Abstract
Erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, is active in glioblastoma. We evaluated erlotinib efficacy in patients with first-relapse glioblastoma and assessed whether response was related to EGFR amplification and/or concomitant use of enzyme-inducing antiepileptic drugs (EIAEDs) in a phase II open-label study of glioblastoma patients in first relapse. Patients took erlotinib daily until progression. Starting dose was 150 mg for patients not taking EIAEDs and 300 mg for patients taking EIAEDs. Tumors were radiographically assessed every 8 weeks. Response was evaluated by investigators and confirmed by an independent radiology facility (IRF). The primary efficacy outcome was the objective response (OR) rate, according to the modified WHO criteria. Enrollment (n = 48) was terminated after a planned interim analysis due to an insufficient number of responses. The IRF confirmed 1 complete and 2 partial responses (PRs), for an OR rate of 6.3% (95% confidence interval [CI]: 1.7–17.0). Investigators determined 1 complete response and 3 PRs, median response duration of 7.0 months, 6-month progression-free survival (PFS) of 20% (95% CI: 10.0–32.4), and median survival of 9.7 months (95% CI: 5.9–11.6). Outcomes were not related to EGFR amplification or EIAED status. Diarrhea and rash were the most common adverse events (AEs); 23% of patients experienced grade 3–4 drug-related AEs. Despite the limited number of responses, 6-month PFS and median survival reached or exceeded the previously reported values for patients undergoing chemotherapy for recurrent glioblastoma. EGFR amplification was not associated with erlotinib activity. Given the large CIs and nonrandomized nature of the study, results should be interpreted cautiously.
Keywords: EGFR amplification, EGFR inhibition, enzyme-inducing antiepileptic drugs, progression-free survival
Glioblastoma is the most aggressive and fatal primary brain tumor in adults. Standard therapy for newly diagnosed glioblastoma includes surgical resection followed by temozolomide (TZ; Temodal, Temodar; Schering-Plough) and radiotherapy followed by adjuvant TZ.1 Median survival for patients treated with this approach is 15 months, with a 2-year survival rate of 26%. After initial disease progression, response to chemotherapy is uncommon, with 6-month progression-free survival (PFS) of 8%–21% and median survival of 6 months.2–4 Thus, there is an urgent need for an effective treatment for recurrent glioblastoma.
The epidermal growth factor receptor (EGFR) is a transmembrane receptor tyrosine kinase whose activation triggers mechanisms that are involved in cell proliferation and survival. EGFR is dysregulated in a variety of epithelial tumors and is linked to increased tumorigenicity.5 Strategies that disrupt EGFR signal transduction, such as the orally active, reversible small molecule tyrosine kinase inhibitors erlotinib (Tarceva; Genentech, Inc.) and gefitinib (Iressa; AstraZeneca Pharmaceuticals), are being evaluated as antitumor therapies.6
Several molecular characteristics of glioblastoma make it a compelling candidate for anti-EGFR therapy. For instance, EGFR is overexpressed in 40%–90% of glioblastomas, and in nearly half of those cases, overexpression is due to amplification of the EGFR gene.7 EGFR amplification is sometimes associated with mutations that are believed to promote cell proliferation, migration, and invasiveness via the RAS-RAF-MAPK pathway and impair apoptosis via the PI3K/AKT pathway.8 The most common of these mutations is EGFRvIII, a variant in which the extracellular ligand-binding domain has been deleted, resulting in constitutive receptor activation.7,9
Recent studies have investigated the activity of EGFR tyrosine kinase inhibitors in glioblastoma and the relationships between response and EGFR expression, with inconsistent results.10–12 In a phase I study, 8 (19.5%) of 41 patients with advanced gliomas responded to erlotinib, and response was associated with EGFR overexpression.10 On the other hand, EGFR expression was not associated with sensitivity to gefitinib in recurrent glioblastoma,12 and a retrospective analysis of tissue from glioblastoma patients treated with erlotinib or gefitinib indicated that response was independent of EGFR amplification but was associated with coexpression of EGFRvIII and PTEN.11 PTEN is a tumor suppressor gene that is frequently mutated in glioblastoma and leads to disinhibition and constitutive activation of the PI3K/Akt signaling pathway.13
In this multicenter phase II study, we examined efficacy, safety, and tolerability of single-agent erlotinib in patients with first-relapse glioblastoma, independent of tumor EGFR amplification status, and in a subgroup of patients with EGFR-amplified tumors. In an effort to increase our understanding of tumor and patient characteristics that predict therapeutic success with anti-EGFR agents, we also evaluated response in terms of EGFR and EGFRvIII expression and the presence of PTEN. Since erlotinib exposure is influenced by enzyme-inducing agents,14 we also evaluated response in the subgroups of patients who were or were not taking concomitant enzyme-inducing antiepileptic drugs (EIAEDs).
Patients and Methods
Patients
Patients were ≥18 years of age with histologically confirmed, bidimensionally measurable (minimum 1 cm) glioblastoma and radiographic evidence of disease progression, as assessed by the investigator and reviewed by an independent radiology facility (IRF; RadPharm, Inc.), according to the modified WHO criteria15,16 on MRI or CT scan performed ≤14 days prior to the study entry. The slides used for the original diagnosis of glioblastoma were required for central pathologic confirmation of histology. Patients with an original histology of a lower-grade glioma and a subsequent diagnosis of glioblastoma were not excluded but must have met all other eligibility requirements.
