Abstract
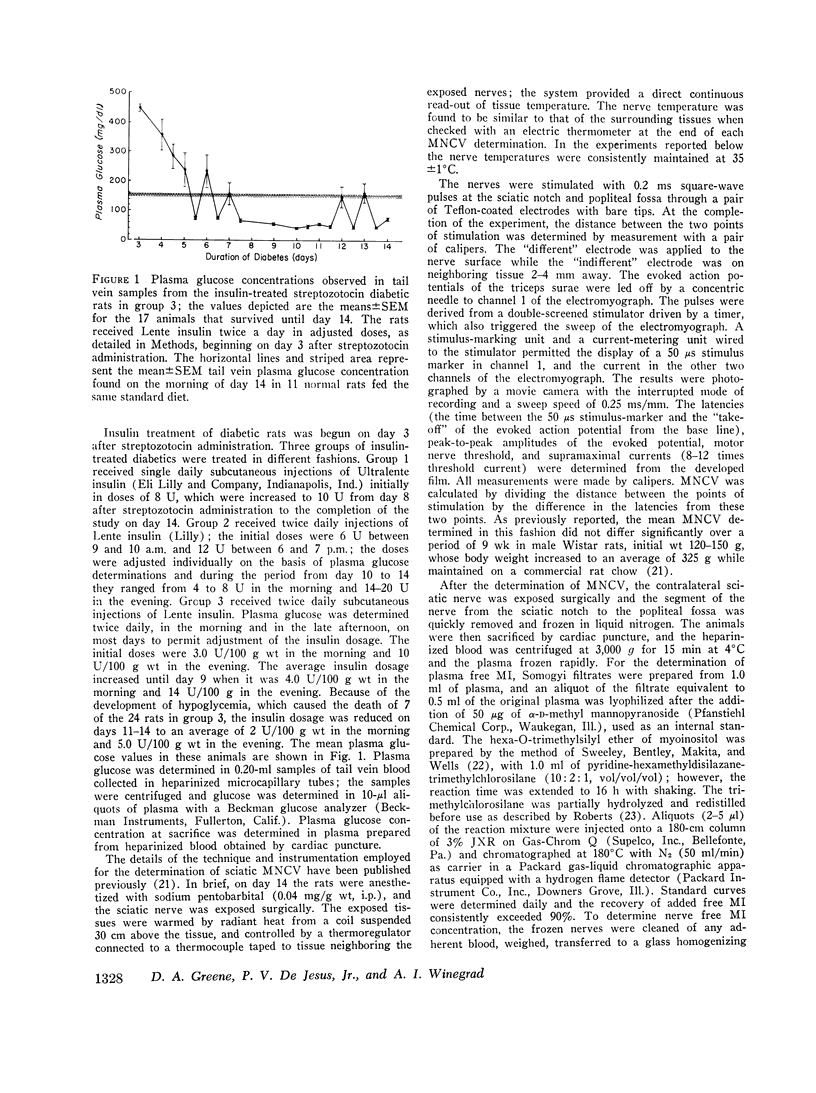
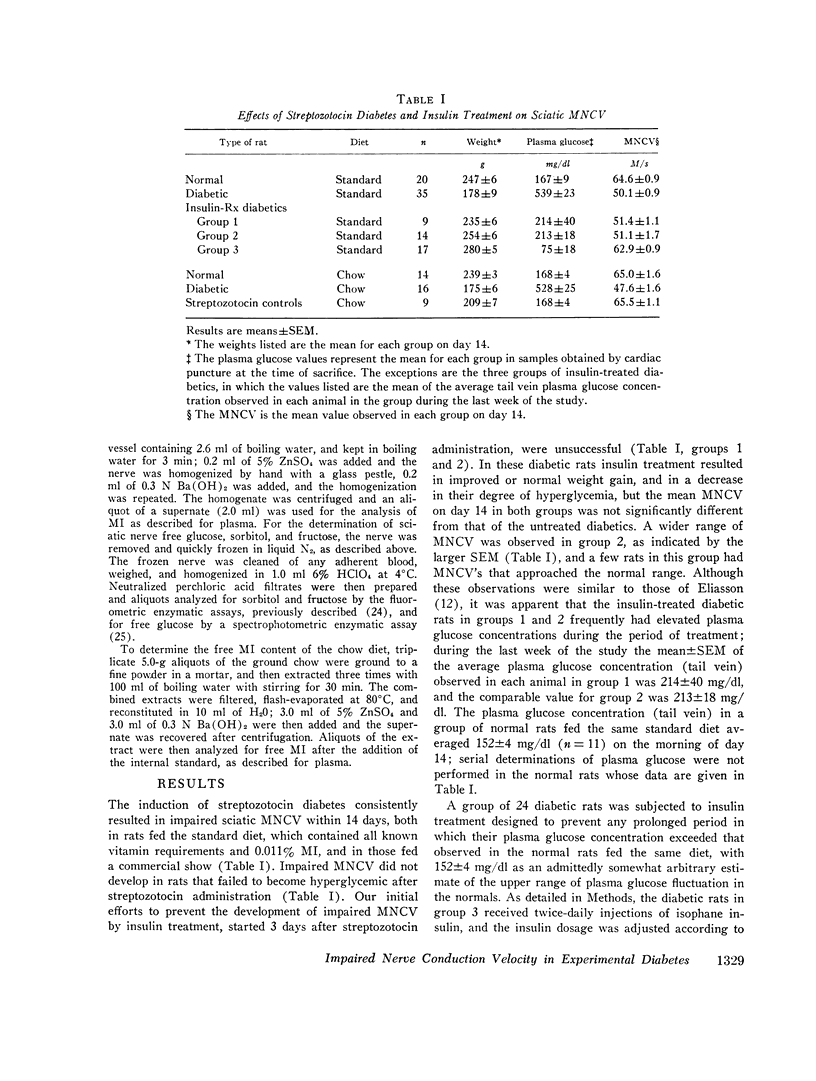
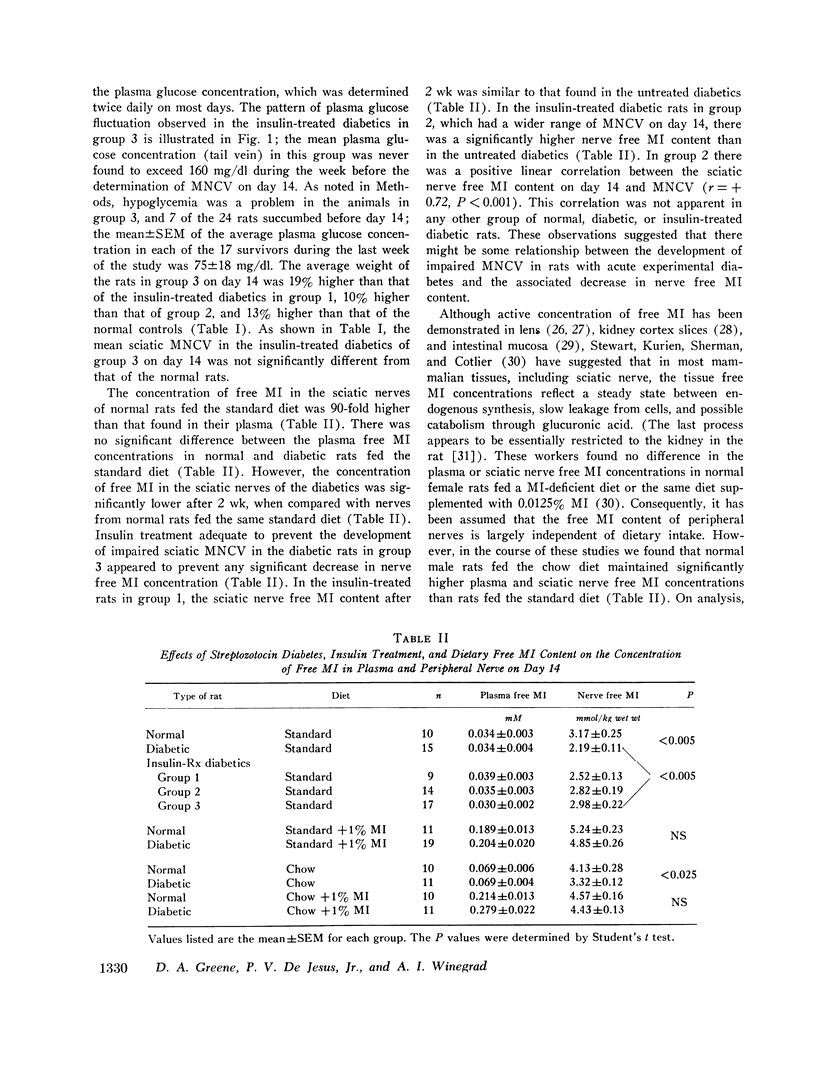
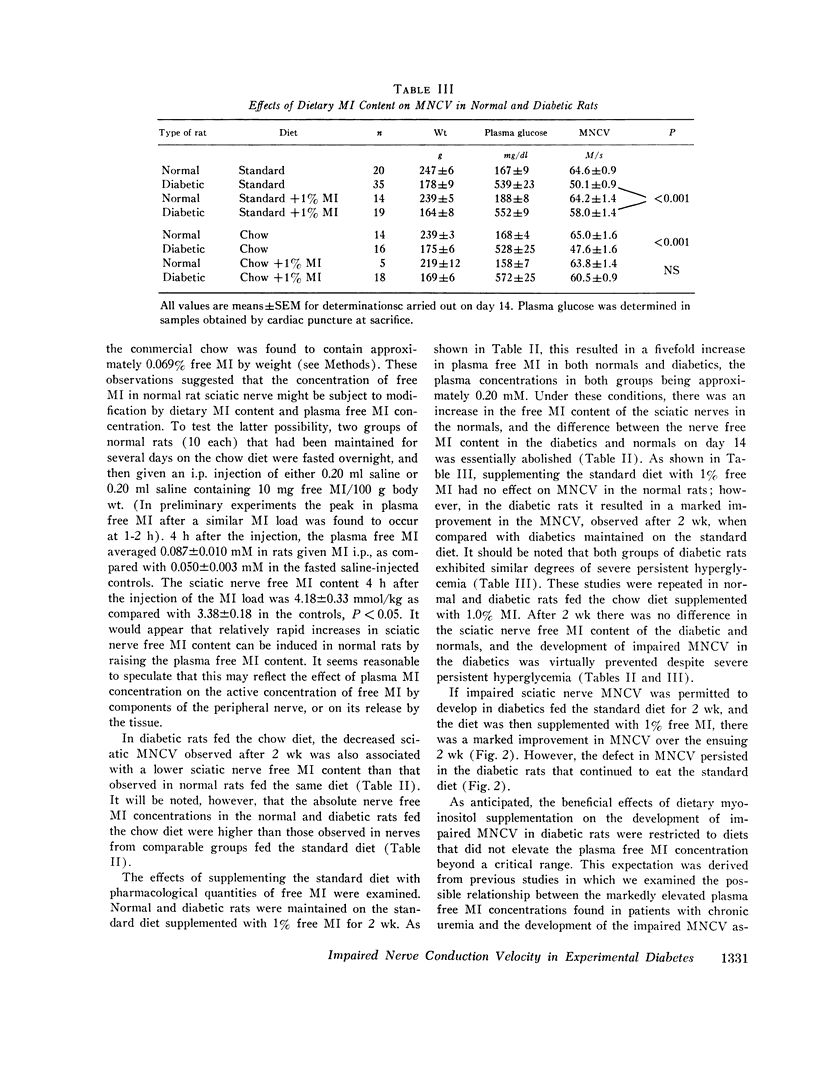
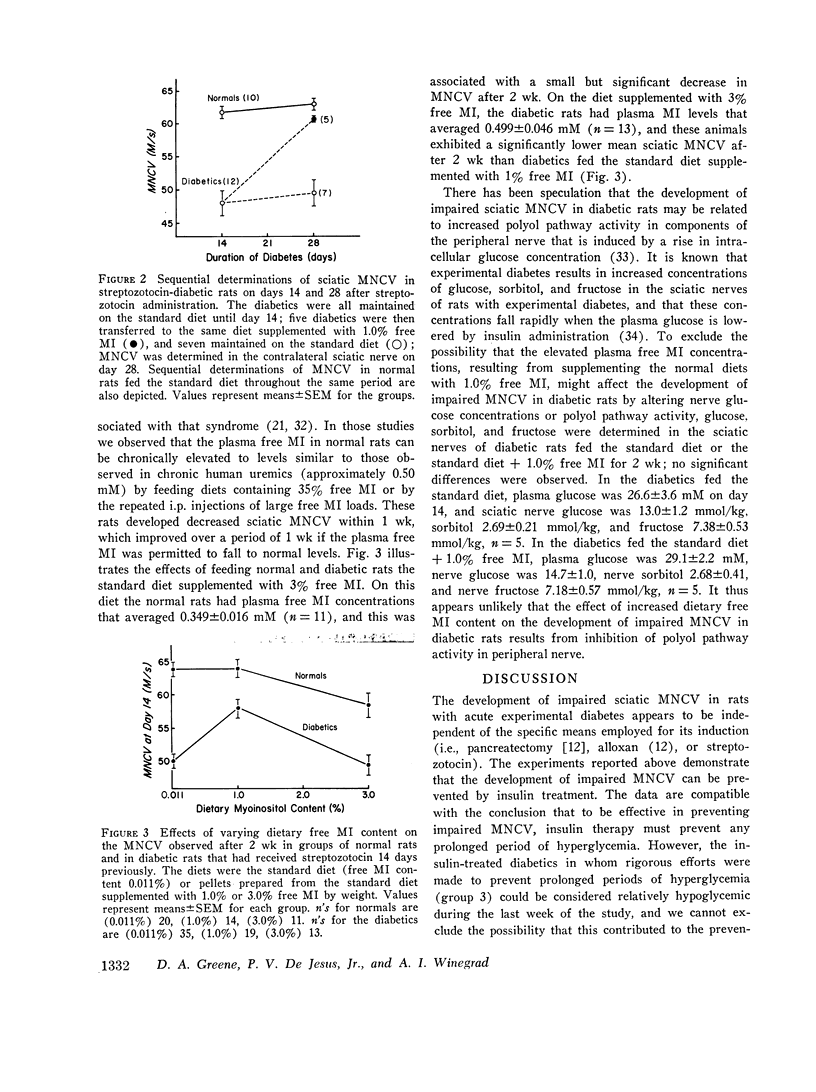
The factors influencing the development of impaired sciatic motor nerve conduction velocity (MNCV) in acute experimental diabetes were examined. Decreased MNCV developed by the 14th day after streptozotocin administration but only in rats which became hyperglycemic. Insulin treatment, begun on day 3, failed to prevent imparied MNCV in diabetic rats in which improved or normal weight gain and a decreased degree of hyperglycemia was induced. However, insulin treatment prevented the development of impaired MNCV in a group of diabetic rats in which the tail vein plasma glucose concentration was never found to exceed 160 mg/dl during days 6 through 14, andin which the mean plus or minus SEM of the average plasma glucose concentration for each animal during the same period was 75 plus or minus 18 mg/dl. In normal rats fed diets containing 0.011% or 0.069% free myoinositol (a presumably normal range), sciatic nerve free myoinositol concentrations were 90- and 60-fold higher than those in plasma. On these diets the development of impaired MNCV in the diabetics was associated with a decrease in nerve free myoinositol as compared with nerves from normals fed the same diet, despite similar plasma levels in the normals and diabetics. Plasma and nerve free myoinositol increased with increasing dietary myoinositol content in both normals and diabetics, and nerve myoinositol content could be acutely increased by an i.p. myoinositol