Abstract
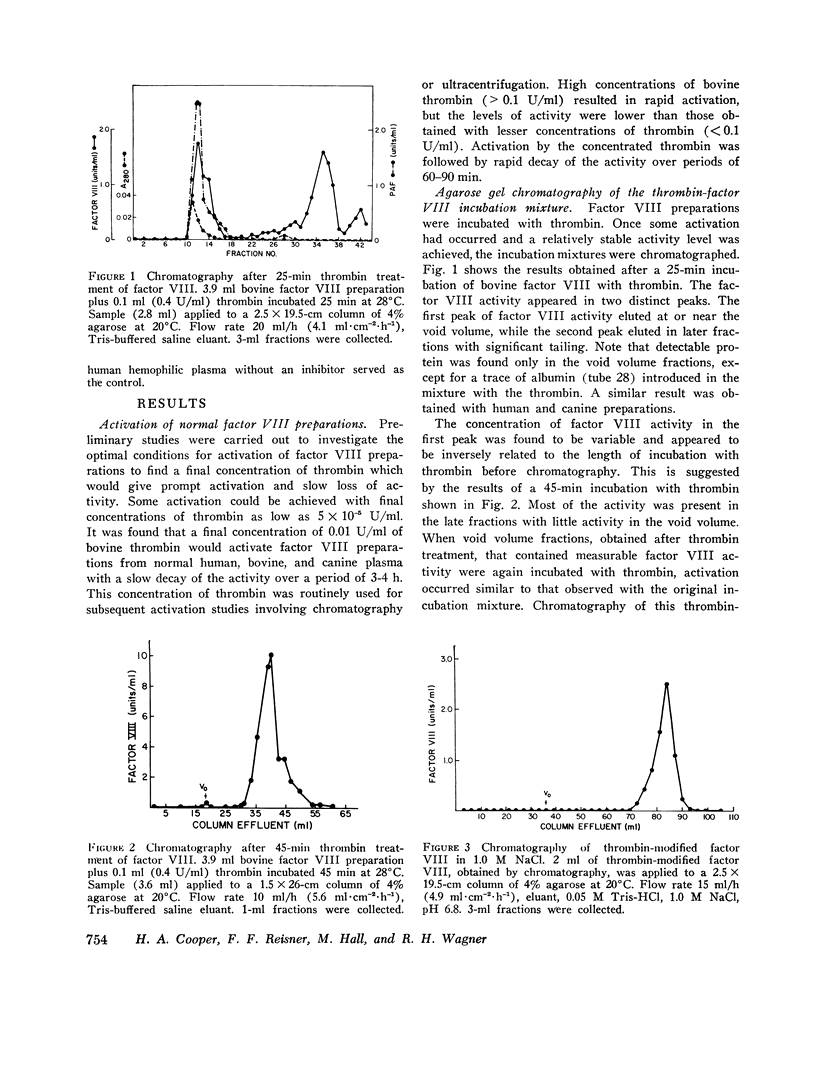
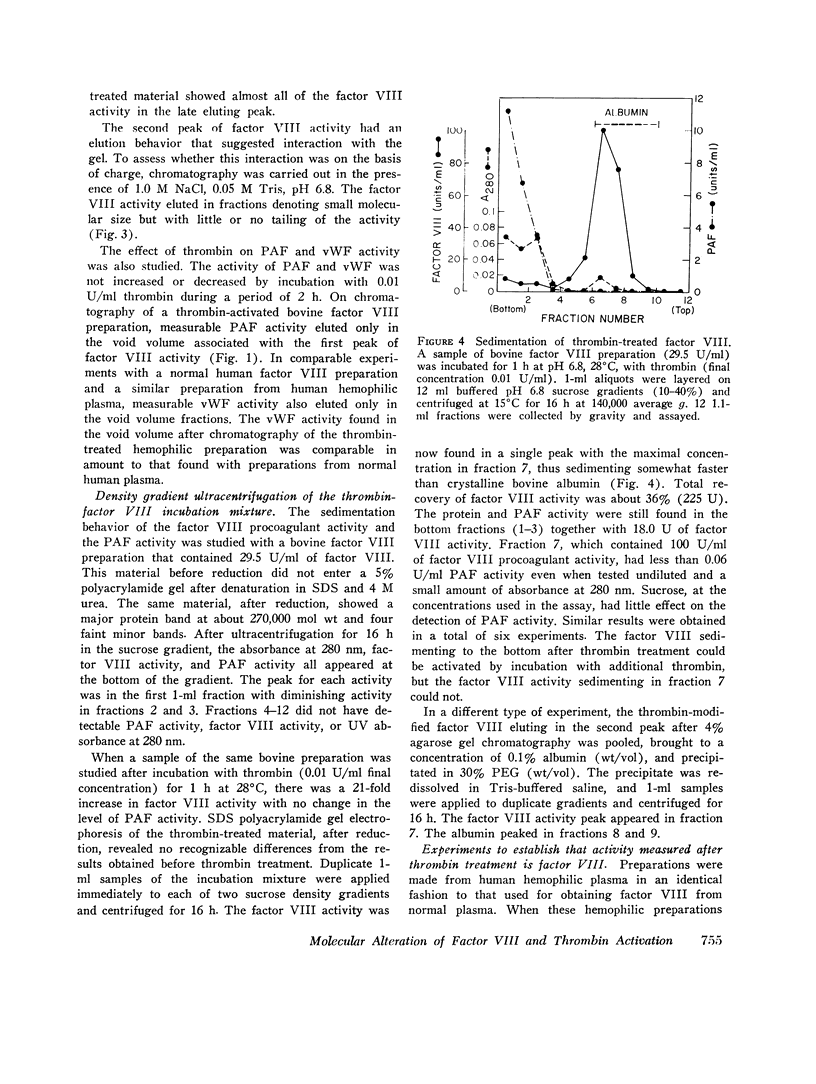
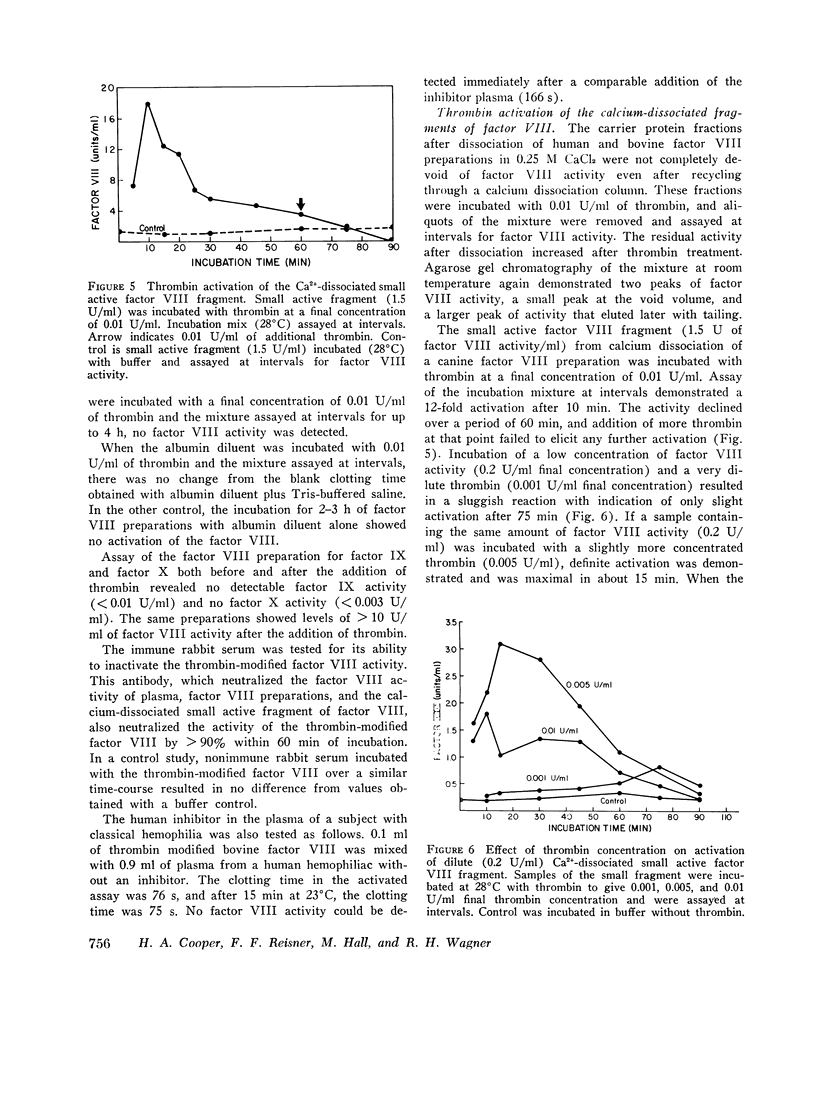
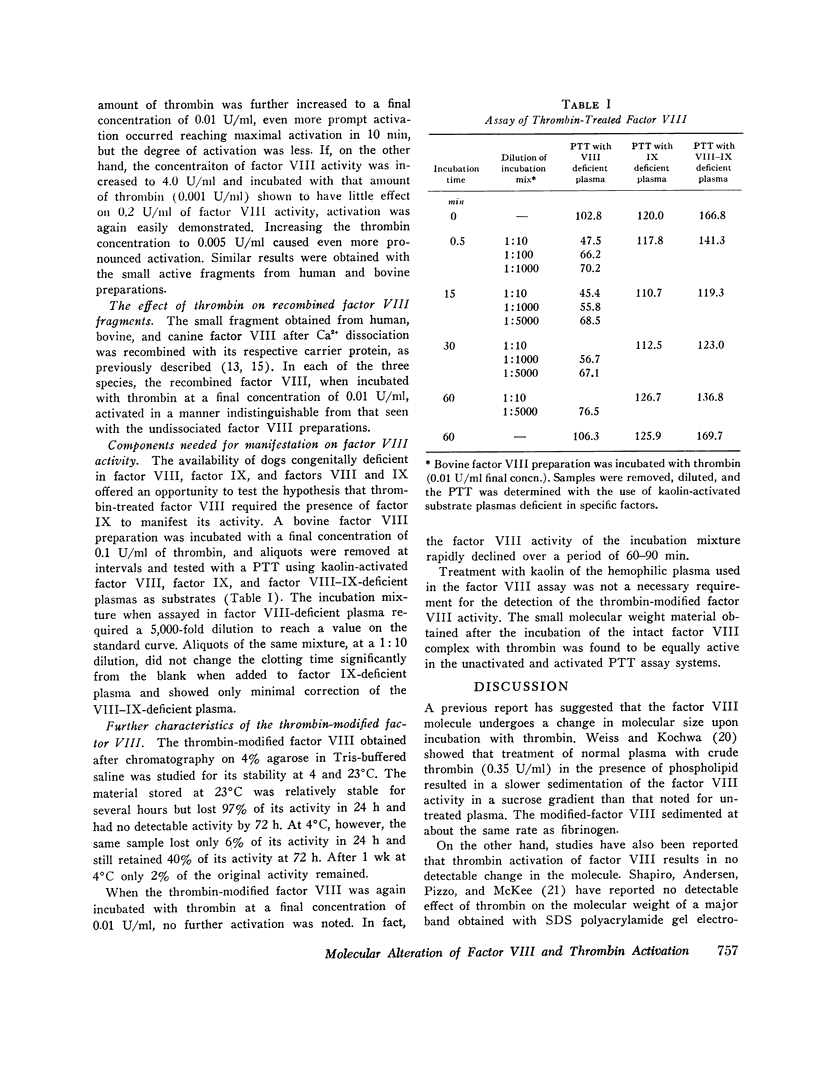
When human, canine, or bovine factor VIII preparations are chromatographed on 4% agarose at ionic strength 0.2, the factor VIII activity elutes as a single peak in the void volume with slight tailing. Incubation of such preparations with dilute (0.01 U/ml) highly purified thrombin results in some activation of factor VIII. Chromatography of such incubation mixtures, under the same conditions as before, results in elution of two peaks of factor VIII activity one in the void volume and one much later with marked tailing. The void volume peak has most of the protein and some factor VIII activity. These void volume fractions also contain all the von Willebrand factor activity of thrombin-treated bovine preparations. Longer treatment with thrombin, or treatment with stronger thrombin, appears to shift much more of the procoagulant activity to the later eluting peak. Also, when the peak of factor VIII activity, found in the void volume after thrombin treatment, was again incubated with dilute thrombin, an increase in factor VIII activity occurred. Chromatography of this incubation mixture demonstrated only a small amount of activity in the void volume, while the bulk of the activity was present in the second peak. On the other hand, thrombin treatment of factor VIII activity from peak 2 caused a rapid decline of activity instead of a further increase. It is proposed that the residual factor VIII activity found in the void volume represents unreacted factor VIII, while the late eluting peak represents thrombin-activated material that is of smaller apparent size. The late eluting peak differs from the small active factor VIII fragment obtained by Ca2+ dissociation, as the latter can be activated by thrombin. A similar set of experiments was performed using ultracentifugation of bovine factor VIII preparations on sucrose density gradients. Results of these experiments agreed completely with those obtained with get chromatography. Preparations made from human hemophilic plasma, by the procudure employed in the purification of human factor VIII, were also incubated with thrombin and chromatographed. von Willebrand factor was again found only in the void volume fractions, but there was no factor VIII activity in any fractions eluted. In other control experiments, activated and unactivated factor VIII fractions did not clot fibrinogen and contained no assayable factor IX or X. The thrombin-modified factor VIII of small size was inactivated by both a naturally occurring human inhibitor to factor VIII and the gamma globulin fraction of a rabbit antisera produced against the calcium-dissociated small active factor VIII fragment.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain J. P., Cooper H. A., Wagner R. H., Brinkhous K. M. Platelets fixed with paraformaldehyde: a new reagent for assay of von Willebrand factor and platelet aggregating factor. J Lab Clin Med. 1975 Feb;85(2):318–328. [PubMed] [Google Scholar]
- BIGGS R., MACFARLANE R. G., DENSON K. W., ASH B. J. THROMBIN AND THE INTERACTION OF FACTORS 8 AND 9. Br J Haematol. 1965 May;11:276–295. doi: 10.1111/j.1365-2141.1965.tb06588.x. [DOI] [PubMed] [Google Scholar]
- Barrow E. M., Graham J. B. Factor VIII (AHF) activity of small size produced by succinylating plasma. Am J Physiol. 1972 Jan;222(1):134–141. doi: 10.1152/ajplegacy.1972.222.1.134. [DOI] [PubMed] [Google Scholar]
- Barrow E. M., Graham J. B. Kidney antihemophilic factor. Partial purification and some properties. Biochemistry. 1968 Nov;7(11):3917–3925. doi: 10.1021/bi00851a020. [DOI] [PubMed] [Google Scholar]
