Abstract
We report the creation of alkylated poly-N-substituted glycine (peptoid) mimics of antimicrobial lipopeptides with alkyl tails ranging from 5 to 13 carbons. In several cases, alkylation significantly improved the selectivity of the peptoids with no loss in antimicrobial potency. Using this technique, we synthesized an antimicrobial peptoid only 5 monomers in length with selective, broad-spectrum antimicrobial activity as potent as previously reported dodecameric peptoids and the antimicrobial peptide pexiganan.
Antimicrobial peptides (AMPs) (1) are ubiquitously found in nature as an integral component of many organisms' first defense against pathogens (10). Unlike conventional antibiotics, AMPs are largely thought to kill bacteria via nonspecific membrane interactions (5), with a reduced probability that bacteria will develop resistance (11, 14, 28). Several peptidomimetic analogs of AMPs, made of β-peptides (29-32), poly(phenyleneethynylene)s (12, 33), and peptoids (6, 9, 26), have successfully captured AMP-like activity in a nonnatural system.
Peptoids are isomers of peptides where the side chain of each residue stems from the backbone nitrogen instead of the α-carbon (Fig. 1). This change makes peptoids protease resistant (24), thereby increasing their bioavailability. Peptoids with α-chiral side chains can fold into three-sided polyproline type-I-like helices that are ideal for mimicking the amphipathic nature of many AMPs (2, 3, 16, 36, 37). The cost-effective and facile synthesis of peptoids (38), as well as their designable structure and advantageous pharmacological properties, makes peptoids excellent candidates to mimic antimicrobial peptides.
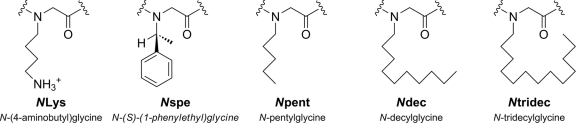
FIG. 1.
Peptoid monomer side chain structures, with full and shorthand names.
Lipopeptides such as daptomycin and the polymyxin and echinocandin families of clinically approved antibiotics are an increasingly important class of antimicrobial peptides. Polymyxin B (34) and trichogin (27) possess fatty acid moieties that are known to be integral to their activities. In recent years, development of linear antimicrobial peptides has shifted to ever-shorter peptides for synthetic and pharmacological reasons (15, 17, 18, 22, 23), but relatively little work has investigated linear AMPs with hydrophobic tails. Fatty acid tails have been attached to linear antimicrobial peptides that are not normally acylated (4, 19-21), in some cases endowing inactive cationic peptides with antimicrobial activity. Peptoid synthesis enables facile incorporation of an alkylamine into the peptoid as an N-terminal alkyl tail, and thus we use this strategy to investigate alkylated peptoids as mimics of antimicrobial lipopeptides.
Design of peptoids.
Previous studies demonstrated the feasibility of peptoid mimicry of AMPs (26), yielding peptoid oligomers 12 to 16 residues in length with low-micromolar, broad-spectrum antibacterial activity and low-to-negligible mammalian cytotoxicity at the bacterial MICs (6, 26). Peptoid 1 [H-(NLys-Nspe-Nspe)4-NH2, where NLys is N-(4-aminobutyl)glycine and Nspe is N-(S)-(1-phenylethyl)glycine] (Table 1) is a dodecameric peptoid with a simple trimer repeat forming an amphipathic helix with one cationic face and two aromatic faces. Using the submonomer approach, we synthesized a series of alkylated analogs of peptoid 1 (38), systematically decreasing the length of the peptoid chain in increments of three monomers (one helical turn), except for the shortest length based on reports of ultrashort AMPs containing two positive charges (15, 17, 22, 23). Alkyl tails were 5, 10, or 13 carbons in length and were incorporated as the side chains of the N-terminal peptoid residue using the appropriate alkylamine for the substitution step. Table 1 lists the names and sequences of the alkylated peptoids investigated (Fig. 1 shows monomer structures).
TABLE 1.
