physiological roles of oxidants in cell function.
Because inflammation plays such an important role in the pathophysiology of vascular disease, and oxidant generation is an inherent participant in that process, anti-inflammatory therapies might achieve antioxidant effects. However, there is no evidence that anti-inflammatory therapy per se with aspirin, nonsteroidal, or steroid anti-inflammatory agents are able to prevent vascular disease progression or excess oxidants in tissues. On the other hand, therapeutic agents that have proven effective in decreasing atherosclerotic plaque in human patients, including HMG CoA reductase inhibitors (statins)89 and metformin92–94, do have both anti-inflammatory and anti-oxidant effects95–99. For example, statins ameliorate endothelial dysfunction mainly by an attenuation of O2−• production by NADPH oxidase. A recent study showed that atorvastatin can decrease COX2-dependent 8-isoprostane generation which causes endothelial dysfunction in SHR100. Atorvastatin also restored NO bioavailability by increasing phosphorylation of extracellular signal-regulated kinase 1/2, Akt, and eNOS, as well as increasing expression of inducible NO synthase levels and decreasing vascular NADPH oxidase-driven O2-• production101. TP antagonists63, 102 and polyphenols103, 104 are two additional examples of therapeutic agents that provide anti-inflammatory effects and vascular protection accompanied by significant improvement in protein oxidation, at least in rodent models.
9.6. Conclusions
ROS are regulators of normal cellular function, but when produced in excess they contribute to the disease process. High levels of •NO produced by iNOS can create the more reactive OONO−, and leukocyte myeloperoxidase produces HOCl, making the accumulation of inflammatory leukocytes possessing these two enzymes within vascular lesions particularly injurious. Oxidants attack specific amino acids within proteins, and these residues are often those that are more reactive because they have evolved to mediate normal enzymatic catalysis or regulation, as in PGI2 synthase, eNOS, MnSOD, and SERCA. Despite the ability of antioxidants to prevent vascular disease in rodents, little evidence exists for their efficacy in patients. Rather, agents that have pleotropic anti-inflammatory effects including statins, metformin, TP antagonists, and polyphenols appear to limit oxidant production because of the integral role of oxidant production in inflammation.
Acknowledgements
The authors' laboratory is supported by National Institutes of Health grants: R01 HL031607, PO1 HL081587, R01 AG27080 and P01 HL068758, and by a Strategic Alliance with the Institut de Recherche Servier. Dr. Tong is the recipient of an American Diabetes Association Junior Faculty Award 7-09-JF-69.
Footnotes
Conflicts of interest: Dr. Cohen is a consultant to Institut de Recherche Servier.
References
- (1).Contreras F, Rivera M, Vasquez J, De la Parte MA, Velasco M. Diabetes and hypertension physiopathology and therapeutics. J Hum Hypertens. 2000;14(Suppl 1):S26–S31. doi: 10.1038/sj.jhh.1000983. [DOI] [PubMed] [Google Scholar]
- (2).Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- (4).Savoia C, Schiffrin EL. Inhibition of the renin angiotensin system: implications for the endothelium. Curr Diab Rep. 2006;6(4):274–8. doi: 10.1007/s11892-006-0060-5. [DOI] [PubMed] [Google Scholar]
- (5).Adachi T, Schoneich C, Cohen RA. S-glutathiolation in redox-sensitive signaling. Drug Discov Today. 2005;2(1):39–46. [Google Scholar]
- (6).Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279(28):29857–62. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- (7).Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 2006;20(3):518–20. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- (8).Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10(11):1200–7. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- (9).Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, Cohen RA. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler Thromb Vasc Biol. 2007;27(4):783–90. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- (10).Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86(5):494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- (11).Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90(11):1205–13. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- (12).Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65(2):495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- (13).Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a Novel Regulator of Nox4 and Cytoskeletal Integrity in Vascular Smooth Muscle Cells. Circulation Research. 2009;105(3):249–59. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279(44):45935–41. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- (15).Martyn KD, Frederick LM, von LK, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18(1):69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- (16).Meng D, Lv DD, Fang J. Insulin-like growth factor-I induces reactive oxygen species production and cell migration through Nox4 and Rac1 in vascular smooth muscle cells. Cardiovasc Res. 2008;80(2):299–308. doi: 10.1093/cvr/cvn173. [DOI] [PubMed] [Google Scholar]
