Abstract
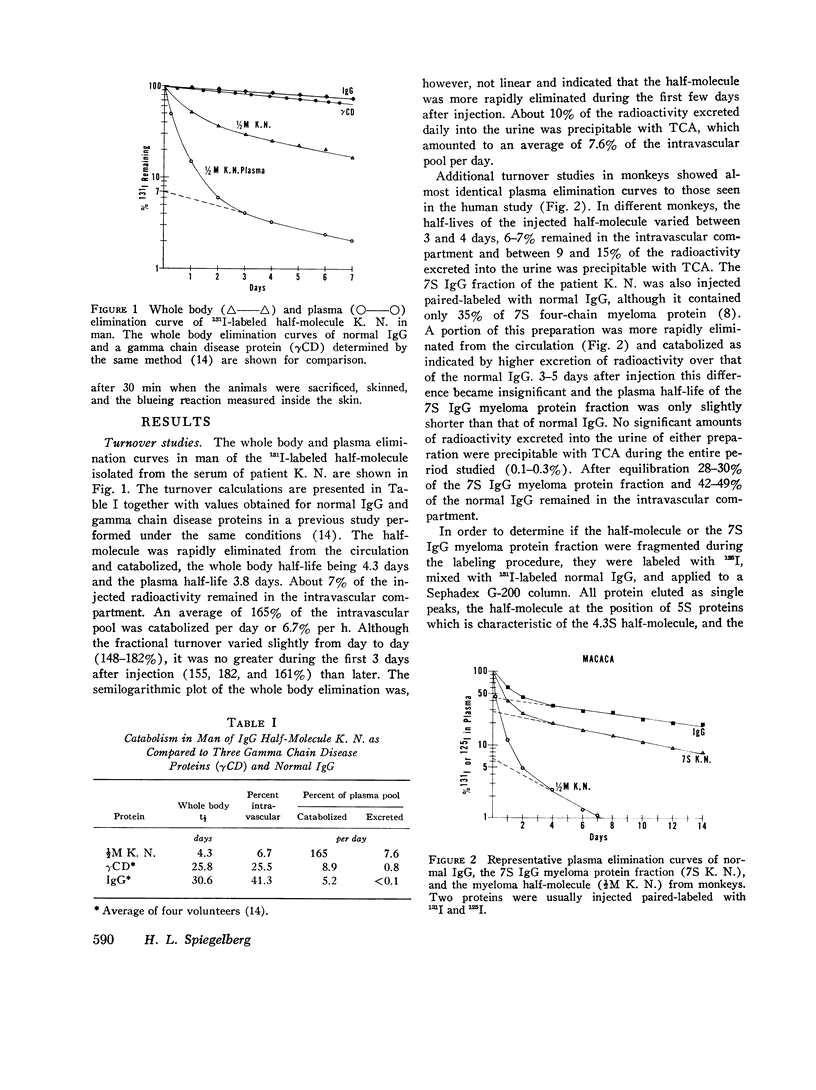
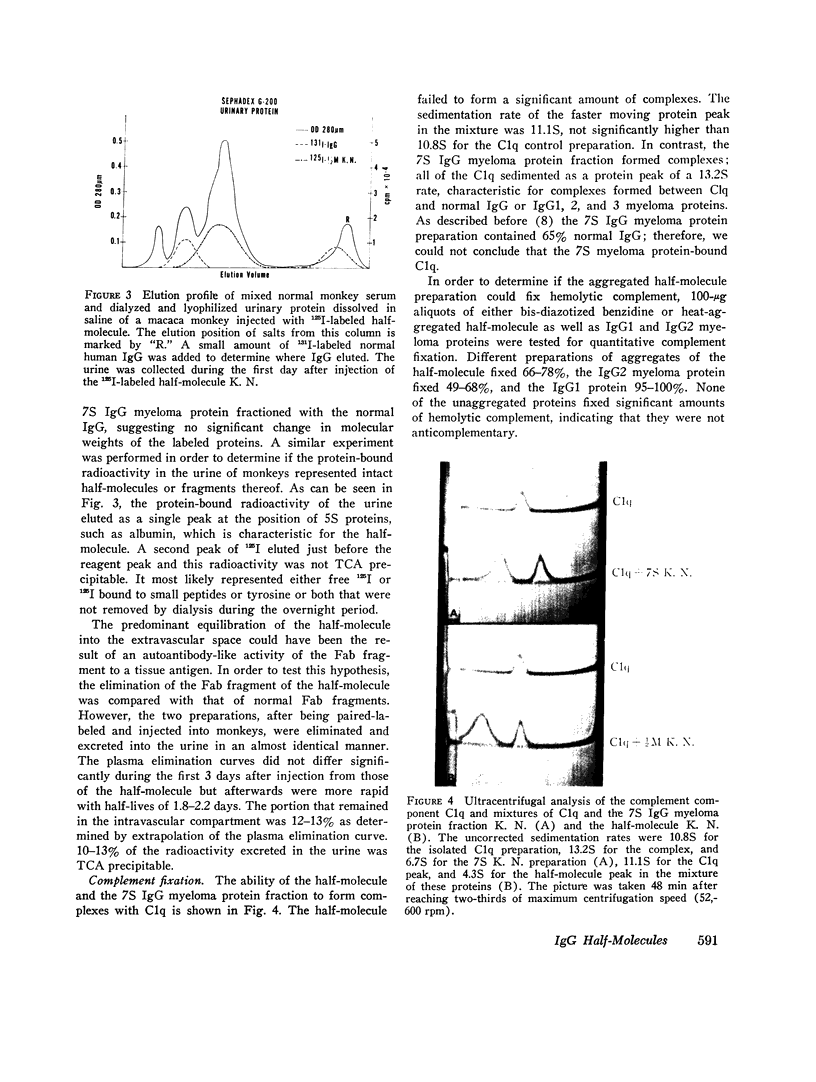
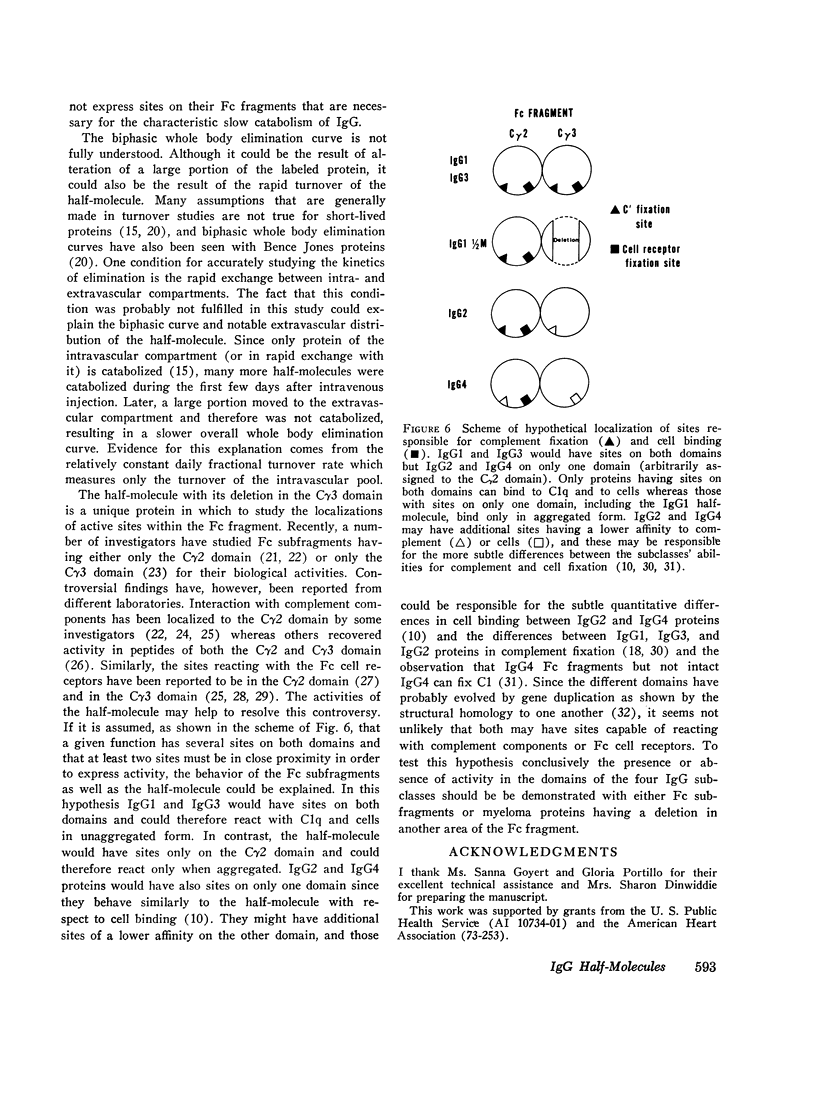
A human IgG1 myeloma protein that has a delection in the third constant domain of the heavy chain (Cgamma3) and forms two-chain half-molecules was studied for its in vivo turnover and its ability to fix C1q and hemolytic complement, to bind to human lymphocytes, neutrophils, and monocytes, and to induce a passive cutaneous reaction in guinea pigs. In both man and monkeys, the half-molecule was rapidly catabolized and in part excreted into the urine. The half-life in man was 4.3 days and the fractional turnover 165% per day; 7.6% of the intravascular pool was excreted into the urine per day. Although the 7S four-chain myeloma protein could not be obtained in a pure form, the elimination from the serum of a partially purified preparation suggested that it was also rapidly catabolized. The unaggregated half-molecule neither formed complexes with C1q, cound to human lymphocytes, neutrophils, and monocytes, nor elicited a reverse passive cutaneous reaction in guinea pigs. In contrast, the aggregated half-molecule fixed hemolytic complement and bound to the human white cells similarly to an intact IgG1 myeloma protein. In order to explain the biological activities of this half-moleculr, it is postulated that IgG1 may have several (at least two) submolecular sites for a given biological activity that are localized on both the Cgamma2 and Cgamma3 domains. Proteins having both sites would be capable of binding to C1q and Fc cell receptors in unaggregated in