Antibacterial, antifungal, and hemolytic activities of alkylated ampetoidsa
| Peptoid or peptide | Sequence | MWb | % ACN at RP-HPLC elutionc | Calculated log D7.4d | MIC (μg/ml) for: |
HD10/HD50e (μg/ml) | SRf | ||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | B. subtilis | C. albicans | |||||||
| 1 | H-(NLys-Nspe-Nspe)4-NH2 | 1,819.3 | 64.2 | −1.11 | 14.7 | 3.7 | 14.7 | 33/145 | 8.9 |
| Npent-1 | H-Npent-(NLys-Nspe-Nspe)4-NH2 | 1,946.5 | 66.0 | 1.88 | 15.5 | 3.9 | 15.5 | 37/128 | 9.5 |
| Ndec-1 | H-Ndec-(NLys-Nspe-Nspe)4-NH2 | 2,016.3 | 72.5 | 4.43 | 31.6 | 4.0 | 4.0 | 13/40 | 3.3 |
| 19mer | H-(NLys-Nspe-Nspe)3-NH2 | 1,368.8 | 60.7 | −1.72 | 45.6 | 2.9 | 28.5 | 274/>365 | 95 |
| Npent-19mer | H-Npent-(NLys-Nspe-Nspe)3-NH2 | 1,495.9 | 63.3 | 1.28 | 30.8 | 1.8 | 23.7 | 100/>190 | 56 |
| Ndec-19mer | H-Ndec-(NLys-Nspe-Nspe)3-NH2 | 1,566.1 | 70.8 | 3.83 | 12.4 | 6.2 | 6.2 | 20/69 | 3.2 |
| 16mer | H-(NLys-Nspe-Nspe)2-NH2 | 918.2 | 47.0 | −2.31 | >126 | >126 | >126 | >252/>252 | NDg |
| Npent-16mer | H-Npent-(NLys-Nspe-Nspe)2-NH2 | 1,045.4 | 59.4 | 0.68 | >266 | 4.1 | 133 | >266/>266 | >65 |
| Ndec-16mer | H-Ndec-(NLys-Nspe-Nspe)2-NH2 | 1,115.5 | 69.8 | 3.23 | 8.8 | 2.2 | 8.8 | 37.8/106 | 17 |
| Ndec-14mer | H-Ndec-(NLys-Nspe-Nspe-NLys)-NH2 | 793.1 | 60.1 | 1.91 | >108 | 6.8 | 108 | >215/>215 | >32 |
| Ntridec-14mer | H-Ntridec-(NLys-Nspe-Nspe-NLys)-NH2 | 835.2 | 68.0 | 3.44 | 14.0 | 1.8 | 14.0 | 73/224 | 41 |
| Pexiganan | GIGKFLKKAKKFGKAFVKILKK-NH2 | 2,477.2 | 49.2 | ND | 7.8 | 3.9 | 124 | 181/>495 | 46 |
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ-NH2 | 2,846.5 | 64.3 | ND | 35.6 | 4.5 | 4.5 | 2.8/17.1 | 0.62 |
See Fig. 1 for a guide to peptoid monomers.
MW, molecular weight.
ACN, acetonitrile; RP-HPLC, reverse-phase high-performance liquid chromatography.
Log D7.4, log (concentration of the solute in octanol/concentration of the solute in water) at pH 7.4.
HD10 and HD50, 10% and 50% hemolytic doses, respectively.
Selectivity ratio (SR) = HD10/B. subtilis MIC.
ND, not determined.
Antibacterial and antifungal activity.
The MICs of the peptoids against bacteria (Gram-negative Escherichia coli and Gram-positive Bacillus subtilis), determined according to CLSI M07-A6 protocols (7), and Candida albicans SC5314, determined according CLSI M27-A2 protocols (25), are shown in Table 1. Data for pexiganan (8), a peptide analog of magainin-2 developed as a topical antimicrobial agent, and melittin, a cytotoxic AMP from bee venom, are included for comparison.