load. By supplementing the diets with 1.0% myoinositol, the difference in nerve myoinositol in normal and diabetic rats on day 14 was abolished; on this diet the development of impaired MNCV in the diabetics was moderated or totally prevented, despite persistent hyperglycemia and elevated nerve sorbitol and fructose concentrations. Insulin treatment that prevented impaired MNCV prevented a decrease in nerve myoinositol in diabetics. These studies suggest that insulin deficiency, and possibly hyperglycemia, are primary factors in the development of imparied MNCV in acute experimental diabetes. However, the development of impaired MNCV appears to be related in some manner to a derangement in the regulation of nerve free myoinositol content, which appears to be subject to modification by increases in plasma myoinositol concentration over a critical range.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Benjamin J. A., Hajra A. K. Biosynthesis of phosphatidylinositol. Ann N Y Acad Sci. 1969 Oct 17;165(2):755–760. doi: 10.1111/j.1749-6632.1970.tb55954.x. [DOI] [PubMed] [Google Scholar]
- Bischoff A. Ultrastructural pathology of periperal nervous system in early diabetes. Adv Metab Disord. 1973;2(Suppl):441–449. doi: 10.1016/b978-0-12-027362-1.50052-2. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M. Changes in myo-inositol permeability in the lens due to cataractous conditions. Biochim Biophys Acta. 1968 Sep 17;163(2):269–272. doi: 10.1016/0005-2736(68)90107-7. [DOI] [PubMed] [Google Scholar]
