Abstract
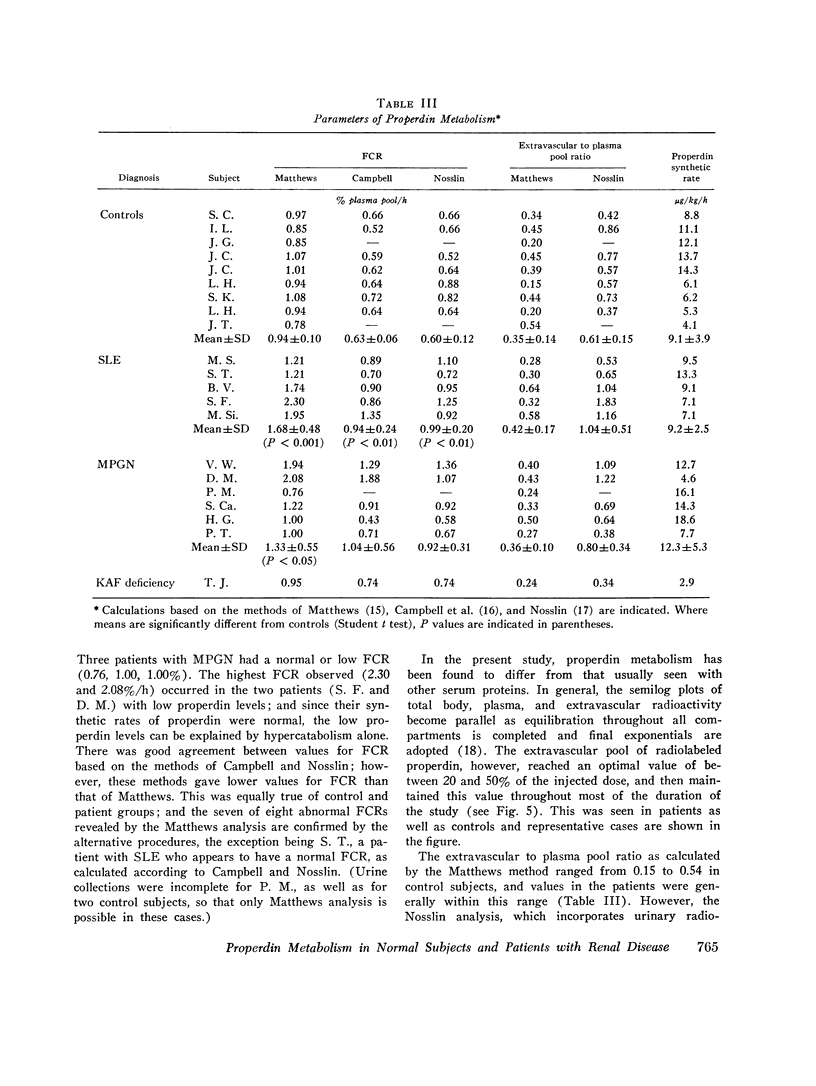
Properdin deposition has been recognized in glomeruli of patients with acute and chronic nephritis and lupus nephritis, and low serum properdin levels have been found in these disorders. These findings suggest that properdin may be involved in the production of glomerular damage and that low properdin levels may be due to hypercatabolism. The study was designed to examine the metabolism of properdin in normal subjects and to look for an abnormality in five patients with systemic lupus erythematosus with renal involvement and in six patients with membranoproliferative glomerulonephritis or dense deposit disease (MPGN). Highly purified human properdin was prepared by elution from zymosan, followed by DEAE-cellulose and carboxymethyl-Sephadex chromatography, and labeled with 125I by the iodine monochloride method. Parameters of metabolism were determined by monitoring plasma and urinary radioactivity at frequent intervals after the intravenous injection of 1-2 muCi of labeled material. The fractional catabolic rate (FCR) of properdin in normal subjects was found to have a very narrow range of 0.78-1.0,% of the plasma pool per hour (mean 0.95%). In systemic lupus erythematosus, the FCR was regularly elevated with a range of 1.21-2.30% (mean 1.70%). In MPGN, FCR was elevated in three patients (1.22, 1.94, and 2.08%) and within or below the normal range in three (0.78, 1.00, and 1.00%). Properdin levels were reduced in two patients who had the highest FCR's noted in the study. Properdin synthetic rates in normals varied from 4.1 to 14.3 mug/kg per h (mean 9.1) and was not found to be reduced in any patient. Properdin catabolism was found to be normal in a patient deficient in the C3b inactivator. These studies show that properdin is hypercatabolized in patients with renal disease and that decreased properdin levels when they occur in these patients can be entirely explained on the basis of this hypercatabolism.