order to obtain half-molecule, must be aggregated in order to obtain this binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R., Isliker H. Studies on the complement-binding site of rabbit immunoglobulin G-11. The reaction of rabbit IgG and its fragments with Clq. Immunochemistry. 1974 May;11(5):243–248. doi: 10.1016/0019-2791(74)90202-x. [DOI] [PubMed] [Google Scholar]
- Bennich H., Natvig J. B., Turner M. W. The C gamma 3 homology region in human IgG subclasses and allotypes. I. Amino acid composition and end-group analysis of pFc' fragments. Scand J Immunol. 1974;3(1):107–115. doi: 10.1111/j.1365-3083.1974.tb01238.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ein D., Waldmann T. A. Metabolic studies of a heavy chain disease protein. J Immunol. 1969 Aug;103(2):345–348. [PubMed] [Google Scholar]
- Ellerson J. R., Yasmeen D., Painter R. H., Dorrington K. J. A fragment corresponding to the C(H)2 region of immunoglobulin G (IgG) with complement fixing activity. FEBS Lett. 1972 Aug 15;24(3):318–322. doi: 10.1016/0014-5793(72)80381-8. [DOI] [PubMed] [Google Scholar]
- FRANEK F., RIHA I. PURIFICATION AND STRUCTURAL CHARACTERIZATION OF 5S GAMMA-GLOBULIN IN NEW-BORN PIGS. Immunochemistry. 1964 Apr;1:49–63. doi: 10.1016/0019-2791(64)90056-4. [DOI] [PubMed] [Google Scholar]
- Froland S. S., Michaelsen T. E., Wisloff F., Natvig J. B. Specificity of receptors for IgG on human lymphocyte-like cells. Scand J Immunol. 1974;3(4):509–517. doi: 10.1111/j.1365-3083.1974.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Hobbs J. R. Immunocytoma o' mice an' men. Br Med J. 1971 Apr 10;2(5753):67–72. doi: 10.1136/bmj.2.5753.