- Cameron D. P., Amherdt M., Leuenberger P., Orci L., Stauffacher W. Microvascular alterations in chronically streptozotocin-diabetic rats. Adv Metab Disord. 1973;2(Suppl):257–269. doi: 10.1016/b978-0-12-027362-1.50033-9. [DOI] [PubMed] [Google Scholar]
- Caspary W. F., Crane R. K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970 Apr 21;203(2):308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- Chopra J. S., Hurwitz L. J. A comparative study of peripheral nerve conduction in diabetes and non-diabetic chronic occlusive peripheral vascular disease. Brain. 1969 Mar;92(1):83–96. doi: 10.1093/brain/92.1.83. [DOI] [PubMed] [Google Scholar]
- Chopra J. S., Hurwitz L. J., Montgomery D. A. The pathogenesis of sural nerve changes in diabetes mellitus. Brain. 1969;92(2):391–418. doi: 10.1093/brain/92.2.391. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, DeJesus P. V., Jr, Winegrad A. I. Raised plasma-myoinositol levels in uraemia and experimental neuropathy. Lancet. 1973 May 26;1(7813):1137–1141. doi: 10.1016/s0140-6736(73)91143-4. [DOI] [PubMed] [Google Scholar]
- Cotlier E. Myo-inositol: active transport by the crystalline lens. Invest Ophthalmol. 1970 Sep;9(9):681–691. [PubMed] [Google Scholar]
- DAUGHADAY W. H., LARNER J. The renal excretion of inositol in normal and diabetic human beings. J Clin Invest. 1954 Mar;33(3):326–332. doi: 10.1172/JCI102901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., FREINKEL N. The distribution of free mesoinositol in mammalian tissues, including some observations on the lactating rat. Biochem J. 1961 Mar;78:606–610. doi: 10.1042/bj0780606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus P. V., Jr, Clements R. S., Jr, Winegrad A. I. Hypermyoinositolemic polyneuropathy in rats. A possible mechanism for uremic polyneuropathy. J Neurol Sci. 1974 Mar;21(3):237–249. doi: 10.1016/0022-510x(74)90170-1. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Johnson W. J., Lambert E. H., O'Brien P. C. Segmental demyelination secondary to axonal degeneration in uremic neuropathy. Mayo Clin Proc. 1971 Jun;46(6):400–431. [PubMed] [Google Scholar]
- ELIASSON S. G. NERVE CONDUCTION CHANGES IN EXPERIMENTAL DIABETES. J Clin Invest. 1964 Dec;43:2353–2358. doi: 10.1172/JCI105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson S. G. Properties of isolated nerve fibres from alloxanized rats. J Neurol Neurosurg Psychiatry. 1969 Dec;32(6):525–529. doi: 10.1136/jnnp.32.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLIATT R. W., WILLISON R. G. Peripheral nerve conduction in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1962 Feb;25:11–18. doi: 10.1136/jnnp.25.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM D., RICHARDSON P. C., SALMON M. V., URICH H. PATHOLOGICAL OBSERVATIONS ON SIX CASES OF DIABETIC NEUROPATHY. Brain. 1964 Jun;87:201–214. doi: 10.1093/brain/87.2.201. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973 Apr 19;288(16):831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Gregersen G. Diabetic neuropathy: influence of age, sex, metabolic control, and duration of diabetes on motor conduction velocity. Neurology. 1967 Oct;17(10):972–980. doi: 10.1212/wnl.17.10.972. [DOI] [PubMed] [Google Scholar]
- Gregersen G. Variations in motor conduction velocity produced by acute changes of the metabolic state in diabetic patients. Diabetologia. 1968 Nov;4(5):273–277. doi: 10.1007/BF01309900. [DOI] [PubMed] [Google Scholar]