Patients were included if they had prior radiotherapy, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, a life expectancy >12 weeks, and archival tissue available for determining EGFR-amplification status and other tumor characteristics. Patients were excluded if they had prior treatment with an anti-EGFR agent, had been treated with more than 1 chemotherapy regimen, had a surgical procedure ≤2 weeks prior to study entry, anticipated a major surgical procedure during the study, or had unstable systemic disease. If receiving corticosteroids, patients must have been on a stable dose for ≥2 weeks prior to MRI at screening. Patients taking EIAEDs continued to do so throughout the study. Radiotherapy, investigational agents other than erlotinib, and chemotherapy were not permitted during the study.
Study Design
We conducted a phase II, open-label, multicenter study of single-agent erlotinib in patients with glioblastoma in first relapse. Appropriate institutional review boards approved the protocol, and all patients provided written informed consent prior to study participation.
The primary goals of the study were to evaluate (i) objective response (OR) to erlotinib, independent of EGFR amplification and in a subgroup of patients with EGFR-amplified tumors, and (ii) safety and tolerability of erlotinib in the study population. Additional objectives included examining the relationship between response and tumor characteristics, including EGFR and EGFRvIII expression and PTEN deletion, and evaluating the impact of concomitant EIAEDs on response rates.
A modified Simon 2-stage study design was used.17 During the first stage, 47 patients who had received at least 1 dose of erlotinib, had at least 1 tumor assessment, and whose EGFR amplification status was known were to be evaluated. The objective of the first stage was to determine if erlotinib had activity independent of EGFR amplification or was active only in patients EGFR-amplified tumors. The outcome of Stage 1 determined whether Stage 2 enrollment would include glioblastoma patients, independent of EGFR-amplification status, or only those patients with EGFR-amplified tumors. If additional patients were to be enrolled independent of EGFR-amplification status, then in Stage 2, an additional 63 patients would be enrolled. If Stage 2 enrollment was to be restricted to patients with EGFR-amplified tumors, enrollment would continue until a total of 41 EGFR-amplified patients were enrolled during Stages 1 and 2.
Treatment
Erlotinib is primarily metabolized by CYP3A4, an important metabolic enzyme; and serum levels of erlotinib are markedly decreased in patients who are taking concomitant drugs that increase CYP3A4 activity (eg, EIAEDs).14 Thus, the starting dose of erlotinib depended upon whether a patient was taking EIAEDs at study entry and was based on preliminary safety reports that the incidence of severe diarrhea and/or cutaneous toxicity was unacceptably high at erlotinib doses exceeding 150 mg/day, but erlotinib in patients taking EIAEDs was well tolerated at doses up to 300 mg/day.
Patients were treated on a continuous, once daily oral dosing schedule of 150 mg (non-EIAED subgroup) or 300 mg (EIAED subgroup) erlotinib, beginning Day 0 (ie, the first day of study drug administration). Dose was escalated in 50 mg increments, beginning 2 weeks after treatment initiation and completion of a toxicity assessment, and was escalated every 2 weeks until the appearance of rash, dose-limiting toxicity, or the maximum allowable dose (200 mg/day for non-EIAED patients and 500 mg/day for EIAED patients) was reached. Patients who experienced a tolerable rash were maintained at their current dose.
Assessments
Screening for study eligibility was performed within 28 days prior to Day 0 and included neurological and physical examinations and laboratory, radiographic (chest x-ray and brain MRI), and pathological assessments. On Day 0, and every 4 weeks thereafter until treatment was discontinued, patients underwent a physical examination and ECOG performance status evaluation, and their laboratory parameters were assessed. Neurological evaluations and radiographic tumor assessments were made every 8 weeks until progression. Erlotinib plasma trough concentrations were measured at 4 and 8 weeks. Reports of adverse events (AEs) were collected every 2 weeks during the first 8 weeks of treatment and every 4 weeks thereafter. Patients were telephoned during treatment week 2 and every 2 weeks thereafter to inquire if rash was present. If a rash was symptomatic or dose limiting, the patient came to the clinic to be assessed, and the rash was photographed. Patients were evaluated for toxicity 30 (±3) days after discontinuing erlotinib and were followed for survival until death or loss to follow-up.
EGFR amplification was determined by fluorescence in situ hybridization and chromogenic in situ hybridization. EGFR, EGFRvIII, and PTEN protein expression was determined using the standard immunohistochemical techniques.
Outcome Measures
The primary outcome measure was OR in all patients and in a subgroup of patients with EGFR-amplified tumors, as determined by the investigator and confirmed by the IRF, according to the modified WHO response criteria. To determine a complete response (CR), a patient could not be taking corticosteroids above the physiologic levels (ie, equivalent to 20 mg/day hydrocortisone). To determine a partial response (PR), patients taking corticosteroids were required to take a stable or lower dose relative to their dose at screening. OR was defined as CRs and PRs that were radiographically confirmed ≥4 weeks after a response was initially determined. In the event of a discrepancy between investigator and IRF assessments, the IRF assessment took precedence.