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs J. R., Jacobs A. A half-molecule GK plasmacytoma. Clin Exp Immunol. 1969 Jul;5(1):199–207. [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Kehoe J. M., Fougereau M. Immunoglobulin peptide with complement fixing activity. Nature. 1969 Dec 20;224(5225):1212–1213. doi: 10.1038/2241212a0. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R., Mushinski J. F., Potter M. 2-chain immunoglobulin A molecules: abnormal or normal intermediates in synthesis. Science. 1968 Mar 22;159(3821):1355–1357. doi: 10.1126/science.159.3821.1355. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Connell G. E., Gotch F. M. Effector activating determinants on IgG. II. Differentiation of the combining sites for C1q from those for cytotoxic K cells and neutrophils by plasmin digestion of rabbits IgG. Immunology. 1974 Feb;26(2):303–310. [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Minta J. O., Painter R. H. A re-examination of the ability of pFc' and Fc' to participate in passive cutaneous anaphylaxis. Immunochemistry. 1972 Nov;9(11):1041–1048. doi: 10.1016/0019-2791(72)90073-0. [DOI] [PubMed] [Google Scholar]
- Okafor G. O., Turner M. W., Hay F. C. Localisation of monocyte binding site of human immunoglobulin G. Nature. 1974 Mar 15;248(445):228–230. doi: 10.1038/248228a0. [DOI] [PubMed] [Google Scholar]
- Prokesová L., Rejnek J. Molecular heterogeneity of newborn piglet IgG. Immunochemistry. 1973 Sep;10(9):607–609. doi: 10.1016/0019-2791(73)90162-6. [DOI] [PubMed] [Google Scholar]