- (17).Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122(4):339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- (18).Touyz RM. Recent advances in intracellular signalling in hypertension. Curr Opin Nephrol Hypertens. 2003;12(2):165–74. doi: 10.1097/00041552-200303000-00007. [DOI] [PubMed] [Google Scholar]
- (19).Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q, Taylor WR, Harrison DG, de LH, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80(1):45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- (20).Schiffrin EL, Touyz RM. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart Circ Physiol. 2004;287(2):H435–H446. doi: 10.1152/ajpheart.00262.2004. [DOI] [PubMed] [Google Scholar]
- (21).Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci USA. 1997;94:14483–8. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lassegue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- (23).Pagano PJ, Ito Y, Tornheim K, Gallop P, Cohen RA. An NADPH oxidase superoxide generating system in the rabbit aorta. Am J Physiol. 1995;268:H2274–H2280. doi: 10.1152/ajpheart.1995.268.6.H2274. [DOI] [PubMed] [Google Scholar]
- (24).Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–8. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- (25).Wang HD, Hope S, Du Y, Quinn MT, Cayatte AJ, Pagano PJ, Cohen RA. Paracrine role of adventitial superoxide anion in mediating spontaneous tone of the isolated rat aorta in angiotensin II-induced hypertension. Hypertension. 1999;33:1225–32. doi: 10.1161/01.hyp.33.5.1225. [DOI] [PubMed] [Google Scholar]
- (26).Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91(5):406–13. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- (27).Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97(8):1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42(2):206–12. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- (29).Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, Salvetti A. Different effect of antihypertensive drugs on conduit artery endothelial function. Hypertension. 2003;41(6):1281–6. doi: 10.1161/01.HYP.0000070956.57418.22. [DOI] [PubMed] [Google Scholar]
- (30).Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San MA, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112(17):2668–76. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- (31).Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580(2):497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- (32).Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112(17):2677–85. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- (33).Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–53. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- (34).Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109(14):1795–801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- (35).Zhou MS, Hernandez S, I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47(1):81–6. doi: 10.1161/01.HYP.0000197182.65554.c7. [DOI] [PubMed] [Google Scholar]
- (36).Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: Bellwether for vascular disease? Cardiovasc Res. 2007;75(4):679–89. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- (37).Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40(4):511–5. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circ. 1998;97(17):1695–701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- (39).Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007;3(1):46–56. doi: 10.1038/ncpendmet0366. [DOI] [PubMed] [Google Scholar]
- (40).Quagliaro L, Piconi L, Assaloni R, Da RR, Maier A, Zuodar G, Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: the distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis. 2005;183(2):259–67. doi: 10.1016/j.atherosclerosis.2005.03.015. [DOI] [PubMed] [Google Scholar]
- (41).Ding H, Aljofan M, Triggle CR. Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J Cell Physiol. 2007;212(3):682–9. doi: 10.1002/jcp.21063. [DOI] [PubMed] [Google Scholar]