- Barton P. G., Hanahan D. J. The preparation and properties of a stable factor V from bovine plasma. Biochim Biophys Acta. 1967 Apr 11;133(3):506–518. doi: 10.1016/0005-2795(67)90555-7. [DOI] [PubMed] [Google Scholar]
- Brinkhous K. M., Davis P. D., Graham J. B., Dodds W. J. Expression and linkage of genes for X-linked hemophilias A and B in the dog. Blood. 1973 Apr;41(4):577–585. [PubMed] [Google Scholar]
- Cooper H. A., Griggs T. R., Wagner R. H. Factor VIII recombination after dissociation by CaCl12. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2326–2329. doi: 10.1073/pnas.70.8.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. A., Wagner R. H. The defect in hemophilic and von Willebrand's disease plasmas studied by a recombination technique. J Clin Invest. 1974 Nov;54(5):1093–1099. doi: 10.1172/JCI107853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G., Duiguid D. L., Jackson C. M. The action of thrombin on blood clotting factor V: conversion of factor V to a prothrombin-binding protein. Biochim Biophys Acta. 1973 May 17;310(1):289–294. doi: 10.1016/0005-2795(73)90034-2. [DOI] [PubMed] [Google Scholar]
- LANGDELL R. D., WAGNER R. H., BRINKHOUS K. M. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assy procedure. J Lab Clin Med. 1953 Apr;41(4):637–647. [PubMed] [Google Scholar]
- Legaz M. E., Schmer G., Counts R. B., Davie E. W. Isolation and characterization of human Factor VIII (antihemophilic factor). J Biol Chem. 1973 Jun 10;248(11):3946–3955. [PubMed] [Google Scholar]
