Abstract
Staphylococcus aureus is the most common cause of nosocomial infections. Multiple antibiotic resistance and severe clinical outcomes provide a strong rationale for development of immunoglobulin-based strategies. Traditionally, novel immunological approaches against bacterial pathogens involve antibodies directed against cell surface-exposed virulence-associated epitopes or toxins. In this study, we generated a monoclonal antibody targeting the housekeeping protein IsaA, a suggested soluble lytic transglycosylase of S. aureus, and tested its therapeutic efficacy in two experimental mouse infection models. A murine anti-IsaA antibody of the IgG1 subclass (UK-66P) showed the highest binding affinity in Biacore analysis. This antibody recognized all S. aureus strains tested, including hospital-acquired and community-acquired methicillin-resistant S. aureus strains. Therapeutic efficacy in vivo in mice was analyzed using a central venous catheter-related infection model and a sepsis survival model. In both models, anti-IsaA IgG1 conferred protection against staphylococcal infection. Ex vivo, UK-66P activates professional phagocytes and induces highly microbicidal reactive oxygen metabolites in a dose-dependent manner, resulting in bacterial killing. The study provides proof of concept that monoclonal IgG1 antibodies with high affinity to the ubiquitously expressed, single-epitope-targeting IsaA are effective in the treatment of staphylococcal infection in different mouse models. Anti-IsaA antibodies might be a useful component in an antibody-based therapeutic for prophylaxis or adjunctive treatment of human cases of S. aureus infections.
Staphylococcus aureus is a nosocomial and community-acquired pathogen that causes several diseases, ranging from minor skin infections to life-threatening wound infections, bacteremia, endocarditis, pneumonia, and toxic shock syndrome (25). The potential of S. aureus to develop multidrug resistance to traditional antibiotics has created renewed interest in using alternative treatment options, such as antibody-based immunotherapy approaches (5, 19, 31, 34). The key factor for developing an antistaphylococcal immunotherapy depends on the identification of those bacterial antigens expressed in vivo that provide protection by the immune system during infection in diverse populations of S. aureus-infected patients (9). Therefore, several studies have investigated the immune response to S. aureus to determine which bacterial antigens are associated with protective antistaphylococcal antibodies (4, 7, 9, 24, 27, 39). However, the significance and specificity of the immune response in S. aureus infections have proven difficult to be elucidated, as a number of clinical trials have recently failed (34). Other immunotherapy approaches target typical virulence factors that may play a central role in the pathogenesis of staphylococci (2, 3, 10, 12, 18, 21, 22, 27, 37, 43, 44), but the functional redundancy of adhesion proteins or the appearance of escape mutants may limit the efficacy of strictly monovalent immunotherapeutic strategies. Some evidence suggests that bacterial cell wall components with immunogenic properties can also serve as potential candidates for immunotherapy development (16, 20).
One such protein involved in cell wall metabolism is the immunodominant staphylococcal antigen A (IsaA). IsaA is a highly immunogenic, noncovalently cell wall-bound lytic transglycosylase (24, 36, 38) that is coregulated with a glycylglycine endopeptidase, LytM (8). Strains of S. aureus lacking IsaA expression are viable, and the paralogue SceD, a second lytic transglycosylase, is able to compensate for the loss (38). All of these pieces of evidence implicate a role for IsaA as a complex regulated factor involved in cell wall growth and division. Hence, the IsaA antigen appears to be not a typical virulence factor but rather a standard cellular housekeeping protein.
The present study was conducted to further clarify the therapeutic potential of antibodies to S. aureus, with a particular focus on IsaA as the target. We recently developed an animal model of S. aureus catheter-induced sepsis in immunocompetent mice that closely mimics the clinicopathological features of human disease (23). By application of this experimental system and a sepsis survival model in mice, the immunotherapeutic potential of a murine monoclonal antibody (MAb) recognizing IsaA was investigated. Both infection models show that passive anti-IsaA antibody application significantly reduces the bacterial burden in host tissues compared to that in untreated animals. In addition, anti-IsaA immunotherapy triggers highly microbicidal reactive oxygen metabolites by phagocytes and killing of S. aureus.
Overall, the data presented within the study prove that the staphylococcal immunodominant antigen IsaA is a promising candidate for antibody-based therapy that could significantly improve the outcome of S. aureus infection in humans.
MATERIALS AND METHODS
Monoclonal antibody production.
Murine monoclonal antibodies were generated by the standard protocol of Synaptic Systems (Goettingen, Germany), using an enzyme-linked immunosorbent assay (ELISA) and Western blot screening. Briefly, three 8- to 10-week-old female BALB/c mice were immunized over a period of 17 days with the purified recombinant IsaA (rIsaA) protein. Cells from knee lymph nodes were fused with the mouse myeloma cell line P3X63Ag.653 (ATCC CRL-1580). The hybridoma elected in this study was cloned two times by limiting dilution. The monoclonal antibody was determined to be of the IgG1 subclass. The IgG1 antibody solution was purified by protein G fast-flow affinity chromatography as described elsewhere (17). Purified anti-IsaA IgG1 MAb (UK-66P) and murine isotype control antibody (IC) were further used.
Biosensor measurements.
To determine the affinity of the monoclonal antibody UK-66P to IsaA, the kinetics of binding of rIsaA to immobilized antibody was determined by means of label-free surface plasmon resonance using a Biacore 2000 system (GE Healthcare Europe GmbH, Freiburg, Germany). Reversible immobilization of the antibody UK-66P was performed using an anti-mouse Fc antibody covalently coupled in high density (18,700 resonance units [RU]) to a CM5 sensor surface according to the manufacturer's instructions (mouse antibody capture kit; GE Healthcare). The average amount of captured antibody UK-66P on the anti-mouse Fc surface corresponds to about 640 RU. A blank anti-mouse Fc surface was used as a control surface for monitoring unspecific binding and performing reference subtraction. Interaction analyses were performed using HBS-EP buffer (10 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM EDTA, 0.005% Tween 20). Sensorgrams were recorded at a flow rate of 30 μl/min at 25°C. Association and dissociation times were set to 3 and 15 min, respectively. The anti-Fc capturing surfaces were regenerated after each cycle by using short pulses of 10 mM glycine, pH 1.7. Affinities (equilibrium dissociation constant [KD]) and rate constants for association (ka) and for dissociation (kd) were calculated using BIAevaluation software 4.0.1, fitting the obtained sensorgrams to a 1:1 Langmuir binding model.