- (42).Liu S, Ma X, Gong M, Shi L, Lincoln T, Wang S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med. 2007;42(6):852–63. doi: 10.1016/j.freeradbiomed.2006.12.025. [DOI] [PubMed] [Google Scholar]
- (43).Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105(14):1656–62. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- (44).Lopez-Lopez JG, Moral-Sanz J, Frazziano G, Gomez-Villalobos MJ, Flores-Hernandez J, Monjaraz E, Cogolludo A, Perez-Vizcaino F. Diabetes induces pulmonary artery endothelial dysfunction by NADPH oxidase induction. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L727–L732. doi: 10.1152/ajplung.90354.2008. [DOI] [PubMed] [Google Scholar]
- (45).Ulker S, McMaster D, McKeown PP, Bayraktutan U. Antioxidant vitamins C and E ameliorate hyperglycaemia-induced oxidative stress in coronary endothelial cells. Diabetes Obes Metab. 2004;6(6):442–51. doi: 10.1111/j.1462-8902.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- (46).Wendt MC, Daiber A, Kleschyov AL, Mulsch A, Sydow K, Schulz E, Chen K, Keaney JF, Jr., Lassegue B, Walter U, Griendling KK, Munzel T. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med. 2005;39(3):381–91. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- (47).Vecchione C, Aretini A, Marino G, Bettarini U, Poulet R, Maffei A, Sbroggio M, Pastore L, Gentile MT, Notte A, Iorio L, Hirsch E, Tarone G, Lembo G. Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circ Res. 2006;98(2):218–25. doi: 10.1161/01.RES.0000200440.18768.30. [DOI] [PubMed] [Google Scholar]
- (48).Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50(2):384–91. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- (49).Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109(6):817–26. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Molnar J, Yu S, Mzhavia N, Pau C, Chereshnev I, Dansky HM. Diabetes induces endothelial dysfunction but does not increase neointimal formation in high-fat diet fed C57BL/6J mice. Circ Res. 2005;96(11):1178–84. doi: 10.1161/01.RES.0000168634.74330.ed. [DOI] [PubMed] [Google Scholar]
- (51).Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91:2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Matsumoto S, Koshiishi I, Inoguchi T, Nawata H, Utsumi H. Confirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabetic mice. Free Radic Res. 2003;37(7):767–72. doi: 10.1080/1071576031000107344. [DOI] [PubMed] [Google Scholar]
- (53).Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- (54).Cohen RA. Dysfunction of vascular endothelium in diabetes mellitus. Circ. 1993;87:V67–V76. [Google Scholar]
- (55).Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136(1):104–10. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003;41(1):143–8. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- (57).Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004;18(3):321–6. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- (58).Kanie N, Kamata K. Contractile responses in spontaneously diabetic mice: I. Involvement of superoxide anion in enhanced contractile response of aorta to norepinephrine in C57BL/KsJ(db/db) mice. General Pharmacology: The Vascular System. 2000;35(6):311–8. doi: 10.1016/s0306-3623(02)00115-5. [DOI] [PubMed] [Google Scholar]
- (59).Luscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–8. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- (60).Luscher TF, Diederich D, Weber E, Vanhoutte PM, Buhler FR. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension. 1988;11:573–8. doi: 10.1161/01.hyp.11.6.573. [DOI] [PubMed] [Google Scholar]
- (61).Diederich D, Yang Z, Buhler FR, Luscher TF. Impaired endothelium-dependent relaxations in hypertensive resistance arteries involve cyclooxygenase pathway. Am J Physiol. 1990;258:H445–H451. doi: 10.1152/ajpheart.1990.258.2.H445. [DOI] [PubMed] [Google Scholar]
- (62).Bayat H, Xu S, Pimentel D, Cohen RA, Jiang B. Activation of thromboxane receptor upregulates interleukin (IL)-1beta-induced VCAM-1 expression through JNK signaling. Arterioscler Thromb Vasc Biol. 2008;28(1):127–34. doi: 10.1161/ATVBAHA.107.150250. [DOI] [PubMed] [Google Scholar]
- (63).Zuccollo A, Shi C, Mastroianni R, Maitland-Toolan KA, Weisbrod RM, Zang M, Xu S, Jiang B, Oliver-Krasinski JM, Cayatte AJ, Corda S, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane A2 receptor antagonist S18886 prevents enhanced atherogenesis caused by diabetes mellitus. Circulation. 2005;112(19):3001–8. doi: 10.1161/CIRCULATIONAHA.105.581892. [DOI] [PubMed] [Google Scholar]
