Abstract
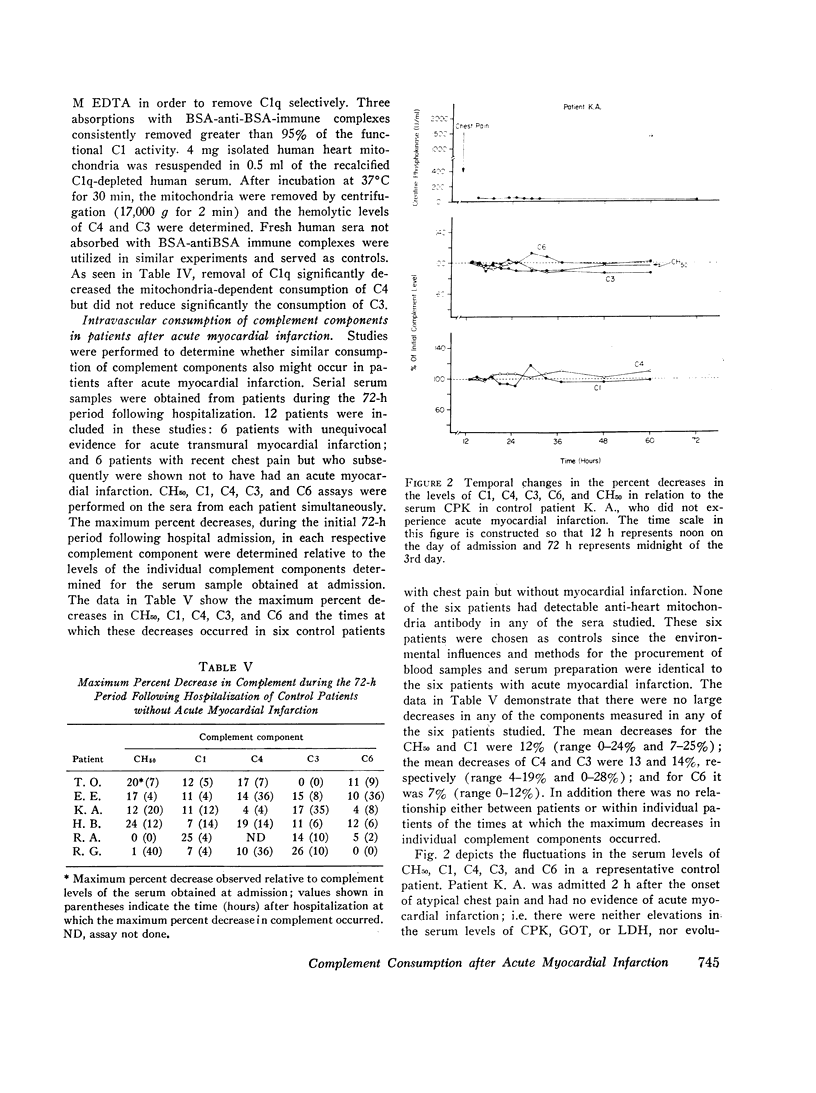
Experiments were conducted to characterize the antibody-independent activation of complement in human serum by isolated human heart mitochondrial membranes in vitro and to determine whether similar patterns of complement consumption occurred in patients after acute myocardial infarction. Direct evidence for the interaction of C1 and heart mitochondrial membranes was obtained by mitochondria-C1 binding and elution experiments. Exposure of normal human sera to isolated human heart mitochondria at 37 degrees C resulted in the consumption of C1, C4, C2, and C3 without significant consumption of the terminal components of the complement system (C6 through C9). The consumption occurred in the absence of detectable anti-heart mitochondria autoantibody, was demonstrated to be calcium dependent, and was inhibited by either 0.01 M EDTA or ethylene glycol bis(bets-aminoethyl ether) N,N,N',N',-tetraacetic acid (EDTA). Although specific absorption of C1q from human sera inhibited the mitochondria-dependent activation of C4, C3 donsumption was not affected. These data indicate that the consumption of C4 and C2 likely occurred due to the mitochondrial membrane-mediated activation of C1, but that the consumption of the C3 did not necessarily involve either the classical or alternative complement pathways. After the in vitro characterization of the mitochondria-dependent activation of the complement system, additional studies were performed to determine whether similar consumption occurred in patients after acute myocaridal infarction. During a 72-h period after hospital admission significant decreases in C1, C4, and C3 occurred in six patients with recent chest pain but no evidence of acute myocardial infarction. These studies suggest that myocardial cell necrosis results in the release of subcellular membrane constituents capable of activating the complement system in the absence of detectable anti-heart autoantibodies; such activation may be responsible in part for the development of acute inflammation and evolution of the infarct size following coronary artery occulusion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLTAX A. J., FISCHEL E. E. Serologic tests for inflammation; serum complement, c-reactive protein and erythrocyte sedimentation rate in myocardial infarction. Am J Med. 1956 Mar;20(3):418–427. doi: 10.1016/0002-9343(56)90127-9. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Immune hemolysis: a simplified method for the preparation of EAC'4 with guinea pig or with human complement. J Immunol. 1967 Aug;99(2):263–268. [PubMed] [Google Scholar]