Bacteria.
The wild-type S. aureus strain MA12 and its isogenic IsaA insertion mutant strain MA12 ΔisaA::Emr (MA12 ΔisaA) were used throughout. The IsaA mutant strain served as an internal control for UK-66P specificity within the ex vivo and in vivo experiments. The protein A mutant Cowan I Δspa::Tcr (Cowan I Δspa) (DU 5889) was used to test cross-reactivity of UK-66P. The strains ANS46 (staphylococcal cassette chromosome mec type III [SCCmec III]), BK2464 (SCCmec II), HDE288 (SCCmec IV), MU50 (vancomycin-resistant S. aureus [VRSA]), MW2 (community-acquired methicillin-resistant S. aureus [CA-MRSA]), and EMSRA-15 (epidemic MRSA) served as additional controls for binding experiments. The strain USA300 (CA-MRSA) was also used for survival analysis. Single colonies of the respective strain were used to inoculate a 25-ml 2× YT (16 g tryptone, 10 g yeast extract, 5 g NaCl) broth culture overnight at 37°C. The culture was washed in phosphate-buffered saline (PBS), and serial dilution was performed to obtain a concentration of 106 or 107 CFU, which was confirmed by quantitative culture analysis. The bacteria were suspended in 0.1 ml or 0.5 ml of physiologic NaCl solution for infection experiments (see below).
Indirect immunofluorescence assay.
S. aureus MA12, its isogenic IsaA insertion mutant strain MA12 ΔisaA, and Cowan I Δspa were grown in Trypticase soy broth (TSB) to mid-log phase (optical density at 600 nm [OD600] of 0.5), and 1 ml of the culture was centrifuged for 5 min at 13,000 × g. The washed bacterial sediment was suspended in 1 ml PBS (10 mM sodium phosphate [pH 7.2], 0.15 M sodium chloride). An aliquot of the cell suspension (100 μl) was mixed with UK-66P in PBS and incubated at room temperature for 15 min. Then, 5 μl of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG(H+L) (Dianova, Hamburg, Germany) was added, and the mixture was incubated at room temperature for 30 min in a dark moisture chamber. After the bacteria were washed three times with 200 μl of PBS, the cells were suspended in 100 μl of PBS and viewed with a Zeiss Axioplan epifluorescence microscope. The images were captured with a low-light Intas MP Focus 5000 camera. The fluorescence and the phase-contrast images were processed in Adobe Photoshop CS2.
Quantitative determination of neutrophil activation and oxidative burst.
For the quantitative determination of murine neutrophil oxidative burst, the commercially available flow cytometry-based Phagoburst test kit was used according to the manufacturer's instruction (Orpegen Pharma, Heidelberg, Germany). The Phagoburst assay allows the determination of neutrophils that oxidize the fluorogenic substrate dihydrorhodamine 123 (DHR123). For oxidative burst analysis, heparinized blood was drawn from mice under general anesthesia by femoral vein puncture. Wild-type S. aureus strain MA12 and IsaA mutant strain MA12 ΔisaA were cultured in LB medium at 37°C and harvested in mid-logarithmic phase. Bacteria were washed twice with PBS and adjusted to 1 × 109 CFU/ml. Blood cells were stimulated with 20 μl of bacteria. Before challenge of neutrophils, bacteria were opsonized with different dilutions of UK-66P antibody (0.3 mg/ml or 0.6 mg/ml) or isotype control antibody (IC; dose equivalent) for 20 min at room temperature. In a forward/side scatter dot plot, the gate was set on granulocytes. The mean fluorescence intensity (MFI) correlating with oxidation quantity per individual neutrophil (oxidative burst) and the percentage of neutrophils (recruitment) having produced reactive oxygen metabolites were analyzed. For that purpose, a negative-control sample was used to set a marker (M1) for fluorescence 1 (FL1) so that less than 1% of the events were positive. In the study samples, the numbers of events above this marker position were counted. Cells were analyzed with a FACSCalibur flow cytometer and CellQuestPro and WinMDI 2.9 software.
Neutrophil intracellular survival assay.
Neutrophil intracellular survival assays were performed as follows. Bacterial cultures were washed twice in PBS, adjusted to 5 × 107 CFU, mixed with 100 μl whole mouse blood, and then incubated at 37°C in a water shaker. Before coincubation, bacteria were opsonized with UK-66P antibody (0.6 mg/ml) or isotype control antibody (IC; dose equivalent) for 20 min at room temperature. Gentamicin (final concentration, 400 μg/ml) and lysostaphin (final concentration, 100 μg/ml) were added after 45 min to kill extracellular bacteria. At 60 min, the contents of samples were withdrawn, centrifuged to pellet the neutrophils, and washed to remove the antibiotic medium. Neutrophils were then lysed in 1% saponin, and CFU were calculated by plating on TSB.
Catheter-related generalized infection in mice.
The Ethics Committee of the Lower Franconia authorities endorsed all animal studies. Age-, gender-, and weight-matched NMRI mice (Charles River, Sulzfeld, Germany) were used in the experiment. Mice were intraperitoneally anesthetized with xylazine (8 mg/kg body weight) and ketamine (100 mg/kg body weight), and a central venous catheter was surgically placed as already described (23). Twenty-four hours after surgery, the mice were inoculated via the catheter with 100 μl of an S. aureus suspension containing 1 × 107 CFU S. aureus bacteria. The bacterial suspension was allowed to dwell within the catheter lumen for 15 min. The content of the catheter was then flushed with 0.2 ml 0.9% saline. Treated mice received intravenously UK-66P (double-dose regimen, i.e., 15 mg/kg in a volume of 100 μl immediately and 24 h after bacterial challenge), and control mice received an isotype control antibody. Body weight and general appearance were assessed daily during the experiment. Five days postinoculation, the mice were euthanized by CO2 inhalation. Aseptically harvested organs were homogenized in 2 ml saline. Furthermore, the location of the catheter in the superior vena cava was confirmed, the explanted catheter irrigated with 2 ml saline, and the irrigation fluid collected. Serial dilutions of the organ homogenates and catheter fluid collections were cultured on mannitol salt phenol red agar plates for at least 48 h at 37°C. The number of bacteria recovered from each organ was plotted versus time postinfection as a Tukey box-and-whisker plot by using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
Sepsis survival model.