Secondary outcomes included response duration, defined as the time from the initial CR or PR to disease progression (>50% increase in the sum of bidimensional tumor or new disease) or death, whichever occurred first; PFS, defined as the time from the initiation of erlotinib treatment until disease progression or death, whichever occurred first; 6-month PFS; and survival, defined as the time from the initiation of erlotinib treatment until death. Exploratory analyses were performed to evaluate the relationship between response and tumor characteristics, including EGFR, EGFRvIII, and PTEN expression. Relations between efficacy, EIAED use, and erlotinib exposure were also explored.
PK outcome measures were the steady-state plasma trough concentrations (Cmin) of erlotinib and its major metabolite, OSI-420, at treatment weeks 4 and 8, approximately 24 hours after the previous dose.
Safety outcomes included the rate, nature, and severity of AEs, graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI-CTCAE v3.0),18 and changes in targeted laboratory parameters.
Statistical Methods
All enrolled patients who received erlotinib were included in efficacy and safety analyses. OR was summarized with 95% Blythe Still-Casella19 exact CIs for all patients and for the EGFR-amplified subgroup. Medians and distribution curves for response duration, PFS, and survival were estimated using the Kaplan–Meier method.20 Patients who discontinued the study before undergoing a postscreening tumor response assessment were considered nonresponders in the OR analysis. For patients whose disease had not progressed, response duration and PFS were censored at the last tumor assessment date. For patients who were alive at the time of analysis, survival was censored at the last contact date.
A modified Simon 2-stage design21 was used to determine whether response was dependent on EGFR amplification. Since the likelihood of spontaneous response was small, the background response rate for all patients was assumed to be ≤3%. Response rates of ≥10% in all patients or ≥15% in patients with EGFR-amplified tumors were considered indicative of clinically meaningful erlotinib activity. A sample of 47–110 patients was required to achieve approximately 92% power, with an overall type I error of approximately 5.5%.
Results
Patient Characteristics
Forty-eight patients were enrolled between July and November 2003. Twenty-three patients (47.9%) had EGFR-amplified tumors, and 20 (41.7%) patients were taking EIAEDs (phenytoin, cabamazepine, oxcarbazepine) at study entry (Table 1). Thirty-two (66.7%) patients were taking corticosteroids at study entry. Disease progression was the leading reason (77.1%) for study discontinuation. No patients were lost to follow-up.
Table 1.
Patient baseline characteristics by EGFR amplification status
| Variable | EGFR amplified (n = 23) | Non-EGFR amplified (n = 25) | Total (n = 48) |
|---|---|---|---|
| Age (yrs) | |||
| Mean (SD) | 54 (9.7) | 51 10.5) | 52 (10.1) |
| Median | 53 | 50 | 51 |
| Range | 38–70 | 37–73 | 37–73 |
| Race/ethnicity (n [%]) | |||
| White | 20 (87.0) | 24 (96.0) | 44 (91.2) |
| Other | 3 (13.0) | 1 (4.0) | 4 (8.4) |
| ECOG performance status (n [%]) | |||
| 0 | 4 (17.4) | 7 (28.0) | 11 (22.9) |
| 1 | 19 (82.6) | 18 (72.0) | 37 (77.1) |
| Months since diagnosis | |||
| Mean (SD) | 7.8 (6.2) | 12.9 (27.4) | 10.5 (20.2) |
| Median | 5.0 | 6.7 | 6.3 |
| Range | 3–29 | 3–143 | 3–143 |
| Initial surgery (n [%]) | |||
| Partial resection | 6 (26.1) | 11 (44.0) | 17 (35.4) |
| Complete resection | 11 (47.8) | 8 (32.0) | 19 (39.6) |
| Biopsy | 6 (26.1) | 6 (24.0) | 12 (25.0) |
| Radiotherapy (n [%]) | 23 (100) | 25 (100) | 48 (100) |
| Prior systemic therapy (n [%]) | 19 (82.6) | 16 (64.0) | 35 (72.9) |
| EIAEDs at study entry (n [%]) | 10 (43.5) | 10 (40) | 20 (41.7) |
Abbreviations: EGFR, epidermal growth factor receptor; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; EIAEDs, enzyme-inducing antiepileptic drugs.
Efficacy
At the interim analysis after Stage 1 enrollment (n = 48), the IRF confirmed 1 PR. Therefore, enrollment did not continue in Stage 2. During the remainder of the study, there were a total of 3 IRF-confirmed responses: 1 CR and 1 PR in the EGFR-amplified subgroup (n = 23) and 1 PR in the non-EGFR-amplified subgroup (n = 25), for an OR rate of 6.3% (95% CI: 1.7–17.0). The patient who had an IRF-confirmed CR enrolled with a tumor that did not meet the minimum 1 cm requirement. However, serial scans prior to study enrollment showed the presence of a new tumor along the resection cavity, and the IRF confirmed that this area resolved, consistent with a CR.
There were 4 investigator-assessed responses, 3 of which were confirmed by the IRF and 1 additional PR in the non-EGFR-amplified subgroup, for an investigator-assessed OR rate of 8.3% (95% CI: 2.9–18.9).