- SOLOMON A., WALDMANN T. A., FAHEY J. L., MCFARLANE A. S. METABOLISM OF BENCE JONES PROTEINS. J Clin Invest. 1964 Jan;43:103–117. doi: 10.1172/JCI104884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Fishkin B. G., Grey H. M. Catabolism of human gammaG-immunoglobulins of different heavy chain subclasses. I. Catabolism of gammaG-myeloma proteins in man. J Clin Invest. 1968 Oct;47(10):2323–2330. doi: 10.1172/JCI105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Fishkin B. G. The catabolism of human G immunoglobulins of different heavy chain subclasses. 3. The catabolism of heavy chain disease proteins and of Fc fragments of myeloma proteins. Clin Exp Immunol. 1972 Apr;10(4):599–607. [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Heath V. C., Lang J. E. Human myeloma IgG half-molecules. Structural and antigenic analyses,. Biochemistry. 1975 May 20;14(10):2157–2163. doi: 10.1021/bi00681a018. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Heath V. C., Lang J. E. IgG half-molecules: clinical and immunologic features in a patient with plasma cell leukemia. Blood. 1975 Mar;45(3):305–313. [PubMed] [Google Scholar]
- Terry W. D. Skin-sensitizing activity related to gamma- polypeptide chain characteristics of human IgG. J Immunol. 1965 Dec;95(6):1041–1047. [PubMed] [Google Scholar]
- Unanue E. R. Antigen-binding cells. I. Their idenification and role in the immune response. J Immunol. 1971 Oct;107(4):1168–1174. [PubMed] [Google Scholar]
- Waldmann T. A., Strober W. Metabolism of immunoglobulins. Prog Allergy. 1969;13:1–110. doi: 10.1159/000385919. [DOI] [PubMed] [Google Scholar]