Age- and gender-matched NMRI mice (Charles River, Sulzfeld, Germany) were used in the experiment. Animals were challenged on day 0 by intravenous injection with 5 × 108 CFU of wild-type S. aureus USA300 and MA12 or IsaA mutant S. aureus MA12 ΔisaA. Treated mice received intravenously UK-66P (double-dose regimen, i.e., 15 mg/kg in a volume of 100 μl immediately and 24 h after bacterial challenge), and control mice received an isotype control antibody. Animals were monitored for 8 days, and lethal disease was recorded.
Statistical analysis.
Statistical analysis between treated and control groups was performed using the nonparametric Mann-Whitney test. Bacterial burdens in the organs were additionally analyzed by Kruskal-Wallis one-way analysis of variance by ranks with Dunn's posttest. The log rank Mantel-Cox test was used to analyze the statistical significance of the survival data. For all comparisons, a P value of <0.05 was considered statistically significant.
RESULTS
Affinity of anti-IsaA monoclonal antibodies.
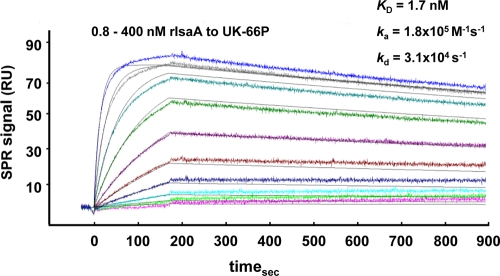
Biosensor analyses of multiple monoclonal antibodies to IsaA were initiated to characterize the binding profiles. Preliminary ELISAs screened a panel of hybridomas for binding to recombinant IsaA (rIsaA) protein. Antibodies that were ELISA positive were applied for a secondary screen. Biacore studies analyzed positive hybridoma clones for high-affinity interaction with rIsaA. By application of the settings described above, a specific anti-IsaA antibody solution (UK-66P) interacted with the 29-kDa rIsaA antigen with a high affinity and a slow off-rate, indicating a strong and highly specific interaction (Fig. 1). In the experiments with serial antibody dilutions in the range of 0.8 to 400 nM, an equilibrium dissociation constant (KD) of 1.7 nM was determined. Rate constants for association (ka) and dissociation (kd) of the interaction between UK-66P and rIsaA were determined to be 1.8 × 105 M−1 s−1 and 3.1 × 10−4 s−1, respectively.
FIG. 1.
Quantitative analysis of the interaction of UK-66P with rIsaA, carried out using surface plasmon resonance (SPR). Various concentrations of rIsaA (0.8 to 400 nM) were flushed over the antibody UK-66P, immobilized on the sensor chip surface. Sensorgrams were recorded at a flow rate of 30 μl/min at 25°C. From these sensorgrams, an equilibrium dissociation constant (KD) of 1.7 nM was determined. Rate constants for association (ka) and dissociation (kd) were determined to be 1.8 × 105 M−1 s−1 and 3.1 × 10−4 s−1, respectively. The figure shows one representative result from two independent experiments yielding identical kinetic constants.
Functional characterization of UK-66P.
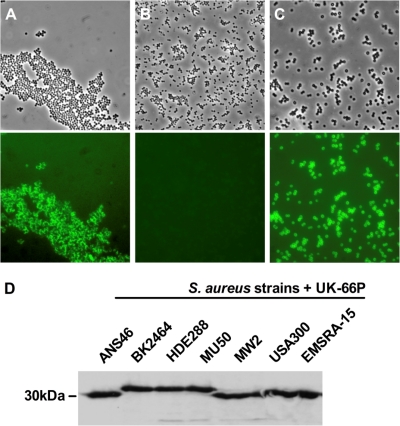
The monoclonal antibody UK-66P was selected for further studies based on its high binding affinity to rIsaA. UK-66P was determined to be of the IgG1 subclass, and indirect immunofluorescence on viable S. aureus showed its ability to bind to the cell surface-exposed IsaA antigen. Strain MA12, the isogenic IsaA knockout strain MA12 ΔisaA, and the protein A knockout strain Cowan I Δspa were used for staining. Bacteria were incubated with UK-66P followed by fluorescein isothiocyanate-labeled anti-mouse Ig, and binding of UK-66P to the bacterial surface was visualized by fluorescence microscopy (Fig. 2A to C). Viable cells of S. aureus MA12 interacted with the IsaA-specific monoclonal antibody UK-66P, while the IsaA mutant strain did not interact, as demonstrated by lack of fluorescence on these cells. The S. aureus protein A knockout strain also bound UK-66P, indicating no cross-reactivity with protein A.
FIG. 2.
Display of UK-66P on the staphylococcal surface and positive binding to representative clinical S. aureus isolates are shown. Binding of FITC-labeled anti-mouse IgG to UK-66P was analyzed by conventional microscopy of S. aureus (top) and corresponding fluorescence microscopy with the data superimposed (bottom). (A) UK-66P binds specifically to wild-type S. aureus MA12. (B) The isogenic mutant strain MA12 ΔisaA failed to bind UK-66P. (C) The S. aureus protein A knockout strain (Cowan I Δspa) binds UK-66P, indicating no antibody cross-reactivity with protein A. Magnification, ×100. (D) Reactivity of UK-66P to IsaA of representative clinical isolates strain ANS46 (SCCmec III), strain BK2464 (SCCmec II), strain HDE288 (SCCmec IV), strain MU50 (vancomycin-resistant S. aureus [VRSA]), strain MW2 (CA-MRSA), strain USA300 (CA-MRSA), and strain EMSRA-15 (epidemic MRSA) was constant, as verified by Western blotting.
The targeted IsaA antigen is found to be conserved among all sequenced methicillin-sensitive and methicillin-resistant staphylococcal strains. Subsequently, binding of UK-66P to a collection of seven representative isolates, i.e., strain ANS46 (SCCmec III), strain BK2464 (SCCmec II), strain HDE288 (SCCmec IV), strain MU50 (vancomycin-resistant S. aureus [VRSA]), strain MW2 (CA-MRSA), strain USA300 (CA-MRSA), and strain EMSRA-15 (epidemic MRSA), was exemplary tested. UK-66P reacted with IsaA of all tested strains, as verified by Western blotting (Fig. 2D).
UK-66P mediates activation of professional phagocytes.