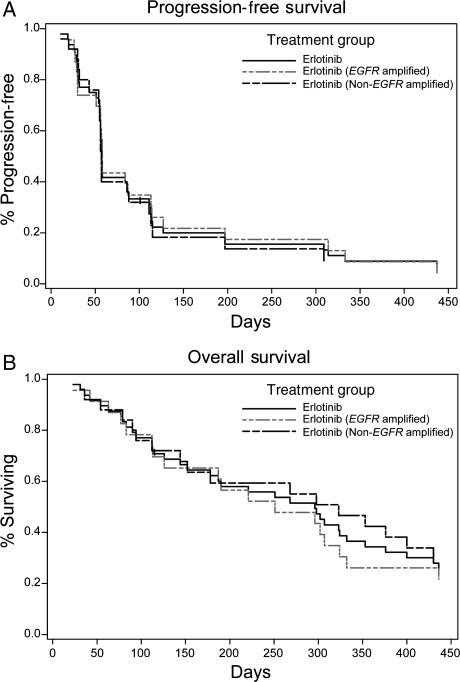
On the basis of investigator assessments, the median response duration was 7.0 months (5.6 months for the 2 EGFR-amplified responders; 9.7 months for the 2 non-EGFR-amplified responders; Table 2). One patient experienced a CR lasting 4.6 months, and 3 patients experienced PRs lasting 6.6, 7.4, and 12.0 months. Ten (43.5%) patients in the EGFR-amplified subgroup and 6 (24.0%) patients in the non-EGFR-amplified subgroup had stable disease (SD). Six-month PFS was similar for the 23 EGFR-amplified (21.7%; 95% CI: 7.9–39.9) and 25 non-EGFR-amplified (18.3%; 95% CI: 6.0–35.9) patients (Fig. 1). Median survival was 8.6 months (95% CI: 4.1–10.7) for EGFR-amplified and 10.6 months (95% CI: 4.7–14.1) for non-EGFR-amplified subgroups (Table 2).
Table 2.
Survival and best investigator-assessed response by EGFR amplification status and EIAED subgroup
| EGFR amplified (n = 23) | Non-EGFR amplified (n = 25) | Total (n = 48) | |
|---|---|---|---|
| Patients evaluated | 23 (100) | 25 (100) | 48 (100) |
| Deaths | 21 (60.9) | 21 (64.0) | 42 (62.5) |
| Patients censored | 2 | 4 | 6 |
| Survival (mos) | |||
| Median | 8.3 | 10.6 | 9.7 |
| 95% CI | 4.1–10.7 | 4.7–14.1 | 5.9–11.6 |
| 25–75 percentile | 3.7, 14.3 | 3.7, 17.8 | 3.7, 16.8 |
| Range | 0.8–24.2a | 1.0–25.6a | 0.8–25.6a |
| Response (n [%]) | |||
| CR | 1 (4.3) | 0 | 1 (2.1) |
| PR | 1 (4.3) | 2 (8.0) | 3 (6.3) |
| SD | 10 (43.5) | 6 (24.0) | 16 (33.3) |
| CR + PR + SD | 12 (52.2) | 8 (32.0) | 20 (41.7) |
| PD | 11 (47.8) | 15 (60.0) | 26 (54.2) |
| Unknown | 0 | 2 (8.0) | 2 (4.2) |
| EIAED (n = 20) | Non-EIAED (n = 28) | ||
| CR | 0 | 1 (3.6) | 1 (2.1) |
| PR | 1 (5.0) | 2 (7.1) | 3 (6.3) |
| SD | 6 (30.0) | 10 (35.7) | 16 (33.3) |
| CR + PR + SD | 7 (35.0) | 13 (46.4) | 20 (41.7) |
| PD | 12 (60.0) | 14 (50.0) | 26 (54.2) |
| Unknown | 1 (5.0) | 1 (3.6) | 2 (4.2) |
Abbreviations: EGFR, epidermal growth factor receptor; CI, confidence interval; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; EIAEDs, enzyme-inducing antiepileptic drugs. Values are n (%) except where otherwise noted. No patients were lost to follow-up. Summary statistics are based on the Kaplan–Meier curves.
aCensored observation.
Fig. 1.
Survival analyses. PFS (A) and overall survival (B) for all randomized patients were analyzed with the Kaplan–Meier methods.
Because of the effects of enzyme-inducing agents on erlotinib exposure and, presumably, antitumor activity, we evaluated response in terms of EIAED use. Response rates were not statistically different for the subgroups of patients who were taking EIAEDs and those who were not taking EIAEDs. There was 1 PR (5.0%) in the EIAED subgroup (n = 20) and 1 CR (3.6%) and 2 PRs (7.1%) in the non-EIAED subgroup (n = 28) (Table 2). Six (30.0%) patients in the EIAED subgroup and 10 (35.7%) patients in the non-EIAED subgroup had SD. Six-month PFS was 25.0% (95% CI: 9.1–44.9) for the EIAED subgroup and 16.1% (95% CI: 5.2–32.3) for the non-EIAED subgroup.
The relationship between response and tumor characteristics, including EGFR expression, EGFRvIII expression, and PTEN deletion, was evaluated (Table 3). However, due to the small number of responses and limited availability of tissue, no meaningful conclusions could be drawn from the molecular subgroup analyses.
Table 3.