- Hauser G. Energy- and sodium-dependent uptake of inositol by kidney cortex slices. Biochem Biophys Res Commun. 1965 Jun 9;19(6):696–701. doi: 10.1016/0006-291x(65)90313-x. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr, Anderson L. Metabolism of myo-inositol in animals. II. Complete catabolism of myo-inositol-14C by rat kidney slices. Arch Biochem Biophys. 1967 Feb;118(2):332–339. doi: 10.1016/0003-9861(67)90357-8. [DOI] [PubMed] [Google Scholar]
- Kinoshita J. H., Barber G. W., Merola L. O., Tung B. Changes in the levels of free amino acids and myo-inositol in the galactose-exposed lens. Invest Ophthalmol. 1969 Dec;8(6):625–632. [PubMed] [Google Scholar]
- Kusama H., Stewart M. A. Levels of myo-inositol in normal and degenerating peripheral nerve. J Neurochem. 1970 Mar;17(3):317–323. doi: 10.1111/j.1471-4159.1970.tb02218.x. [DOI] [PubMed] [Google Scholar]
- LAWRENCE D. G., LOCKE S. Motor nerve conduction velocity in diabetes. Arch Neurol. 1961 Nov;5:483–489. doi: 10.1001/archneur.1961.00450170021003. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Sutherland D. E., Steffes M. W., Leonard R. J., Najarian J. S., Michael A. F., Brown D. M. Pancreatic islet transplantation. Effects on the glomerular lesions of experimental diabetes in the rat. Diabetes. 1974 Sep;23(9):748–753. doi: 10.2337/diab.23.9.748. [DOI] [PubMed] [Google Scholar]
- Noël P., Lauvaux J. P., Pirart J. Upper limbs diabetic neuropathy: a clinical and electrophysiological study. Horm Metab Res. 1971 Nov;3(6):386–392. doi: 10.1055/s-0028-1094127. [DOI] [PubMed] [Google Scholar]