Full text
PDF
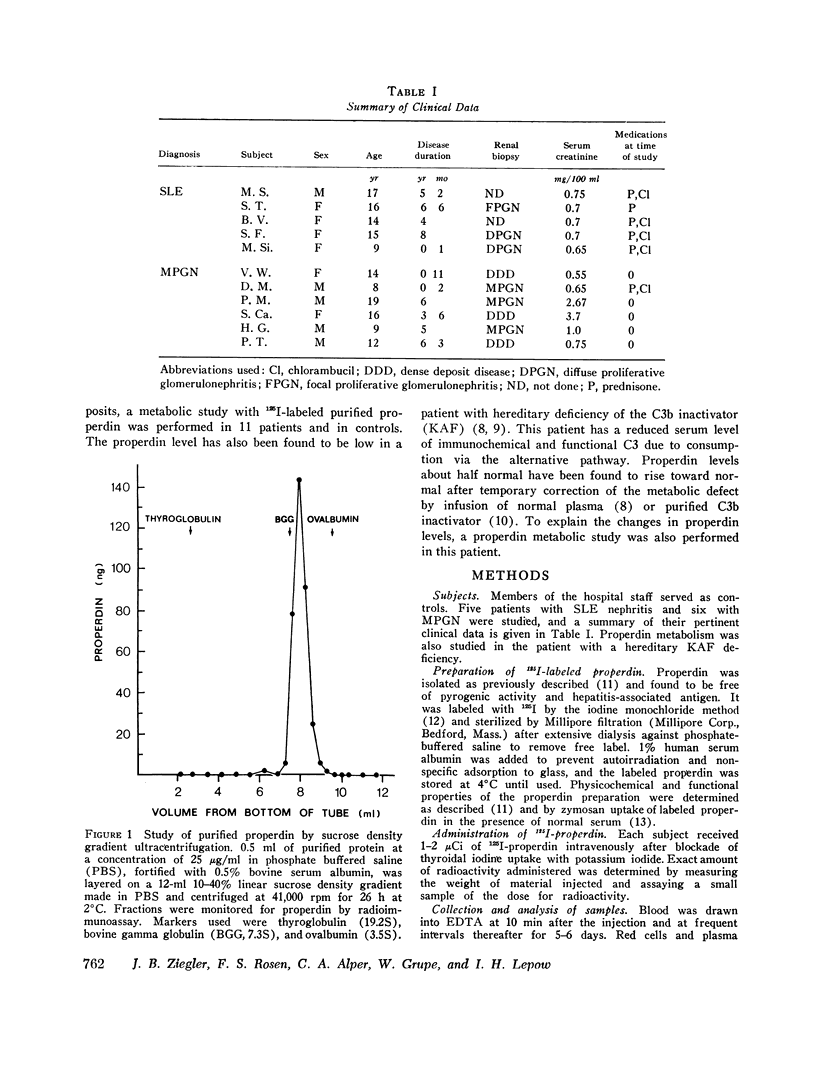
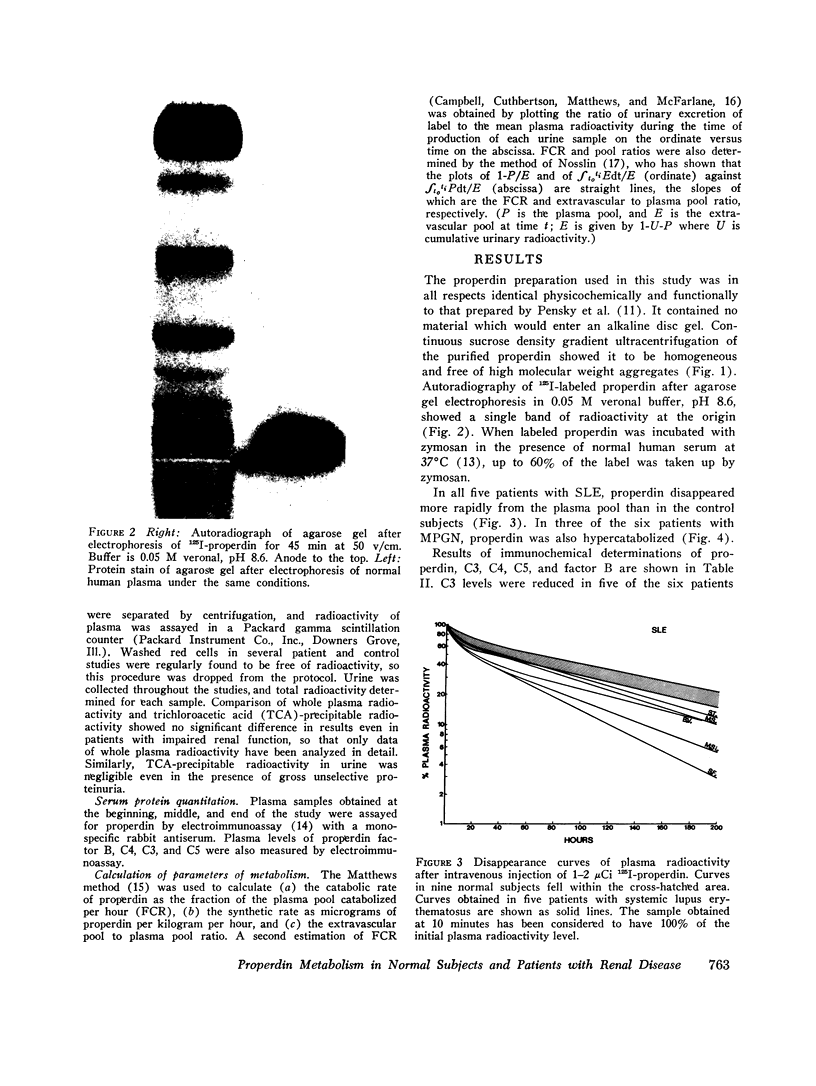
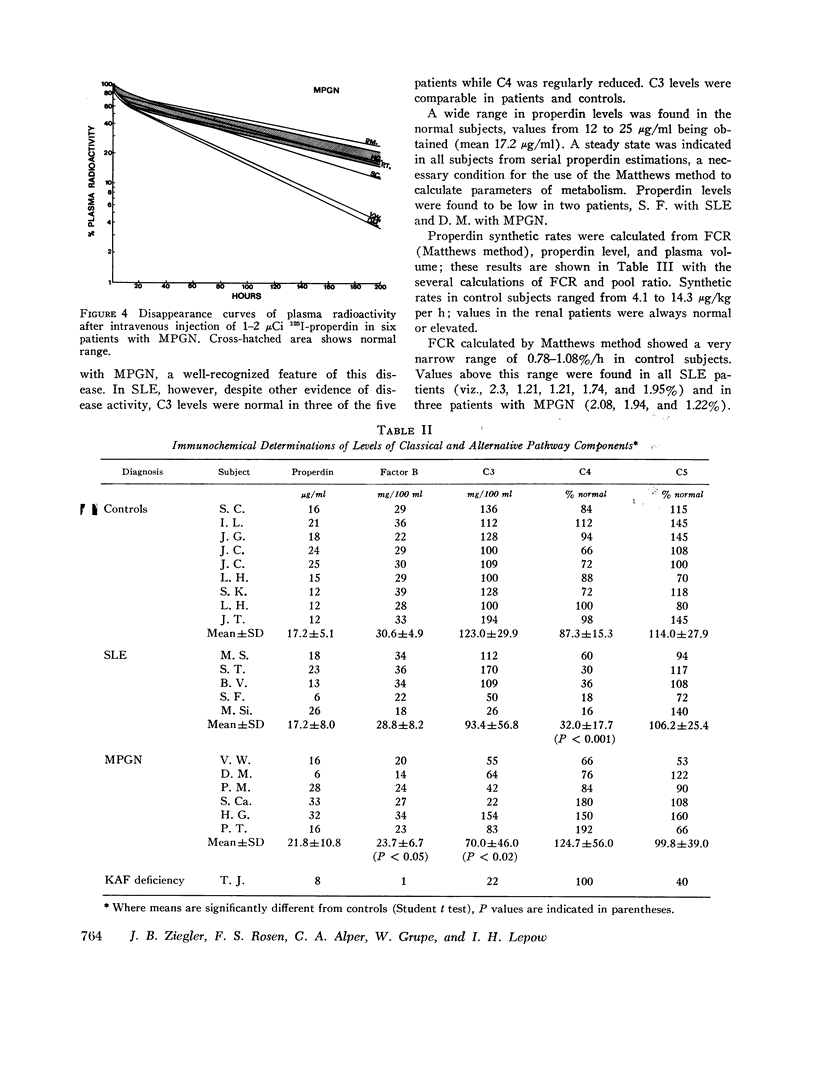
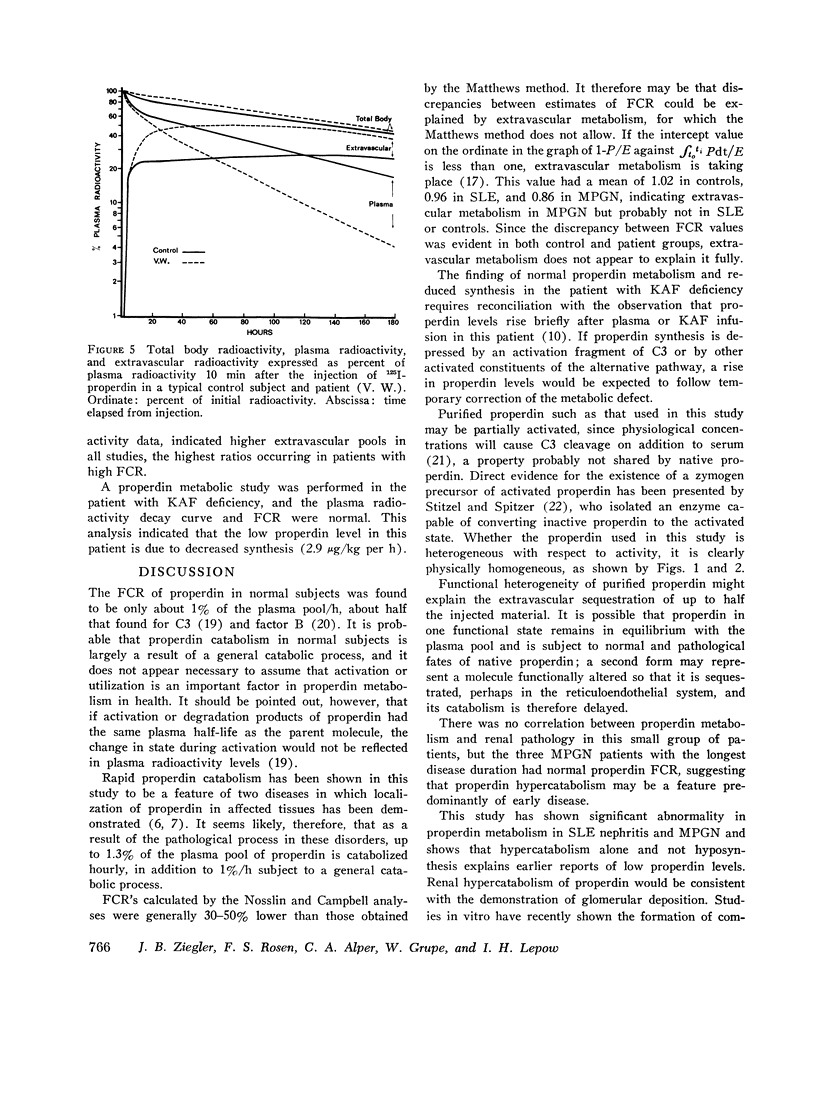

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPER C. A., FREEMAN T., WALDENSTROEM J. THE METABOLISM OF GAMMA GLOBULINS IN MYELOMA AND ALLIED CONDITIONS. J Clin Invest. 1963 Dec;42:1858–1868. doi: 10.1172/JCI104870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson N., Alper C. A., Lachmann P. J., Rosen F. S., Jandl J. H. Deficiency of C3 inactivator in man. J Immunol. 1971 Jul;107(1):19–27. [PubMed] [Google Scholar]
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Studies in vivo and in vitro on an abnormality in the metabolism of C3 in a patient with increased susceptibility to infection. J Clin Invest. 1970 Nov;49(11):1975–1985. doi: 10.1172/JCI106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL R. M., CUTHBERTSON D. P., MATTHEWS C. M., MCFARLANE A. S. Behaviour of 14C- and 131I-labelled plasma proteins in the rat. Int J Appl Radiat Isot. 1956 Jul;1(1-2):66–84. doi: 10.1016/0020-708x(56)90020-5. [DOI] [PubMed] [Google Scholar]