Response by tumor characteristics
| Outcome | EGFR (FISH) |
EGFR (IHC) |
EGFRvIII |
PTEN |
||||
|---|---|---|---|---|---|---|---|---|
| Positive (n = 23) | Negative/unknown (n = 25) | Positive (n = 15) | Negative (n = 20) | Positive (n = 24) | Negative (n = 19) | Wild type (n = 23) | Deletion (n = 14) | |
| CR + PR | 8.7 | 8.0 | 13.3 | 5.0 | 8.3 | 5.3 | 13.0 | 0 |
| CR + PR + SD | 52.2 | 32.0 | 60.0 | 30.0 | 37.5 | 42.1 | 39.1 | 50.0 |
| 6-Month PFS | 21.7 | 18.3 | 26.7 | 10.0 | 20.8 | 15.8 | 13.0 | 21.4 |
Abbreviations: EGFR, epidermal growth factor receptor; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; CR, complete response; PR, partial response; SD, stable disease; PFS, progression-free survival. Values are the percentage of evaluated patients. P > .05 for all comparisons.
Pharmacokinetics
Erlotinib exposure was assessed in 28 patients (non-EIAED, n = 15; EIAED, n = 13) with samples taken during study weeks 4 and 8 and included patients who remained on a low dose (150 mg for non-EIAED patients and 300–350 mg for EIAED patients) and those who had successfully escalated their dose (200 mg for non-EIAED patients, up to 400–500 mg for EAID patients; Table 4). Minimum clearance (Cmin) for the EIAED subgroup (413.2 ± 368.2 ng/mL) was approximately 66.0% lower than that of the non-EIAED subgroup (1092.6 ± 630.9 ng/mL). Dose escalations to 450–500 mg/day did not adequately compensate for increased drug clearance in the EIAED subgroup, and patients taking EIAEDs had less exposure to the study drug than those who were not taking EIAEDs. The effect of corticosteroids on erlotinib concentration was not evaluated.22
Table 4.
Erlotinib dose by EIAED group
| EIAED (n = 20) | Non-EIAED (n = 28) | Total (n = 48) | |
|---|---|---|---|
| Total dose (g) | |||
| Mean (SD) | 31.0 (34.4) | 15.8 (20.2) | 22.1 (27.8) |
| Median | 19.0 | 10.4 | 13.2 |
| Range | 3.0–147.0 | 2.9–107.1 | 2.90–147.0 |
| Daily dose (mg) | |||
| Mean (SD) | 337.4 (59.8) | 168.7 (19.2) | 239.0 (93.4) |
| Median | 300.0 | 169.7 | 188.0 |
| Range | 300–477 | 125–198 | 125–477 |
| Days on study drug | |||
| Mean (SD) | 82.5 (70.7) | 91.0 (103.0) | 87.4 (90.2) |
| Median | 57.5 | 56.5 | 57.0 |
| Range | 10–308 | 18–540 | 10–540 |
| Highest dose (mg; n [%]) | |||
| 500 | 3 (15.0) | n/a | 3 (6.3) |
| 450 | 2 (10.0) | n/a | 2 (4.2) |
| 400 | 1 (5.0) | n/a | 1 (2.1) |
| 350 | 2 (10.0) | n/a | 2 (4.2) |
| 300 | 12 (60.0) | n/a | 12 (25.0) |
| 200 | n/a | 18 (64.3) | 18 (37.5) |
| 150 | n/a | 10 (35.7) | 10 (20.8) |
Abbreviations: EIAED, enzyme-inducing antiepileptic drug; SD, standard deviation; n/a, not analyzed.
Safety and Tolerability
Forty-six (95.8%) patients experienced at least 1 AE that was potentially related to erlotinib. Fifteen (31.3%) patients were reported to have experienced NCI-CTCAE grade 3, 4, or 5 AEs that were potentially related to the study drug. The most common study drug-related AEs included rash (85.4%), diarrhea (62.5%), fatigue (27.1%), dry skin, (27.1%), headache (12.5%), exfoliative dermatitis (10.4%), and nausea (10.4%) (Table 5). The incidence of serious (grade 3 or 4) diarrhea and rash were 4.0% and 18.8%, respectively. No cases of suspected drug-related interstitial lung disease were reported.
Table 5.
Incidence of potentially drug-related adverse events occurring in ≥10% of patients by EIAED subgroup according to MedDRA system organ class and preferred terminology
| Event (n [%]) | EIAED (n = 20) | Non-EIAED (n = 28) | Total (n = 48) |
|---|---|---|---|
| Gastrointestinal disorders | |||
| Diarrhea | 10 (50.0) | 20 (71.4) | 30 (62.5) |
| Nausea | 0 | 5 (17.9) | 5 (10.4) |
| Fatigue | 7 (35.0) | 6 (21.4) | 13 (27.1) |
| Headache | 1 (5.0) | 5 (17.9) | 6 (12.5) |
| Skin/subcutaneous tissue disorders | 19 (95.0) | 24 (85.7) | 43 (89.6) |
Abbreviations: EIAED, enzyme-inducing antiepileptic drug; SD, standard deviation.