- Preston G. M. Peripheral neuropathy in the alloxan-diabetic rat. J Physiol. 1967 Apr;189(2):49P–50P. [PMC free article] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Sherman W. R., Stewart M. A., Kurien M. M., Goodwin S. L. The measurement of myo-inositol, myo-inosose-2 and scyllo-inositol in mammalian tissues. Biochim Biophys Acta. 1968 May;158(2):197–205. doi: 10.1016/0304-4165(68)90131-1. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Kurien M. M., Sherman W. R., Cotlier E. V. Inositol changes in nerve and lens of galactose fed rats. J Neurochem. 1968 Sep;15(9):941–946. doi: 10.1111/j.1471-4159.1968.tb11636.x. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Sherman W. R., Kurien M. M., Moonsammy G. I., Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967 Nov;14(11):1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Travis S. F., Morrison A. D., Clements R. S., Jr, Winegrad A. I., Oski F. A. Metabolic alterations in the human erythrocyte produced by increases in glucose concentration. The role of the polyol pathway. J Clin Invest. 1971 Oct;50(10):2104–2112. doi: 10.1172/JCI106704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma S. D., Chakrapani B., Reddy V. N. Intraocular transport of myoinositol. II. Accumulation in the rabbit lens in vitro. Invest Ophthalmol. 1970 Oct;9(10):794–800. [PubMed] [Google Scholar]
- White G. L., Schellhase H. U., Hawthorne J. N. Phosphoinositide metabolism in rat superior cervical ganglion, vagus and phrenic nerve: effects of electrical stimulation and various blocking agents. J Neurochem. 1974 Jan;22(1):149–158. doi: 10.1111/j.1471-4159.1974.tb12191.x. [DOI] [PubMed] [Google Scholar]