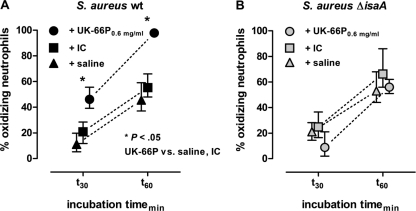
As the monoclonal antibody UK-66P recognizes IsaA on the surface of S. aureus, secondary experiments were performed to determine if the antibody UK-66P could also act as an opsonizing agent, resulting in the engulfment of bacteria by activated neutrophils. To quantify phagocytosis, we scored the percentage of oxidizing neutrophils. Neutrophils in whole mouse blood were coincubated with either wild-type or IsaA mutant S. aureus in the presence of UK-66P, isotype control antibody (IC), or saline at 30 and 60 min (Fig. 3A and B). At both time points, the fraction of oxidizing neutrophils in wild-type bacteria was significantly higher after coincubation with UK-66P than after coincubation with the isotype control (Mann-Whitney test, P < 0.05). More precisely, in the presence of UK-66P (0.6 mg/ml), 46.1% and 97.7% of neutrophils were activated during coincubation periods of 30 and 60 min, respectively (Fig. 3A). The mean percentages of oxidizing neutrophils were 20.9% and 55.2% with the IC after 30- and 60-min incubation times, respectively. In the absence of antibodies (saline), the mean percentages of oxidizing neutrophils were 11.8% and 45.5% after incubation times of 30 min and 60 min, respectively. Specificity controls followed the same experimental setup except for the use of IsaA mutant S. aureus (Fig. 3B). Under these conditions, no UK-66P-dependent increase in the percentage of activated neutrophils was detectable, compared to the results for IC- and saline-primed neutrophils. In the presence of UK-66P (0.6 mg/ml), 8.8% and 56% of neutrophils were activated during coincubation periods of 30 and 60 min, respectively (Fig. 3B). The mean percentages of oxidizing neutrophils were 24.8% and 66.2% with the IC after incubation times of 30 and 60 min, respectively. In the control incubated with saline, the mean percentages of oxidizing neutrophils were 21% and 53% after incubation times of 30 and 60 min, respectively.
FIG. 3.
UK-66P activates neutrophils. Mouse blood (100 μl) was incubated with 5 × 107 CFU of UK-66P-opsonized wild-type S. aureus MA12 or IsaA mutant S. aureus MA12 ΔisaA. Controls included isotype control antibody (IC)-opsonized and unopsonized (saline) bacteria. The percentages of activated and oxidizing neutrophils were determined using a DHR123/R123 assay with flow cytometric analysis. (A) At 30 and 60 min, the fraction of oxidizing neutrophils was significantly higher for the wild-type (wt) bacteria coincubated with UK-66P than for the IC- and saline-coincubated bacteria (Mann-Whitney test, P < 0.05). (B) As a specificity control, the respective percentages of oxidizing neutrophils were similar for UK-66P-, IC-, and saline-coincubated IsaA mutant bacteria. Error bars represent SDs.
Oxidative burst of neutrophils in response to stimulation with UK-66P.
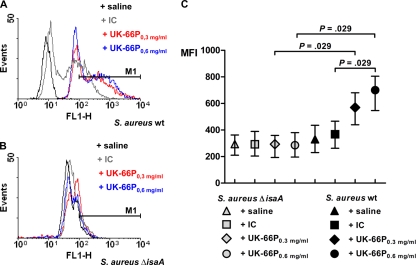
Since increased UK-66P-dependent neutrophil activation does not necessarily imply enhanced bacterial elimination, we measured the quantity of superoxide production per individual neutrophil during phagocytosis of S. aureus in professional phagocytes. Neutrophils in whole mouse blood were incubated with UK-66P-opsonized wild-type or IsaA mutant S. aureus. The production of reactive oxygen metabolites was determined via flow cytometric analysis by measuring the conversion of the fluorogenic substrate DHR123 to fluorescent rhodamine 123 (R123). Oxidative burst induced by wild-type S. aureus in the absence of extrinsic opsonin (saline) was defined as the baseline mean fluorescence intensity (MFI of 331). The results show that the oxidation quantity by wild-type S. aureus in the presence of UK-66P at 0.6 mg/ml was significantly higher than that after costimulation with IC (MFIs of 688 and 366, respectively; Mann-Whitney test, P = 0.029) (Fig. 4A and C). Furthermore, the production of superoxide was dose dependent, as half of the UK-66P dose (0.3 mg/ml) did not significantly increase the oxidation quantity compared to that with IC (MFIs of 564 and 366, respectively; Mann-Whitney test, P = 0.057) (Fig. 4A and C). The UK-66P-dependent specificity was demonstrated by a failure to produce a significant potentiation of oxidative burst following costimulation with the mutant S. aureus strain lacking IsaA expression. The level of oxidative burst was equal to that for isotype control antibody-opsonized or nonopsonized (saline) mutant bacteria, with MFIs of 284 and 287 for UK-66P at doses of 0.3 and 0.6 mg/ml, respectively, an MFI of 294 for IC, and an MFI of 290 for saline (Fig. 4B and C). Altogether, the oxidative burst activity per neutrophil stimulated with wild-type S. aureus in the presence of adequate UK-66P was approximately 2-fold higher than that for the controls. These results suggest that UK-66P is not only an activator for professional phagocytes but also a stimulus for oxidative burst activity in a dose-dependent fashion.
FIG. 4.
Oxidative burst of neutrophils is significantly enhanced in response to UK-66P-opsonized S. aureus. The oxidative burst activity of native mouse blood neutrophils was determined using a DHR123/R123 assay and flow cytometric analysis. (A) Oxidative burst was monitored by observing the fluorescence events (M1) in an FL1 overlay histogram. Wild-type S. aureus MA12-stimulated neutrophils with the addition of saline, isotype control antibody (IC), or UK-66P at concentrations of 0.3 mg/ml and 0.6 mg/ml. (B) As a specificity control for UK-66P, the oxidative burst was additionally monitored for IsaA mutant S. aureus MA12 ΔisaA-stimulated neutrophils with the addition of saline, IC, or UK-66P at concentrations of 0.3 mg/ml and 0.6 mg/ml. (C) Mean fluorescence intensity (MFI) of UK-66P-opsonized bacteria at concentrations of 0.3 and 0.6 mg/ml (equivalent to 15 mg/kg and 30 mg/kg body weight, respectively) compared to those for IC- or saline-opsonized wild-type and IsaA mutant bacteria. Significant differences are denoted (Mann-Whitney test). Error bars represent SDs.