- Charlesworth J. A., Williams D. G., Sherington E., Lachmann P. J., Peters D. K. Metabolic studies of the third component of complement and the glycine-rich beta glycoprotein in patients with hypocomplementemia. J Clin Invest. 1974 Jun;53(6):1578–1587. doi: 10.1172/JCI107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensky J., Hinz C. F., Jr, Todd E. W., Wedgwood R. J., Boyer J. T., Lepow I. H. Properties of highly purified human properdin. J Immunol. 1968 Jan;100(1):142–158. [PubMed] [Google Scholar]
- Gewurz H., Pickering R. J., Naff G., Snyderman R., Mergenhagen S. E., Good R. A. Decreased properdin activity in acute glomerulonephritis. Int Arch Allergy Appl Immunol. 1969;36(6):592–598. doi: 10.1159/000230780. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The role of properdin in the alternate pathway of complement activation. J Exp Med. 1974 Jan 1;139(1):44–57. doi: 10.1084/jem.139.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- McLean R. H., Michael A. F. Properdin anc C3 proactivator: alternate pathway components in human glomerulonephritis. J Clin Invest. 1973 Mar;52(3):634–644. doi: 10.1172/JCI107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta J. O., Lepow I. H. Studies on the sub-unit structure of human properdin. Immunochemistry. 1974 Jul;11(7):361–368. doi: 10.1016/0019-2791(74)90189-x. [DOI] [PubMed] [Google Scholar]
- Nosslin B. Analysis of disappearance time-curves after single injection of labelled proteins. Ciba Found Symp. 1972;9:113–130. doi: 10.1002/9780470719923.ch7. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., ROSS O. A., TODD E. W., WARDLAW A. C. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Lambert P. H., Miescher P. A. Properdin levels in systemic lupus erythematosus and membranoproleferative glomerulonephritis. Clin Exp Immunol. 1974 Apr;16(4):575–581. [PMC free article] [PubMed] [Google Scholar]
- Rothfield N., Ross H. A., Minta J. O., Lepow I. H. Glomerular and dermal depostion of properdin in systemic lupus erythematosus. N Engl J Med. 1972 Oct 5;287(14):681–685. doi: 10.1056/NEJM197210052871402. [DOI] [PubMed] [Google Scholar]
- Stitzel A. E., Spitzer R. E. The utilization of properdin in the alternate pathway of complement activation: isolation of properdin convertase. J Immunol. 1974 Jan;112(1):56–62. [PubMed] [Google Scholar]
- Westberg N. G., Naff G. B., Boyer J. T., Michael A. F. Glomerular deposition of properdin in acute and chronic glomerulonephritis with hypocomplementemia. J Clin Invest. 1971 Mar;50(3):642–649. doi: 10.1172/JCI106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. B., Alper C. A., Rosen R. S., Lachmann P. J., Sherington L. Restoration by purified C3b inactivator of complement-mediated function in vivo in a patient with C3b inactivator deficiency. J Clin Invest. 1975 Mar;55(3):668–672. doi: 10.1172/JCI107975. [DOI] [PMC free article] [PubMed] [Google Scholar]