- (64).Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA. The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes. 2006;55(1):110–9. [PubMed] [Google Scholar]
- (65).Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44(4):381–6. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- (66).Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, Saez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41(5):1096–101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- (67).Tanito M, Nakamura H, Kwon YW, Teratani A, Masutani H, Shioji K, Kishimoto C, Ohira A, Horie R, Yodoi J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid Redox Signal. 2004;6(1):89–97. doi: 10.1089/152308604771978381. [DOI] [PubMed] [Google Scholar]
- (68).Tagami S, Kondo T, Yoshida K, Hirokawa J, Ohtsuka Y, Kawakami Y. Effect of insulin on impaired antioxidant activities in aortic endothelial cells from diabetic rabbits. Metabolism. 1992;41(10):1053–8. doi: 10.1016/0026-0495(92)90285-i. [DOI] [PubMed] [Google Scholar]
- (69).Scheede-Bergdahl C, Penkowa M, Hidalgo J, Olsen DB, Schjerling P, Prats C, Boushel R, Dela F. Metallothionein-mediated antioxidant defense system and its response to exercise training are impaired in human type 2 diabetes. Diabetes. 2005;54(11):3089–94. doi: 10.2337/diabetes.54.11.3089. [DOI] [PubMed] [Google Scholar]
- (70).Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol. 1999;154(5):1359–65. doi: 10.1016/S0002-9440(10)65390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Schmidt P, Youhnovski N, Daiber A, Balan A, Arsic M, Bachschmid M, Przybylski M, Ullrich V. Specific nitration at tyrosine-430 revealed by high resolution mass spectrometry as basis for redox regulation of bovine prostacyclin synthase. J Biol Chem. 2003;278:12813–9. doi: 10.1074/jbc.M208080200. [DOI] [PubMed] [Google Scholar]
- (72).Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H2 receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- (73).Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol. 2003;285(4):H1396–H1403. doi: 10.1152/ajpheart.00096.2003. [DOI] [PubMed] [Google Scholar]
- (74).Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schoneich C, Cohen RA. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am J Physiol Heart Circ Physiol. 2006;290(6):H2220–H2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- (75).Rosenthal R, Huffman K, Fisette L, Damphousse C, Callaway W, Malfroy B, Doctrow S. Orally available Mn porphyrins with superoxide dismutase and catalase activities. Journal of Biological Inorganic Chemistry. 2009;14(6):979–91. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic Biol Med. 2008;45(6):756–62. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Adachi T, Matsui R, Xu S, Kirber M, Lazar HL, Sharov VS, Schoneich C, Cohen RA. Antioxidant improves smooth muscle sarco/endoplasmic reticulum Ca(2+)-ATPase function and lowers tyrosine nitration in hypercholesterolemia and improves nitric oxide-induced relaxation. Circ Res. 2002;90(10):1114–21. doi: 10.1161/01.res.0000019757.57344.d5. [DOI] [PubMed] [Google Scholar]
- (78).Aruoma OI, Evans PJ, Kaur H, Sutcliffe L, Halliwell B. An evaluation of the antioxidant and potential pro-oxidant properties of food additives and of trolox C, vitamin E and probucol. Free Radic Res Commun. 1990;10(3):143–57. doi: 10.3109/10715769009149883. [DOI] [PubMed] [Google Scholar]
- (79).Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension. 2001;37:781–6. doi: 10.1161/01.hyp.37.2.781. [DOI] [PubMed] [Google Scholar]
- (80).Hoffmann DS, Weydert CJ, Lazartigues E, Kutschke WJ, Kienzle MF, Leach JE, Sharma JA, Sharma RV, Davisson RL. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension. 2008;51(4):1058–65. doi: 10.1161/HYPERTENSIONAHA.107.107219. [DOI] [PubMed] [Google Scholar]
- (81).Jagadeesha DK, Lindley TE, Deleon J, Sharma RV, Miller F, Bhalla RC. Tempol therapy attenuates medial smooth muscle cell apoptosis and neointima formation after balloon catheter injury in carotid artery of diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289(3):H1047–H1053. doi: 10.1152/ajpheart.01071.2004. [DOI] [PubMed] [Google Scholar]
- (82).Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77(6):1053–63. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- (83).Trujillo J, Cruz C, Tovar A, Vaidya V, Zambrano E, Bonventre JV, Gamba G, Torres N, Bobadilla NA. Renoprotective mechanisms of soy protein intake in the obese Zucker rat. Am J Physiol Renal Physiol. 2008;295(5):F1574–F1582. doi: 10.1152/ajprenal.90385.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).DeRubertis FR, Craven PA, Melhem MF, Salah EM. Attenuation of renal injury in db/db mice overexpressing superoxide dismutase: evidence for reduced superoxide-nitric oxide interaction. Diabetes. 2004;53(3):762–8. doi: 10.2337/diabetes.53.3.762. [DOI] [PubMed] [Google Scholar]