The incidence of serious (grade 3 or 4) AEs (SAEs) was comparable in the EIAED (25%) and non-EIAED subgroups (28.6%). Four SAEs reported by 3 patients (gastrointestinal bleeding, increase in international normalized ratio, sepsis, hematemesis) were considered by the investigator to be related to erlotinib treatment. One SAE (sepsis) led to discontinuation of erlotinib, and 2 (increased international normalized ratio, hematemesis) led to temporary interruption of erlotinib.
Forty-two of the 48 (87.5%) participating patients had died as of database lock (February 16, 2006). Thirty deaths (70.0%) were attributed by the investigator to progressive glioblastoma. Five deaths were due to cardiac arrest, sepsis, pulmonary embolism, pneumonia, and respiratory failure. One death was attributed to an SAE (sepsis) that was assessed by the investigator to be erlotinib related.
Discussion
This phase II, open-label trial evaluated erlotinib efficacy in unselected patients with recurrent glioblastoma. The IRF-confirmed OR rate was 6.3%, and the best investigator-assessed outcomes included an OR rate of 8.3%, with a median response duration of 7.0 months; 20% 6-month PFS; and median survival of 9.7 months. Thirty-three percent of the patients had SD as their best response.
Our results are consistent with previous trials of erlotinib in glioblastoma and add to a growing body of evidence that erlotinib, alone or in combination with cytotoxic agents, has limited activity in unselected patients with glioblastoma (Table 6). SD is the most frequently reported response, occurring in 13%–50% of treated patients, suggesting that erlotinib has cytostatic activity in glioblastoma. Other reported outcomes vary across studies. Raizer et al.23 and Vogelbaum et al.24 evaluated efficacy of single-agent erlotinib in phase II trials of patients with recurrent glioblastoma who were not taking EIAEDs. The best response observed by Raizer et al. was SD in 4 of 30 (13.3%) patients, whereas Vogelbaum et al. reported a 50% response rate (4 PR and 4 SD) in an interim analysis of 16 patients. In a phase I trial of erlotinib alone or in combination with TZ in patients with malignant gliomas,14 8 of 57 (14%) patients had a PR. Most recently, van den Bent et al.25 reported the results of a phase II, randomized trial of erlotinib versus TZ or carmustine in patients with recurrent glioblastoma.25 The best response observed for 54 patients who were treated with erlotinib (9 of whom were taking EIAEDs) was PR in 2 (3.7%) patients and SD in 9 (16.7%) patients. Six-month PFS was 11.4%, median PFS was 1.8 months, and OS was 7.7 months. Six-month PFS was greater in the TZ/carmustine group (24.1%), whereas median PFS, median OS, 6-month OS, and 1-year OS were similar across treatment groups. In another recent phase II study, de Groot et al.26 evaluated erlotinib in combination with carboplatin in 43 patients with recurrent glioblastoma who were not taking EIAEDs. A PR was observed in 1 (2.3%) patient, and 20 (47%) patients had SD. Median PFS was 2.1 months, 6-month PFS was 14%, and median survival was 6.9 months. As carboplatin and TZ have been shown to have activity in glioblastoma,4,27 it is notable that survival values were superior when erlotinib was administered as a single-agent compared with when it was combined with those cytotoxic agents. This suggests that concomitant chemotherapy may have an antagonistic effect on erlotinib.26 On the other hand, Sathornsumetee et al.28 reported 24% 6-month PFS and a radiographic response in 12 of 25 (48%) patients with recurrent glioblastoma who were treated with erlotinib in combination with bevacizumab (Avastin; Genentech, Inc.), a monoclonal antibody against the vascular endothelial growth factor receptor.
Table 6.
Efficacy outcomes of erlotinib/gefitinib treatment in recurrent glioblastoma
| Authors | Patients (n) | Erlotinib (gefitinib; mg/d) | Response (% C/P/S) | 6-m PFS (%) | Median PFS (mos) | Median OS (mos) |
|---|---|---|---|---|---|---|
| Raizer et al.23 | Recurrent GBM (30) | 150 | −/−/13 | – | 2.8 | – |
| Vogelbaum et al.24 | Progressive/recurrent GBM (16) | 150 | –/25/25 | – | – | – |
| Van den Bent et al.25 | Progressive GBM (54) | 150–500a | 0/4/17 | 11 | 1.8 | 7.7 |
| de Groot et al.26 | Progressive GBM (43) | 150–200 (plus carboplatin every 4 weeks) | 0/2/47 | 14 | 2.1 | 6.9 |
| Sathornsumetee et al.28 | Recurrent GBM (25) | 200–650a (plus bevacizumab every 2 weeks) | 48% (unspecified) | 24 | – | – |
| Rich et al.12 | Recurrent GBM (53) | 500–1000a | 0/0/42 | 13 | 1.9b | 9.1 |
| Lieberman et al.29 | Recurrent GBM (38) | 500–1500a | 0/5/– | 9 | – | – |
| Yung et al. | Recurrent GBM (48) | 150–300a | 2c/6/33 | 20 | – | 9.7 |
Abbreviations: C, complete; P, partial, S, stable; PFS, progression-free survival; OS, overall survival; GBM, glioblastoma; dash (–) indicates outcome was not reported.
aIncludes patients taking enzyme-inducing antiepileptic drugs (EIAEDS).
bFree of progression or event requiring removal from the protocol.
cIndependent radiology facility assessed.