- Braunwald E., Maroko P. R. The reduction of infarct size--an idea whose time (for testing) has come. Circulation. 1974 Aug;50(2):206–209. doi: 10.1161/01.cir.50.2.206. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Dalmasso A. P., Müller-Eberhard H. J. Hemolytic activity of lipoprotein-depleted serum and the effect of certain anions on complement. J Immunol. 1966 Nov;97(5):680–685. [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. C3 leukotactic factors produced by a tissue protease. J Exp Med. 1969 Sep 1;130(3):505–518. doi: 10.1084/jem.130.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. H., Ward P. A. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971 Apr 1;133(4):885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. G., Kindmark C. O., Trell E. Y., Wollheim F. A. Sequential changes of plasma proteins after myocardial infarction. Scand J Clin Lab Invest Suppl. 1972;124:117–126. doi: 10.3109/00365517209102759. [DOI] [PubMed] [Google Scholar]
- Kelley R. E., Olson M. S., Pinckard R. N. Characterization of anti-heart mitochondria autoantibodies produced in dogs following myocardial infarction. Circ Res. 1974 Dec;35(6):862–870. doi: 10.1161/01.res.35.6.862. [DOI] [PubMed] [Google Scholar]
- Libby P., Maroko P. R., Bloor C. M., Sobel B. E., Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest. 1973 Mar;52(3):599–607. doi: 10.1172/JCI107221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroko P. R., Braunwald E. Modification of myocardial infarction size after coronary occlusion. Ann Intern Med. 1973 Nov;79(5):720–733. doi: 10.7326/0003-4819-79-5-720. [DOI] [PubMed] [Google Scholar]
- Pinckard R. N., Olson M. S., Kelley R. E., DeHeer D. H., Palmer J. D., O'Rourke R. A., Goldfein S. Antibody-independent activation of human C1 after interaction with heart subcellular membranes. J Immunol. 1973 May;110(5):1376–1382. [PubMed] [Google Scholar]
- Pinckard R. N., Olson M. S., O'Rourke R. A., Palmer J. D., Kelley R. E., Goldfein S. Development of complement-fixing 19S, anti-heart mitochondria autoantibody, following myocardial infarction in dogs. Circ Res. 1971 Sep;29(3):276–285. doi: 10.1161/01.res.29.3.276. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Bresnahan G. F., Shell W. E., Yoder R. D. Estimation of infarct size in man and its relation to prognosis. Circulation. 1972 Oct;46(4):640–648. doi: 10.1161/01.cir.46.4.640. [DOI] [PubMed] [Google Scholar]