- MARGOLIS J. The kaolin clotting time; a rapid one-stage method for diagnosis of coagulation defects. J Clin Pathol. 1958 Sep;11(5):406–409. doi: 10.1136/jcp.11.5.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh J., Messel H., McDonagh R. P., Jr, Murano G., Blombäck B. Molecular weight analysis of fibrinogen and fibrin chains by an improved sodium dodecyl sulfate gel electrophoresis method. Biochim Biophys Acta. 1972 Jan 26;257(1):135–142. doi: 10.1016/0005-2795(72)90262-0. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I., Schiffman S., Chong M. M. Formation of intrinsic factor-X activator activity, with special reference to the role of thrombin. Br J Haematol. 1971 Dec;21(6):643–660. doi: 10.1111/j.1365-2141.1971.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Owen W. G., Wagner R. H. Antihemophilic factor: separation of an active fragment following dissociation by salts or detergents. Thromb Diath Haemorrh. 1972 Jul 31;27(3):502–515. [PubMed] [Google Scholar]
- PAPAHADJOPOULOS D., HOUGIE C., HANAHAN D. J. PURIFICATION AND PROPERTIES OF BOVINE FACTOR V: A CHANGE OF MOLECULAR SIZE DURING BLOOD COAGULATION. Biochemistry. 1964 Feb;3:264–270. doi: 10.1021/bi00890a021. [DOI] [PubMed] [Google Scholar]
- PENICK G. D. Some factors that influence utilization of antihemophilic activity during clotting. Proc Soc Exp Biol Med. 1957 Nov;96(2):277–281. doi: 10.3181/00379727-96-23454. [DOI] [PubMed] [Google Scholar]
- RAPAPORT S. I., SCHIFFMAN S., PATCH M. J., AMES S. B. The importance of activation of antihemophilic globulin and proaccelerin by traces of thrombin in the generation of intrinsic prothrombinase activity. Blood. 1963 Feb;21:221–236. [PubMed] [Google Scholar]
- Rick M. E., Hoyer L. W. Activation of low molecular weight fragment of antihaemophilic factor (factor VIII) by thrombin. Nature. 1974 Nov 29;252(5482):404–405. doi: 10.1038/252404a0. [DOI] [PubMed] [Google Scholar]
- Rick M. E., Hoyer L. W. Immunologic studies of antihemophilic factor (AHF, factor VIII). V. Immunologic properties of AHF subunits produced by salt dissociation. Blood. 1973 Nov;42(5):737–747. [PubMed] [Google Scholar]