Previous studies of gefitinib activity in glioblastoma12,29,30 have reported response rates similar to those reported in erlotinib studies, and event-/progression-free and overall survival values have not exceeded those of historical controls. It is notable that despite the small number of responses observed in the present study, 6-month PFS and median survival values reached or exceeded historical values for patients undergoing chemotherapy for recurrent glioblastoma.2
Although erlotinib and gefitinib appear to have some activity in patients with recurrent glioblastoma, neither the present study nor any of the previous, cited studies included a control group; thus, any benefits attributable to anti-EGFR therapy cannot be unequivocally determined. It may be that the responses observed in the present study were due to patient characteristics, including the degree of disease progression at enrollment, performance status, and previous treatment. Thus, it will be important to determine the patient and/or tumor characteristics that are associated with response to EGFR inhibitors. To that end, de Groot et al.26 determined that KPS, extent of resection, and the number of prior regimens were associated with response to erlotinib; and Rich et al.12 reported that the presence of diarrhea during gefitinib therapy was a positive predictor for overall survival, whereas the development of skin toxicity during therapy and extent of resection were associated with event-free survival. Van den Bent et al.25 also reported that skin toxicity of grade 2 or greater was associated with prolonged PFS and OS. In the present study, survival might have been biased due to next-line treatment following progression; however, we are unable to address that possibility, as information on subsequent treatment was not collected.
Pseudoprogression is a concern for all clinical trials conducted in the setting of recurrent disease and may have influenced OR rates in the present study. There are currently no accepted criteria to absolutely rule in or rule out pseudoprogression. Notably, all on-study scans were independently reviewed, and investigator-evaluated responses were confirmed by the independent facility.
Response was not associated with EGFR amplification in this study. This is consistent with previous reports10,31 and indicates that EGFR amplification is neither sufficient nor necessary for erlotinib activity in glioblastoma. It is likely that EGFR amplification and other EGFR and/or tumor characteristics interact to sensitize tumors with anti-EGFR therapies. For instance, in one study of patients with recurrent glioblastoma,11 those with tumors that coexpressed EGFRvIII and wild-type PTEN were the most likely to respond to EGFR inhibitors; and the activation of the PKB/AKT pathway was associated with resistance to erlotinib.10 Neither did EGFR expression appear to be necessary or sufficient for response to erlotinib. For instance, we observed cases in which immunohistochemically EGFR-negative patients demonstrated a response or disease stabilization. This finding is consistent with other studies and emphasizes that there are other, unidentified factors or compensatory signals that affect response to single-agent erlotinib.
Molecular characteristics have been associated with sensitivity to anti-EGFR therapies in other tumor types. For example, mutations in exons 19–21 of the EGFR tyrosine kinase domain are associated with response to EGFR inhibitors in non-small-cell lung cancer (NSCLC).32,33 Molecular analyses have failed to reveal the mutation in glioblastoma,31,34 which may account for the decreased sensitivity to EGFR inhibitors in glioblastoma. Mutations in the RAS oncogene increase RAS signaling and tumorigenicity and are associated with resistance to anti-EGFR therapies in NSCLC and colorectal cancer.32,35–37 Higher levels of activated RAS have been observed in glioblastoma specimens compared with normal brain tissue or low-grade gliomas.8 Thus, RAS mutations may confer resistance to anti-EGFR therapies in glioblastoma. In the present study, the expression of EGFR, EGFRvIII, and PTEN protein alone was not associated with response. Our ability to draw meaningful conclusions from histological analyses was restricted by the small number of responses and limited availability of tissue.
PK analysis demonstrated that patients who were taking EIAEDs had limited exposure to erlotinib, with trough concentrations one-third the level of non-EIAED patients and what was anticipated based on historical controls. Although the protocol mandated that dose escalation was to continue until the development of intolerable rash or dose-limiting toxicity, dose escalation was accomplished in only a small minority of patients in the EIAED subgroup. The slow escalation schedule may have accounted for tumor progression prior to the development of skin toxicity and, hence, the failure to achieve high plasma drug levels. The potential confounder of inadequate dosing could be eliminated from future studies by including only patients who do not require EIAEDs. This is feasible now that antiseizure medication that does not affect CYP3A4, an important metabolic enzyme, is available.
The nature and incidence of AEs were consistent with the previous evaluations of erlotinib38–40 and other anti-EGFR agents.41 Rash and diarrhea were the most commonly reported AEs and likely resulted from anti-EGFR activity in normal EGFR-expressing tissue. No new drug-related safety issues were identified.
In summary, single-agent erlotinib had limited activity in recurrent glioblastoma. However, despite the limited number of responses observed and the compromised levels of erlotinib exposure in the EIAED subgroup, 6-month PFS and median survival in first-relapse glioblastoma patients treated with erlotinib reached or exceeded historical PFS and survival values for patients undergoing chemotherapy for recurrent glioblastoma.2–4 The challenge for future studies is to identify clinical and disease characteristics that sensitize glioblastoma tumors to anti-EGFR therapies and select patients who would derive the greatest benefit from erlotinib alone or in combination with other therapies.
Funding
This study was financed by the Accelerate Brain Cancer Cure, a 501(c)3 tax-exempt nonprofit organization (T.F.C., J.J.V., M.D.P., W.K.A.Y.) and a grant from Genentech, Inc., South San Francisco, California.