Effect of UK-66P on the bactericidal activity of professional phagocytes against S. aureus.
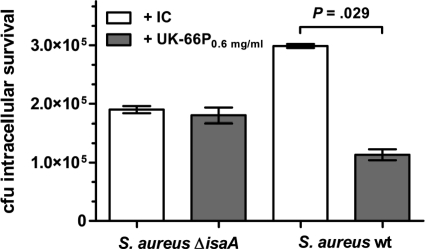
Using an ex vivo system, we determined if the increased oxidant activity of UK-66P translates to enhanced bacterial killing by an innate immune mechanism. After the addition of either UK-66P- or IC-opsonized wild-type or IsaA mutant S. aureus to 100 μl whole mouse blood for 30 min, samples were plated to enumerate survivors after neutrophil lysis. The initial inoculum applied consisted of 5 × 107 bacteria. Opsonization of bacteria with UK-66P significantly enhanced bacterial killing by whole-blood neutrophils compared to that for IC-opsonized bacteria (mean CFU ± standard deviation [SD], 1.13 × 105 ± 9.38 × 103 and 2.99 × 105 ± 3.65 × 103, respectively; Mann-Whitney test, P = 0.0286) (Fig. 5). This effect was not explainable by differences in phagocytosis rate, since levels of uptake of wild-type S. aureus were fairly comparable in the presence of either UK-66P or IC (data not shown). Therefore, differences in phagocytotic killing were clearly attributable to binding of UK-66P to IsaA, since UK-66P- and IC-treated IsaA mutant S. aureus produced similar results (mean CFU ± SD, 1.8 × 105 ± 1.3 × 104 and 1.9 × 105 ± 6.1 × 103, respectively) (Fig. 5).
FIG. 5.
Effect of UK-66P on survival of S. aureus within neutrophils in whole mouse blood. Mouse blood (100 μl) was incubated with 5 × 107 CFU of UK-66P-opsonized wild-type S. aureus MA12 or IsaA mutant S. aureus MA12 ΔisaA. Controls included isotype control antibody (IC)-opsonized bacteria. The number of neutrophil-associated CFU was determined by serial dilution and plating on TSB. UK-66P-opsonized wild-type S. aureus bacteria were killed significantly better than IC-opsonized bacteria (mean CFU ± SD, 1.13 × 105 ± 9.38 × 103 and 2.99 × 105 ± 3.65 × 103, respectively; Mann-Whitney test, P = 0.0286). UK-66P- and IC-opsonized IsaA mutant S. aureus produced similar results (mean CFU ± SD, 1.8 × 105 ± 1.3 × 104 and 1.9 × 105 ± 6.1 × 103, respectively). Error bars represent SDs.
Therapeutic efficacy of UK-66P in a catheter-related sepsis model.
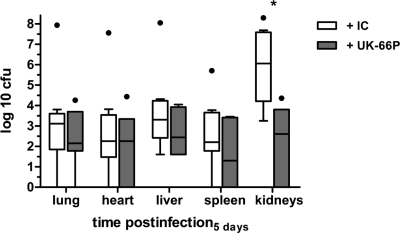
In order to stress the importance of the ex vivo results, we tested the therapeutic efficacy of UK-66P in a sublethal catheter-related sepsis model in mice. Bacterial challenge and treatment were executed 24 h after microsurgical implantation of a catheter into the internal jugular vein of mice. Animals with weight loss greater than 5% after surgery compared to baseline values were excluded from the study to avoid compounding effects of anesthesia or surgical preparation. All elected mice were challenged with 1 × 107 CFU S. aureus MA12 via the catheter. Mice were then treated via the catheter with UK-66P (7 mice; double-dose regimen, i.e., 15 mg/kg in a volume of 100 μl immediately and 24 h after bacterial challenge to yield an effective dose of 30 mg/kg) or IC (9 mice; dose and volume equivalent). Mice were sacrificed 5 days after S. aureus challenge. Numbers of viable bacteria in the liver, lung, heart, spleen, and kidneys following therapy with either UK-66P or IC were quantitated and graphed against time postinfection (Fig. 6). In addition, the catheters were collected and indwelling bacteria obtained. Bacteria from isotype control antibody-treated mice preferentially colonized the kidneys over the infection period. Analysis of the bacterial load in this organ system revealed a significant difference in the numbers of recovered wild-type S. aureus bacteria after UK-66P and IC treatment (Kruskal-Wallis test with Dunn's posttest, P < 0.05), with a greater recovery of bacteria in IC-treated animals. The protective effect of the UK-66P treatment became apparent in the kidneys, resulting in approximately 3 log CFU reduction compared to IC. In contrast, the CFU in lung, liver, heart, and spleen did not differ between UK-66P- and IC-treated mice. The specific infection burden within each organ system is presented in Table 1. In addition, the number of bacteria obtained from the catheters in the IC group was not significantly different from the number of bacteria obtained from catheters in the UK-66P treatment group (mean CFU [range], 2.8 × 105 [1.0 × 105-3.1 × 106] and 1.3 × 106 [1.3 × 105-2.3 × 106], respectively).
FIG. 6.
Bacterial burden after UK-66P and isotype control antibody treatment in lung, heart, liver, spleen, and kidneys 5 days after infection with S. aureus MA12 in the catheter-related infection model. The mice (7 or 9 per group) were inoculated via catheter with 1 × 107 CFU bacteria. *, P < 0.05 compared with IC-treated mice, by Kruskal-Wallis testing with posthoc Dunn's multiple-comparison testing. Data are graphed as a box-and-whisker plot.
TABLE 1.
Multiorgan infection caused by S. aureus after UK-66P and isotype control antibody treatment in the catheter-related sepsis model
| Organ | Mean CFU (range) after S. aureus MA12 treatment with: |
Pa | |
|---|---|---|---|
| Isotype control | UK-66P | ||
| Kidneys | 1.1 × 106 (7.5 × 101-1.4 × 108) | 4.0 × 102 (0-4.2 × 107) | <0.05* |
| Lung | 1.3 × 103 (0-8.6 × 107) | 1.4 × 102 (0-1.8 × 104) | NS |
| Liver | 2.0 × 103 (4.0 × 101-1.1 × 108) | 2.8 × 102 (0-4.0 × 104) | NS |
| Spleen | 1.6 × 102 (0-5.1 × 105) | 2.0 × 101 (0-2.9 × 103) | NS |
| Heart | 1.8 × 102 (0-3.6 × 107) | 1.8 × 102 (0-3.3 × 105) | NS |
*, significant difference by Kruskal-Wallis test with Dunn's posttest; NS, not significant.