Previous studies have shown peptoid 1 to be potent against both Gram-negative and Gram-positive bacteria, and, with increasing tail length, the more hydrophobic analogues of peptoid 1 retain or start losing their antibacterial activity but increase their antifungal potency. In contrast, alkylated peptoids with base chain lengths of nine, six, and four residues all showed significant improvements in potency against both bacteria and fungi relative to the unalkylated peptoid. The higher hydrophobicities required for antifungal activity may result from differences in composition between bacterial (more anionic) and eukaryotic (more zwitterionic) membranes. Therefore, the hydrophobic contribution to fungal membrane adsorption is more important than that to bacterial membrane adsorption. Notably, alkylated peptoids as short as five residues (e.g., Ntridec-14mer, where Ntridec is N-tridecylglycine) showed potent antimicrobial activity. We therefore tested the antibacterial activity of peptoid 1 and Ntridec-14mer against a broad spectrum of pathogenic bacteria. As shown in Table 2, Ntridec-14mer was generally comparable in potency to pexiganan and peptoid 1 against the strains tested.
TABLE 2.
Broad-spectrum antibacterial activity of pexiganan and selected ampetoids against biosafety level 2 (BSL2) and BSL3 pathogenic bacteria
| Strain | Gram staining | MICs (μg/ml) of: |
||
|---|---|---|---|---|
| Pexiganan | Peptoid 1 | Ntridec-14mer | ||
| Proteus vulgaris ATCC 49132 | − | 32.0 | 40.0 | 43.0 |
| Pseudomonas aeruginosa ATCC 27853 | − | 4.0 | 10.0 | 10.8 |
| Proteus mirabilis ATCC 35659 | − | >128 | >160 | 172 |
| Klebsiella pneumoniae ATCC 33495 | − | 8.0 | 20.0 | 10.8 |
| Enterobacteraerogenes ATCC 35029 | − | 32.0 | 20.0 | >172 |
| Escherichia coli ATCC 25922 | − | 8.0 | 5.0 | 10.8 |
| Serratiamarcescens ATCC 13880 | − | >128 | 160 | 172 |
| Staphylococcus aureus ATCC 29213 | + | 32.0 | 5.0 | 5.4 |
| Staphylococcus aureus VAN1a | + | 16.0 | 5.0 | 5.4 |
| Staphylococcus aureus VAN2a | + | 8.0 | 5.0 | 5.4 |
| Staphylococcus aureus NRS100 (COL) | + | 16.0 | 5.0 | 5.4 |
| Staphylococcus aureus NRS119 | + | 64.0 | 5.0 | 5.4 |
| Staphylococcus aureus NRS120 | + | 64.0 | 10.0 | 5.4 |
| Enterococcusfaecalis ATCC 29212 | + | 32.0 | 5.0 | 10.8 |
| Enterococcusfaecalis 99a | + | 128 | 10.0 | 21.6 |
| Enterococcusfaecium 106a | + | 4.0 | 5.0 | 5.4 |
Vancomycin resistant.
Hemolytic activity.
Lytic activity against human erythrocytes, measured as previously reported (6), was used to gauge mammalian cytotoxicity. Since these compounds are mildly selective for Gram-positive bacteria (Tables 1 and 2), we calculated a selectivity ratio (SR) (Table 1) for each compound as the quotient of the B. subtilis MIC and the 10% hemolytic dose (a measure of serious toxicity). Several compounds, such as 19mer, Npent-19mer (Npent, N-pentylglycine), Ndec-16mer (Ndec, N-decylglycine), Ndec-14mer, and Ntridec-14mer, exhibited selectivities ranging from 17 to 95, significantly better than the parent peptoid 1 (SR of 8.9). As expected from an increase in hydrophobicity (6), for peptoids with the same number of residues, hemolytic activity increased with the length of the attached alkyl tail, as is readily apparent for the acylated analogs of 19mer (Table 1). However, alkylation was not always sufficient to cause hemolytic activity, as both Npent-16mer and Ndec-14mer exhibited no hemolysis even at concentrations higher than 200 μg/ml.