- (85).Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97(1):22–8. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Ting HH, Timimi FK, Haley EA, Roddy MA, Ganz P, Creager MA. Vitamin C restores endothelium-dependent vasodilation in patients with hypercholesterolemia. Circ. 1996;94(8):I–402. [Google Scholar]
- (87).Levine GN, Frei B, Koulouris SN, Gerhard MD, Keaney JF, Jr., Vita JA. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circ. 1996;93(6):1107–13. doi: 10.1161/01.cir.93.6.1107. [DOI] [PubMed] [Google Scholar]
- (88).Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997;278(20):1682–6. [PubMed] [Google Scholar]
- (89).Farmer JA, Gotto AM. The Heart Protection Study: expanding the boundaries for high-risk coronary disease prevention. The American Journal of Cardiology. 2003;92(1, Supplement 1):3–9. doi: 10.1016/s0002-9149(03)00503-4. [DOI] [PubMed] [Google Scholar]
- (90).Keli SO, Hertog MGL, Feskens EJM, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke. Arch Intern Med. 1996;154:637–42. [PubMed] [Google Scholar]
- (91).Leng GC, Lee AJ, Fowkes FGR, Horrobin D, Jepson RG, Lowe GDO, Rumley A, Skinner ER, Mowat BF. Randomized control trial of antioxidants in intermittent claudication. Vascular Medicine. 1997;2:279–85. doi: 10.1177/1358863X9700200401. [DOI] [PubMed] [Google Scholar]
- (92).Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37(5):1344–50. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- (93).Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GM. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258(3):250–6. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- (94).de Aguiar LG, Bahia LR, Villela N, Laflor C, Sicuro F, Wiernsperger N, Bottino D, Bouskela E. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance 6. Diabetes Care. 2006;29(5):1083–9. doi: 10.2337/diacare.2951083. [DOI] [PubMed] [Google Scholar]
- (95).Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104(15):1767–72. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- (96).Sumi D, Hayashi T, Thakur NK, Jayachandran M, Asai Y, Kano H, Matsui H, Iguchi A. A HMG-CoA reductase inhibitor possesses a potent anti-atherosclerotic effect other than serum lipid lowering effects--the relevance of endothelial nitric oxide synthase and superoxide anion scavenging action. Atherosclerosis. 2001;155(2):347–57. doi: 10.1016/s0021-9150(00)00597-9. [DOI] [PubMed] [Google Scholar]
- (97).Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol (United States) 2002;20(1):61–9. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- (98).Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG, IV, Schlettner U, Neumann D, Brownlee M, Freeman MB, Goldman MH. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo: Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004:M404421200. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- (99).Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Matsumura T, Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55(1):120–7. [PubMed] [Google Scholar]
- (100).Virdis A, Colucci R, Versari D, Ghisu N, Fornai M, Antonioli L, Duranti E, Daghini E, Giannarelli C, Blandizzi C, Taddei S, Del TM. Atorvastatin prevents endothelial dysfunction in mesenteric arteries from spontaneously hypertensive rats: role of cyclooxygenase 2-derived contracting prostanoids. Hypertension. 2009;53(6):1008–16. doi: 10.1161/HYPERTENSIONAHA.109.132258. [DOI] [PubMed] [Google Scholar]
- (101).Virdis A, Colucci R, Versari D, Ghisu N, Fornai M, Antonioli L, Duranti E, Daghini E, Giannarelli C, Blandizzi C, Taddei S, Del TM. Atorvastatin prevents endothelial dysfunction in mesenteric arteries from spontaneously hypertensive rats: role of cyclooxygenase 2-derived contracting prostanoids. Hypertension. 2009;53(6):1008–16. doi: 10.1161/HYPERTENSIONAHA.109.132258. [DOI] [PubMed] [Google Scholar]
- (102).Cayatte AJ, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:1724–8. doi: 10.1161/01.atv.20.7.1724. [DOI] [PubMed] [Google Scholar]
- (103).Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55(8):2180–91. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- (104).Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets NADPH oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–84. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]