UK-66P treatment protects animals against lethal challenge with S. aureus.
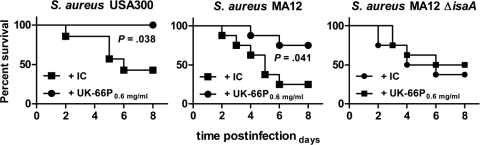
In order to corroborate the obtained results, a different experimental model was introduced. Effective immunotherapy for S. aureus should protect mice against a lethal challenge of S. aureus. Furthermore, immunotherapy must be effective against a wide range of clinically relevant isolates. Therefore, S. aureus strain USA300 was additionally included. USA300 is one of the most frequent causes of community-associated infections in the United States (26), and protection against this strain is of crucial importance for immunotherapeutic efforts. To test whether UK-66P immunotherapy protects against lethal-challenge infections, mice were treated intravenously with UK-66P or isotype control antibody. Challenges of 5 × 108 CFU of wild-type S. aureus USA300, wild-type MA12, and the IsaA mutant (MA12 ΔisaA) were administered intravenously, and mice were monitored for 8 days. Isotype control antibody treatment in wild-type S. aureus USA300 and MA12 had no effect, with 57% and 75% mortality rates, respectively, over the study period (Fig. 7). In contrast, UK-66P treatment protected against challenge with S. aureus USA300 (0% mortality rate; log rank Mantel-Cox test, P = 0.038) and S. aureus MA12 (25% mortality rate; log rank Mantel-Cox test, P = 0.041). Clearly, the immunotherapeutic potential of UK-66P was demonstrated by the fact that mice challenged with S. aureus MA12 ΔisaA were not protected by anti-IsaA antibodies, compared to results for isotype control antibody-treated mice (50 and 38% mortality rates, respectively; log rank Mantel-Cox test, P = 0.55). These results suggest that passive immunotherapy with UK-66P monoclonal antibody can generate increased protection against lethal challenge.
FIG. 7.
Immunotherapy with UK-66P generates protection against lethal S. aureus challenge. Mice (7 or 8 per group) were given UK-66P antibody preparation or isotype control antibody (IC). Animals were challenged with 5 × 108 CFU of wild-type S. aureus USA300, MA12, or IsaA mutant S. aureus MA12 ΔisaA by intravenous injection, and then they were monitored for 8 days. The significance of protection compared to that for animals receiving control IgG1 was measured with the log rank Mantel-Cox test.
DISCUSSION
S. aureus causes a wide variety of diseases, ranging from superficial skin lesions to life-threatening invasive infections, and those at high risk include individuals with short-term or permanent states of immunosuppression, such as patients undergoing surgery, cancer patients, intensive care unit (ICU) patients, and patients with dialysis dependence, diabetes, or HIV (28, 30). There is still a high mortality rate associated with severe S. aureus infections, especially those caused by multiple-antibiotic-resistant strains. Therefore, active or passive immunotherapy has been regarded as a promising adjunctive treatment approach that can bolster the immune response and circumvent rising rates of antimicrobial drug resistance. Experimental evidence strongly supports the concept, but successful clinical trials are still pending (35).
In the present report, the therapeutic efficacy of the mouse monoclonal antibody UK-66P, binding the immunodominant antigen IsaA, has been evaluated in vivo. The UK-66P hyperimmune preparation, passively applied in two different experimental mouse models, significantly lowered S. aureus burden in kidneys and protected mice against lethal challenge. The kidneys are the predominant infection site in mice after intravenous challenge with S. aureus (32, 39). Lowering the bacterial load in this single organ system limits the establishment of infectious foci and thereby curbs the severity of staphylococcal infections. The specific IgG1 subclass preparation was chosen for the experimental studies, as the secondary IgG1 immune response seems to be crucial in S. aureus infections (16, 29). High-affinity binding kinetics of antibodies was demonstrated by Biacore analysis.
Further investigation was conducted to clarify the UK-66P antibody mode of action. Obviously, UK-66P has no direct blocking function to IsaA. Instead, binding of UK-66P to IsaA on the cell surface triggers phagocytosis and subsequent intracellular killing of S. aureus. The bactericidal activity of neutrophils after UK-66P treatment correlates with augmentation in the respiratory burst of these cells. The observation of neutrophil killing upon exposure to high concentrations of superoxide and hydrogen peroxide is concordant with other studies (13). Likewise, mice lacking NADPH oxidase activity inefficiently clear S. aureus infections (33). The ex vivo results of the antibody-mediated phagocytosis transferred to the catheter-related infection and sepsis model may explain the effective reduction of S. aureus bacteria in the treated hosts, with improved survival. Phagocytosis and intracellular killing of S. aureus decrease the initial bacterial proportion, helping to displace the infective balance toward the host organism (1). In addition, the percentage of surviving S. aureus bacteria inside various cells, including phagocytes, contributes to the pathogenesis of staphylococcal infections (11). In our ex vivo studies, the UK-66P immunotherapy reduced the number of viable S. aureus bacteria within phagocytes by one-third compared with the results for the control. In this context, a recent study examined the comparative lethality of clinical S. aureus isolates in a mouse septic model, and lowering the infectious dose of viable bacteria by approximately one-third led to a marked decrease in mortality within a given time (40).
Originally, IsaA was identified in a screening approach for immunodominant antigens that may serve as vaccine candidates or targets for passive immunotherapy (24). The surface-associated IsaA antigen is found to be conserved among all sequenced staphylococcal strains, including community-associated methicillin-resistant S. aureus strains, such as USA300 (41). Moreover, we demonstrated binding of UK-66P to IsaA of major clinical S. aureus lineages. In vivo data suggest that antibody reactivity raised to IsaA tends to be a ubiquitous mechanism in host immune defense against invasive S. aureus infections (9, 24). Importantly, reactive IgG titers against IsaA are significantly increased in serum samples obtained from individuals with confirmed disease compared to those from healthy individuals. Furthermore, comparison of IgG titers against IsaA from healthy noncarriers and carriers showed that there are significantly increased reactive titers in the latter group (6). We hypothesize that the amount of anti-IsaA-specific antibody represents one important component that modulates the outcome of human systemic S. aureus infection. Nevertheless, the significance of the antibody response to IsaA in a human infection has not yet been defined conclusively.