Short alkylated peptoids may employ modes of killing that are similar to those of nonalkylated, full-length antimicrobial peptoids and peptides (22, 35). In solution, alkylated AMPs may form micelles (22), intertwined peptide-tail structures (13), and nanostructures (23). While ongoing studies in our laboratory are investigating the effect and extent of peptoid oligomerization, micellization may play a role in assembling alkylated peptoids into areas of high local concentration, allowing them to exert their microbicidal effects at lower bulk concentrations relative to unalkylated parent compounds of the same length. Micellization or alternate aggregation methods almost certainly occur, as demonstrated by the great disparity between the low variation in high-performance liquid chromatography (HPLC) elution times (a measurement of hydrophobicity [6]) and the greatly variable calculated log D7.4 (log [concentration of the solute in octanol/concentration of the solute in water]) values in Table 1.
The antimicrobial activities of Ndec-16mer and Ntridec-14mer were comparable to that of peptoid 1, and both demonstrated improved selectivity, with molecular weights 61% and 46% of that of peptoid 1, respectively. Thus, these short peptoid mimics of antimicrobial lipopeptides preserve the potent, broad-spectrum antimicrobial activity of our previously reported antimicrobial peptoids but with substantially lower molecular weights and improved selectivity (6, 26). This approach will be useful in designing future peptoid and other peptidomimetic compounds with therapeutic potential against bacterial and fungal infections.
Acknowledgments
We thank David Steinhorn for his assistance with hemolysis assays.
N.P.C. was supported in part by a Department of Homeland Security Graduate Fellowship. T.M.M. was supported by the Jean Dreyfus Boissevain Undergraduate Scholarship for Excellence in Chemistry and a Northwestern Undergraduate Research Grant. A.E.B. acknowledges support from NIH grants 1R01 HL067984 and 1R01 AI072666.
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Andreu, D., and L. Rivas. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415-433. [DOI] [PubMed] [Google Scholar]
- 2.Armand, P., K. Kirshenbaum, A. Falicov, R. L. Dunbrack, Jr., K. A. Dill, R. N. Zuckermann, and F. E. Cohen. 1997. Chiral N-substituted glycines can form stable helical conformations. Folding Des. 2:369-375. [DOI] [PubMed] [Google Scholar]
- 3.Armand, P., K. Kirshenbaum, R. A. Goldsmith, S. Farr-Jones, A. E. Barron, K. T. V. Truong, K. A. Dill, D. F. Mierke, F. E. Cohen, R. N. Zuckermann, and E. K. Bradley. 1998. NMR determination of the major solution conformation of a peptoid pentamer with chiral side chains. Proc. Natl. Acad. Sci. U. S. A. 95:4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avrahami, D., and Y. Shai. 2003. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to d,l-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry 42:14946-14956. [DOI] [PubMed] [Google Scholar]
- 5.Bessalle, R., A. Kapitkovsky, A. Gorea, I. Shalit, and M. Fridkin. 1990. All-D-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 274:151-155. [DOI] [PubMed] [Google Scholar]
- 6.Chongsiriwatana, N. P., J. A. Patch, A. M. Czyzewski, M. T. Dohm, A. Ivankin, D. Gidalevitz, R. N. Zuckermann, and A. E. Barron. 2008. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 105:2794-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. CLSI, Wayne, PA.