Acknowledgments
We acknowledge Bert Lum and Mubashira Malik for PK analysis and Fong Clow and Linda Gau for statistical analyses. Genentech, Inc. and OSI Pharmaceuticals, Inc. provided support for the clinical trial and manuscript preparation.
Conflict of interest statement. W.K.A.Y. has advisory relationship with Genentech. M.R.G. was DSMC for a Genentech supported clinical trial. W.K.A.Y., M.R.G., and T.F.C. have received honorarium from Genentech. B.K. is an employee of Genentech, Inc., obtains salary from Genentech, and owns stock in Roche.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neurooncology. 2006;8:189–193. doi: 10.1215/15228517-2005-010. doi:10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 4.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. doi:10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arteaga CL. Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist. 2002;7(suppl):31–39. doi: 10.1634/theoncologist.7-suppl_4-31. [DOI] [PubMed] [Google Scholar]
- 6.Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. doi: 10.1200/JCO.2003.01.504. doi:10.1200/JCO.2003.01.504. [DOI] [PubMed] [Google Scholar]
- 7.Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- 8.Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–1453. doi: 10.1097/00006123-199912000-00034. doi:10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 10.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 11.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. doi:10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 12.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. doi:10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 13.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. doi:10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prados MD, Lamborn KR, Chang S, et al. Phase 1 study of erlotinib HCl alone and combined with temozolomide in patients with stable or recurrent malignant glioma. Neurooncology. 2006;8:67–78. doi: 10.1215/S1522851705000451. doi:10.1215/S1522851705000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 16.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. doi:10.1002/1097-0142(19810101)47:1<207::AID-CNCR2820470134>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Thall PF, Simon R, Ellenberg SS, Shrager R. Optimal two-stage designs for clinical trials with binary response. Stat Med. 1988;7:571–579. doi: 10.1002/sim.4780070504. doi:10.1002/sim.4780070504. [DOI] [PubMed] [Google Scholar]
- 18.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. doi:10.1002/sim.4780070504. [DOI] [PubMed] [Google Scholar]
- 19.Cassella G. Refining binomial confidence intervals. Can J Stat. 1986;14:113–129. doi:10.2307/3314658. [Google Scholar]
- 20.Meier P, Kaplan E. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Simon R. Optimal two-stage designs for Phase II clinical trials. Control Clin Trials. 1988;10:1–10. doi: 10.1016/0197-2456(89)90015-9. doi:10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 22.Lu JF, Eppler SM, Wolf J, et al. Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther. 2006;80:136–145. doi: 10.1016/j.clpt.2006.04.007. doi:10.1016/j.clpt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Raizer JJ, Abrey LE, Wen P, et al. A phase II trial of erlotinib (OSI-774) in patients (pts) with recurrent malignant gliomas (MG) not on EIAEDs. J Clin Oncol. 2004;145:1502. [Google Scholar]
- 24.Vogelbaum MA, Peereboom D, Stevens G, Barnett G, Brewer C. Phase II trial of the EGFR tyrosine kinase inhibitor erlotinib for single agent therapy of recurrent glioblastoma multiforme: interim results. J Clin Oncol. 2004;22:1558. [Google Scholar]
- 25.van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. doi:10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Groot JF, Gilbert MR, Aldape K, et al. Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol. 2008;90:89–97. doi: 10.1007/s11060-008-9637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prados MD, Warnick RE, Mack EE, et al. Intravenous carboplatin for recurrent gliomas. A dose-escalating phase II trial. Am J Clin Oncol. 1996;19:609–612. doi: 10.1097/00000421-199612000-00016. doi:10.1097/00000421-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Sathornsumetee S, Vredenburgh JJ, Rich JN, et al. Phase II study of bevacizumab and erlotinib in patients with recurrent glioblastoma multiforme [abstract] J Clin Oncol. 2008;26:13008. [Google Scholar]
- 29.Lieberman FS, Cloughesy T, Fine HA, et al. NABTC phase I/II trial of ZD-1839 for recurrent malignant gliomas and unresectable meningiomas [abstract] J Clin Oncol. 2004;22:1510. [Google Scholar]
- 30.Uhm JH, Ballman KV, Giannini C, et al. Phase II study of ZD1839 in patients with newly diagnosed grade 4 astrocytoma [abstract] J Clin Oncol. 2004;22:1505. [Google Scholar]
- 31.Lassman AB, Rossi MR, Raizer JJ, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American Brain Tumor Consortium Trials 01-03 and 00-01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. doi:10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 32.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small- cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. doi:10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 33.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 34.Marie Y, Carpentier AF, Omuro AM, et al. EGFR tyrosine kinase domain mutations in human gliomas. Neurology. 2005;64:1444–1445. doi: 10.1212/01.WNL.0000158654.07080.B0. [DOI] [PubMed] [Google Scholar]
- 35.Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–1169. doi: 10.1038/sj.bjc.6603685. doi:10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. doi:10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 37.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. doi:10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 38.Gordon AN, Finkler N, Edwards RP, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15:785–792. doi: 10.1111/j.1525-1438.2005.00137.x. doi:10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 39.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. doi:10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 40.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. doi:10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 41.Baselga J, Albanell J, Ruiz A, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. doi:10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]