Selection of a protective target antigen is the key factor in the development of an effective vaccine or passive immunization strategy. Traditionally, virulence-associated factors have been regarded as promising vaccine candidates due to their potential role in pathogenesis. This concept is especially questionable for S. aureus, since the pathogen expresses a broad range of toxins and adhesins that are important in different phases of disease but are not solely essential for virulence, as others may compensate for a loss of function, e.g., by blocking antibodies. Alternatively, naturally raised antibodies in humans exposed to staphylococcal invasive infections may select antigens as potential targets for immunotherapy (6, 24, 27, 46). However, as native protective immunity to staphylococcal infections does not exist to a significant degree and individuals with colonization of invasive infections elicit a unique immune response to different S. aureus proteins, selection of a universal protective antigen is highly challenging (14, 42). Moreover, certain patients develop recurrent infections, with the same strain in up to 35% of cases, despite a broad spectrum of existing antistaphylococcal antibodies (15). On the other hand, those patients that become infected with their own strain have a significantly greater chance to survive invasive S. aureus infections (45).
Although our approach has been demonstrated to be effective in two animal model systems, clinical trials are still lacking. Very recently, we have identified the antigen binding region of UK-66P and grafted the mouse complementarity-determining regions into human variable regions that were joined to human constant regions (unpublished data). Studies on binding and functional activity of the humanized antibody are in progress. However, some important issues remain to be addressed before this antibody can proceed to clinical trials. For example, the optimal treatment regimen has to be determined, as does the general accessibility of IsaA in all strains, including those of different capsule types.
Together, the results presented in this study have demonstrated that the immunotherapy strategy focusing on IsaA, targeting only a single epitope of S. aureus, is effective in reducing bacteria in experimental infections, due to enhanced opsonophagocytosis with effective intracellular killing. Based on the mechanism of action, the ability of therapeutic antibodies to work cooperatively with the immune system in this way has important implications for the selection of IsaA as a target for immunotherapy of staphylococcal infections. Further analysis of the humanized antibody will help to establish the suitability of this immunotherapy.
Acknowledgments
We are indebted to H. Merkert and U. Wallner for expert technical assistance and to D. O'Callaghan for critical reading of the manuscript and helpful remarks. We thank Bhanu Sinha (Institute of Hygiene and Microbiology, University of Würzburg, Germany) for providing the protein A mutant strain.
This study was supported by a grant from the Else-Kröner-Fresenius Stiftung and by grants from the Bundesministerium für Wirtschaft und Technologie (BMWi) Exist, the Deutsche Forschungsgemeinschaft (SFB TR34), and EU StaphDynamics.
Footnotes
Published ahead of print on 18 October 2010.
REFERENCES
- 1.Aderem, A. 2003. Phagocytosis and the inflammatory response. J. Infect. Dis. 187(Suppl. 2):S340-S345. [DOI] [PubMed] [Google Scholar]
- 2.Bubeck Wardenburg, J., T. Bae, M. Otto, F. R. Deleo, and O. Schneewind. 2007. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13:1405-1406. [DOI] [PubMed] [Google Scholar]
- 3.Bubeck Wardenburg, J., and O. Schneewind. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnie, J. P., R. C. Matthews, T. Carter, E. Beaulieu, M. Donohoe, C. Chapman, P. Williamson, and S. J. Hodgetts. 2000. Identification of an immunodominant ABC transporter in methicillin-resistant Staphylococcus aureus infections. Infect. Immun. 68:3200-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall, A., E. Dadachova, and L. A. Pirofski. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695-703. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, S. R., K. J. Brummell, M. J. Horsburgh, P. W. McDowell, S. A. Mohamad, M. R. Stapleton, J. Acevedo, R. C. Read, N. P. Day, S. J. Peacock, J. J. Mond, J. F. Kokai-Kun, and S. J. Foster. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J. Infect. Dis. 193:1098-1108. [DOI] [PubMed] [Google Scholar]
- 7.Dryla, A., S. Prustomersky, D. Gelbmann, M. Hanner, E. Bettinger, B. Kocsis, T. Kustos, T. Henics, A. Meinke, and E. Nagy. 2005. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin. Diagn. Lab. Immunol. 12:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubrac, S., and T. Msadek. 2004. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J. Bacteriol. 186:1175-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etz, H., D. B. Minh, T. Henics, A. Dryla, B. Winkler, C. Triska, A. P. Boyd, J. Sollner, W. Schmidt, U. von Ahsen, M. Buschle, S. R. Gill, J. Kolonay, H. Khalak, C. M. Fraser, A. von Gabain, E. Nagy, and A. Meinke. 2002. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 99:6573-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fattom, A., S. Fuller, M. Propst, S. Winston, L. Muenz, D. He, R. Naso, and G. Horwith. 2004. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23:656-663. [DOI] [PubMed] [Google Scholar]
- 11.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 12.Hall, A. E., P. J. Domanski, P. R. Patel, J. H. Vernachio, P. J. Syribeys, E. L. Gorovits, M. A. Johnson, J. M. Ross, J. T. Hutchins, and J. M. Patti. 2003. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect. Immun. 71:6864-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 14.Holtfreter, S., T. T. Nguyen, H. Wertheim, L. Steil, H. Kusch, Q. P. Truong, S. Engelmann, M. Hecker, U. Volker, A. van Belkum, and B. M. Broker. 2009. Human immune proteome in experimental colonization with Staphylococcus aureus. Clin. Vaccine Immunol. 16:1607-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, S. S., D. J. Diekema, D. K. Warren, G. Zuccotti, P. L. Winokur, S. Tendolkar, L. Boyken, R. Datta, R. M. Jones, M. A. Ward, T. Aubrey, A. B. Onderdonk, C. Garcia, and R. Platt. 2008. Strain-relatedness of methicillin-resistant Staphylococcus aureus isolates recovered from patients with repeated infection. Clin. Infect. Dis. 46:1241-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishizaka, A., K. Kojima, Y. Sakiyama, S. Matsumoto, K. Kuwajima, Y. Wagatsuma, R. Shibata, and K. Joh. 1992. Hyper-response of serum IgG1 to Staphylococcus aureus peptidoglycan in patients with hyper-IgE syndrome. Clin. Exp. Immunol. 87:53-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungbauer, A., C. Tauer, M. Reiter, M. Purtscher, E. Wenisch, F. Steindl, A. Buchacher, and H. Katinger. 1989. Comparison of protein A, protein G and copolymerized hydroxyapatite for the purification of human monoclonal antibodies. J. Chromatogr. 476:257-268. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman, D. 2006. Veronate (Inhibitex). Curr. Opin. Invest. Drugs 7:172-179. [PubMed] [Google Scholar]