- 8.Ge, Y., D. MacDonald, M. M. Henry, H. I. Hait, K. A. Nelson, B. A. Lipsky, M. A. Zasloff, and K. J. Holroyd. 1999. In vitro susceptibility to pexiganan of bacteria isolated from infected diabetic foot ulcers. Diagn. Microbiol. Infect. Dis. 35:45-53. [DOI] [PubMed] [Google Scholar]
- 9.Goodson, B., A. Ehrhardt, S. Ng, J. Nuss, K. Johnson, M. Giedlin, R. Yamamoto, W. H. Moos, A. Krebber, M. Ladner, M. B. Giacona, C. Vitt, and J. Winter. 1999. Characterization of novel antimicrobial peptoids. Antimicrob. Agents Chemother. 43:1429-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. U. S. A. 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock, R. E. W., and H.-G. Sahl. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551-1557. [DOI] [PubMed] [Google Scholar]
- 12.Ishitsuka, Y., L. Arnt, J. Majewski, S. Frey, M. Ratajczek, K. Kjaer, G. N. Tew, and K. Y. C. Lee. 2006. Amphiphilic poly(phenyleneethynylene)s can mimic antimicrobial peptide membrane disordering effect by membrane insertion. J. Am. Chem. Soc. 128:13123-13129. [DOI] [PubMed] [Google Scholar]
- 13.Japelj, B., M. Zorko, A. Majerle, P. Pristovsek, S. Sanchez-Gomez, G. Martinez de Tejada, I. Moriyon, S. E. Blondelle, K. Brandenburg, J. Andrae, K. Lohner, and R. Jerala. 2007. The acyl group as the central element of the structural organization of antimicrobial lipopeptide. J. Am. Chem. Soc. 129:1022-1023. [DOI] [PubMed] [Google Scholar]
- 14.Jenssen, H., P. Hamill, and R. E. W. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamysz, W., C. Silvestri, O. Cirioni, A. Giacometti, A. Licci, A. Della Vittoria, M. Okroj, and G. Scalise. 2007. In vitro activities of the lipopeptides palmitoyl (Pal)-Lys-Lys-NH2 and Pal-Lys-Lys alone and in combination with antimicrobial agents against multiresistant Gram-positive cocci. Antimicrob. Agents Chemother. 51:354-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirshenbaum, K., A. E. Barron, R. A. Goldsmith, P. Armand, E. K. Bradley, K. T. V. Truong, K. A. Dill, F. E. Cohen, and R. N. Zuckermann. 1998. Sequence-specific polypeptoids: a diverse family of heteropolymers with stable secondary structure. Proc. Natl. Acad. Sci. U. S. A. 95:4303-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laverty, G., M. McLaughlin, C. Shaw, S. P. Gorman, and B. F. Gilmore. 2010. Antimicrobial activity of short, synthetic cationic lipopeptides. Chem. Biol. Drug Des. 75:563-569. [DOI] [PubMed] [Google Scholar]
- 18.Lipinski, C. A., F. Lombardo, B. W. Dominy, and P. J. Feeney. 1997. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 23:3-25. [DOI] [PubMed] [Google Scholar]
- 19.Lockwood, N. A., J. R. Haseman, M. V. Tirrell, and K. H. Mayo. 2004. Acylation of SC4 dodecapeptide increases bactericidal potency against Gram-positive bacteria, including drug-resistant strains. Biochem. J. 378:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majerle, A., J. Kidric, and R. Jerala. 2003. Enhancement of antibacterial and lipopolysaccharide binding activities of a human lactoferrin peptide fragment by the addition of acyl chain. J. Antimicrob. Chemother. 51:1159-1165. [DOI] [PubMed] [Google Scholar]
- 21.Mak, P., J. Pohl, A. Dubin, M. S. Reed, S. E. Bowers, M. T. Fallon, and W. M. Shafer. 2003. The increased bactericidal activity of a fatty acid-modified synthetic antimicrobial peptide of human cathepsin G correlates with its enhanced capacity to interact with model membranes. Int. J. Antimicrob. Agents 21:13-19. [DOI] [PubMed] [Google Scholar]
- 22.Makovitzki, A., D. Avrahami, and Y. Shai. 2006. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. U. S. A. 103:15997-16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makovitzki, A., J. Baram, and Y. Shai. 2008. Antimicrobial lipopolypeptides composed of palmitoyl di- and tricationic peptides: in vitro and in vivo activities, self-assembly to nanostructures, and a plausible mode of action. Biochemistry 47:10630-10636. [DOI] [PubMed] [Google Scholar]
- 24.Miller, S. M., R. J. Simon, S. Ng, R. N. Zuckermann, J. M. Kerr, and W. H. Moos. 1995. Comparison of the proteolytic susceptibilities of homologous L-amino acid, D-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Dev. Res. 35:20-32. [Google Scholar]
- 25.NCCLS. 2002. Method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 2nd ed. NCCLS, Wayne, PA.