- Schiffman S., Rapaport S. I., Chong M. M. The mandatory role of lipid in the interaction of factors 8 and 9. Proc Soc Exp Biol Med. 1966 Dec;123(3):736–740. doi: 10.3181/00379727-123-31590. [DOI] [PubMed] [Google Scholar]
- Schmer G., Kirby E. P., Teller D. C., Davie E. W. The isolation nd characterization of bovine factor VIII (antihemophilic factor). J Biol Chem. 1972 Apr 25;247(8):2512–2521. [PubMed] [Google Scholar]
- Shapiro G. A., Andersen J. C., Pizzo S. V., McKee P. A. The subunit structure of normal and hemophilic factor VIII. J Clin Invest. 1973 Sep;52(9):2198–2210. doi: 10.1172/JCI107405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THERRIAULT D. G., GRAY J. L., JENSEN H. Influence of thrombin on rate of prothrombin conversion. Proc Soc Exp Biol Med. 1957 Jun;95(2):207–211. doi: 10.3181/00379727-95-23169. [DOI] [PubMed] [Google Scholar]
- Vogel C. N., Parfitt H. E., Jr, Kingdon H. S., Lundblad R. L. Preparation of modified bovine factor VIII with enhanced biological activity using insoluble-trypsin columns. Thromb Diath Haemorrh. 1973 Nov;30(2):229–234. [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Ware A. G., Murphy R. C., Seegers W. H. The Function of Ac-Globulin in Blood Clotting. Science. 1947 Dec 19;106(2764):618–619. doi: 10.1126/science.106.2764.618-a. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss H. J., Hoyer I. W. Von Willebrand factor: dissociation from antihemophilic factor procoagulant activity. Science. 1973 Dec 14;182(4117):1149–1151. doi: 10.1126/science.182.4117.1149. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Kochwa S. Molecular forms of antihaemophilic globulin in plasma, cryoprecipitate and after thrombin activation. Br J Haematol. 1970 Jan;18(1):89–100. doi: 10.1111/j.1365-2141.1970.tb01421.x. [DOI] [PubMed] [Google Scholar]