- 19.Keller, M. A., and E. R. Stiehm. 2000. Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 13:602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly, J. 2000. Immunotherapy against antibiotic-resistant bacteria: the Russian experience with an antistaphylococcal hyperimmune plasma and immunoglobulin. Microbes Infect. 2:1383-1392. [DOI] [PubMed] [Google Scholar]
- 21.Kelly-Quintos, C., L. A. Cavacini, M. R. Posner, D. Goldmann, and G. B. Pier. 2006. Characterization of the opsonic and protective activity against Staphylococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect. Immun. 74:2742-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuklin, N. A., D. J. Clark, S. Secore, J. Cook, L. D. Cope, T. McNeely, L. Noble, M. J. Brown, J. K. Zorman, X. M. Wang, G. Pancari, H. Fan, K. Isett, B. Burgess, J. Bryan, M. Brownlow, H. George, M. Meinz, M. E. Liddell, R. Kelly, L. Schultz, D. Montgomery, J. Onishi, M. Losada, M. Martin, T. Ebert, C. Y. Tan, T. L. Schofield, E. Nagy, A. Meineke, J. G. Joyce, M. B. Kurtz, M. J. Caulfield, K. U. Jansen, W. McClements, and A. S. Anderson. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz, U., C. Huttinger, T. Schafer, W. Ziebuhr, A. Thiede, J. Hacker, S. Engelmann, M. Hecker, and K. Ohlsen. 2008. The alternative sigma factor sigma B of Staphylococcus aureus modulates virulence in experimental central venous catheter-related infections. Microbes Infect. 10:217-223. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz, U., K. Ohlsen, H. Karch, M. Hecker, A. Thiede, and J. Hacker. 2000. Human antibody response during sepsis against targets expressed by methicillin resistant Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 29:145-153. [DOI] [PubMed] [Google Scholar]
- 25.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 26.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Doring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 28.Menon, K. V., M. S. Whiteley, P. Burden, and R. B. Galland. 1999. Surgical patients with methicillin resistant Staphylococcus aureus infection: an analysis of outcome using P-POSSUM. J. R. Coll. Surg. Edinb. 44:161-163. [PubMed] [Google Scholar]
- 29.Monteil, M. A., A. S. Kaniuk, and J. R. Hobbs. 1990. Staphylococcal opsonization and anti-Staphylococcus aureus IgG subclass antibodies in patients with severe or recurrent S. aureus infections. FEMS Microbiol. Immunol. 2:259-262. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, G. J., R. Pararajasingam, A. Nasim, M. J. Dennis, and R. D. Sayers. 2001. Methicillin-resistant Staphylococcus aureus infection in vascular surgical patients. Ann. R. Coll. Surg. Engl. 83:158-163. [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlsen, K., and U. Lorenz. 2007. Novel targets for antibiotics in Staphylococcus aureus. Future Microbiol. 2:655-666. [DOI] [PubMed] [Google Scholar]
- 32.Patti, J. M. 2004. A humanized monoclonal antibody targeting Staphylococcus aureus. Vaccine 22(Suppl. 1):S39-S43. [DOI] [PubMed] [Google Scholar]
- 33.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 34.Projan, S. J., M. Nesin, and P. M. Dunman. 2006. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr. Opin. Pharmacol. 6:473-479. [DOI] [PubMed] [Google Scholar]
- 35.Rupp, M. E., H. P. Holley, Jr., J. Lutz, P. V. Dicpinigaitis, C. W. Woods, D. P. Levine, N. Veney, and V. G. Fowler, Jr. 2007. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 51:4249-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakata, N., S. Terakubo, and T. Mukai. 2005. Subcellular location of the soluble lytic transglycosylase homologue in Staphylococcus aureus. Curr. Microbiol. 50:47-51. [DOI] [PubMed] [Google Scholar]
- 37.Schaffer, A. C., R. M. Solinga, J. Cocchiaro, M. Portoles, K. B. Kiser, A. Risley, S. M. Randall, V. Valtulina, P. Speziale, E. Walsh, T. Foster, and J. C. Lee. 2006. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect. Immun. 74:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stapleton, M. R., M. J. Horsburgh, E. J. Hayhurst, L. Wright, I. M. Jonsson, A. Tarkowski, J. F. Kokai-Kun, J. J. Mond, and S. J. Foster. 2007. Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J. Bacteriol. 189:7316-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stranger-Jones, Y. K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 103:16942-16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao, M., H. Yamashita, K. Watanabe, and T. Nagatake. 1999. Possible virulence factors of Staphylococcus aureus in a mouse septic model. FEMS Immunol. Med. Microbiol. 23:135-146. [DOI] [PubMed] [Google Scholar]
- 41.Tenover, F. C., L. K. McDougal, R. V. Goering, G. Killgore, S. J. Projan, J. B. Patel, and P. M. Dunman. 2006. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 44:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verkaik, N. J., H. A. Boelens, C. P. de Vogel, M. Tavakol, L. G. Bode, H. A. Verbrugh, A. van Belkum, and W. J. van Wamel. 2010. Heterogeneity of the humoral immune response following Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 29:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vernachio, J., A. S. Bayer, T. Le, Y. L. Chai, B. Prater, A. Schneider, B. Ames, P. Syribeys, J. Robbins, and J. M. Patti. 2003. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob. Agents Chemother. 47:3400-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vernachio, J. H., A. S. Bayer, B. Ames, D. Bryant, B. D. Prater, P. J. Syribeys, E. L. Gorovits, and J. M. Patti. 2006. Human immunoglobulin G recognizing fibrinogen-binding surface proteins is protective against both Staphylococcus aureus and Staphylococcus epidermidis infections in vivo. Antimicrob. Agents Chemother. 50:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 46.Vytvytska, O., E. Nagy, M. Bluggel, H. E. Meyer, R. Kurzbauer, L. A. Huber, and C. S. Klade. 2002. Identification of vaccine candidate antigens of Staphylococcus aureus by serological proteome analysis. Proteomics 2:580-590. [DOI] [PubMed] [Google Scholar]