- 26.Patch, J. A., and A. E. Barron. 2003. Helical peptoid mimics of magainin-2 amide. J. Am. Chem. Soc. 125:12092-12093. [DOI] [PubMed] [Google Scholar]
- 27.Peggion, C., F. Formaggio, M. Crisma, R. F. Epand, R. M. Epand, and C. Toniolo. 2003. Trichogin: a paradigm for lipopeptaibols. J. Pept. Sci. 9:679-689. [DOI] [PubMed] [Google Scholar]
- 28.Peschel, A., and H.-G. Sahl. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529-536. [DOI] [PubMed] [Google Scholar]
- 29.Porter, E. A., X. Wang, H. S. Lee, B. Weisblum, and S. H. Gellman. 2000. Non-haemolytic beta-amino-acid oligomers. Nature 404:565. [DOI] [PubMed] [Google Scholar]
- 30.Porter, E. A., B. Weisblum, and S. H. Gellman. 2002. Mimicry of host-defense peptides by unnatural oligomers: antimicrobial beta-peptides. J. Am. Chem. Soc. 124:7324-7330. [DOI] [PubMed] [Google Scholar]
- 31.Porter, E. A., B. Weisblum, and S. H. Gellman. 2005. Use of parallel synthesis to probe structure-activity relationships among 12-helical beta-peptides: evidence of a limit on antimicrobial activity. J. Am. Chem. Soc. 127:11516-11529. [DOI] [PubMed] [Google Scholar]
- 32.Seebach, D., M. Overhand, F. N. M. Kuehnle, and B. Martinoni. 1996. Beta-peptides. Synthesis by Arndt-Eistert homologation with concomitant peptide coupling. Structure determination by NMR and CD spectroscopy and by x-ray crystallography. Helical secondary structure of a beta-hexapeptide in solution and its stability towards pepsin. Helv. Chim. Acta 79:913-941. [Google Scholar]
- 33.Tew, G. N., D. Liu, B. Chen, R. J. Doerksen, J. Kaplan, P. J. Carroll, M. L. Klein, and W. F. DeGrado. 2002. De novo design of biomimetic antimicrobial polymers. Proc. Natl. Acad. Sci. U. S. A. 99:5110-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2001. N-terminal modifications of polymyxin B nonapeptide and their effect on antibacterial activity. Peptides 22:1675-1681. [DOI] [PubMed] [Google Scholar]
- 35.Uchida, M., G. McDermott, M. Wetzler, M. A. Le Gros, M. Myllys, C. Knoechel, A. E. Barron, and C. A. Larabell. 2009. Soft X-ray tomography of phenotypic switching and the cellular response to antifungal peptoids in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 106:19375-19380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu, C. W., K. Kirshenbaum, T. J. Sanborn, J. A. Patch, K. Huang, K. A. Dill, R. N. Zuckermann, and A. E. Barron. 2003. Structural and spectroscopic studies of peptoid oligomers with alpha-chiral aliphatic side chains. J. Am. Chem. Soc. 125:13525-13530. [DOI] [PubMed] [Google Scholar]
- 37.Wu, C. W., T. J. Sanborn, K. Huang, R. N. Zuckermann, and A. E. Barron. 2001. Peptoid oligomers with alpha-chiral, aromatic side chains: sequence requirements for the formation of stable peptoid helices. J. Am. Chem. Soc. 123:6778-6784. [DOI] [PubMed] [Google Scholar]
- 38.Zuckermann, R. N., J. M. Kerr, S. B. H. Kent, and W. H. Moos. 1992. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 114:10646-10647. [Google Scholar]