Abstract
The diploid genome of the pathogenic yeast Candida albicans exhibits a high degree of heterozygosity. Genomic alterations that result in a loss of heterozygosity at specific loci may affect phenotypes and confer a selective advantage under certain conditions. Such genomic rearrangements can also occur during the construction of C. albicans mutants and remain undetected. The SAP2 gene on chromosome R encodes a secreted aspartic protease that is induced and required for growth of C. albicans when proteins are the only available nitrogen source. In strain SC5314, the two SAP2 alleles are functionally divergent because of differences in their regulation. Basal expression of the SAP2-2 allele, but not the SAP2-1 allele, provides the proteolytic degradation products that serve as inducers for full SAP2 induction. A triple mutant lacking the SAP4, SAP5, and SAP6 genes, which are located on chromosome 6, has previously been reported to have a growth defect on proteins, suggesting that one of the encoded proteases is required for SAP2 expression. Here we show that this sap4Δ sap5Δ sap6Δ mutant has become homozygous for chromosome R and lost the SAP2-2 allele. Replacement of one of the SAP2-1 copies in this strain by SAP2-2 and its regulatory region restored the ability of the sap4Δ sap5Δ sap6Δ mutant to utilize proteins as the sole nitrogen source. This is an illustrative example of how loss of heterozygosity at a different genomic locus can cause the mutant phenotype attributed to targeted deletion of a specific gene in C. albicans.
The yeast Candida albicans is a harmless colonizer of the gastrointestinal tract in most healthy people, but it may also become a pathogen and cause infections of many different body locations, especially in immunocompromised patients. C. albicans can efficiently adapt to alterations in its surroundings, for example, when it encounters a new host niche. This is achieved by sensing changes in the environment and reprogramming gene expression in an appropriate fashion (2). In addition, genomic alterations can facilitate adaptation to specific conditions in a host, as exemplified by the selection of strains that have acquired mutations conferring drug resistance during long-term therapy of oral candidiasis in AIDS patients (52).
The diploid genome of C. albicans consists of 8 pairs of homologous chromosomes that exhibit a high degree of heterozygosity (22). In some cases, it has been demonstrated that allelic differences result in functional differences in the encoded proteins. For example, the two alleles of the ALS3 gene of the sequenced C. albicans model strain SC5314 contain different copy numbers of a tandem repeat sequence and differ in their capacities to mediate adhesion to host cells (35). Other examples are the polymorphic CDR2 alleles of other strains, which encode efflux pumps that differ in their drug transport efficiencies (19). Mitotic recombination or gene conversion can result in loss of heterozygosity (LOH) for certain chromosomal regions, and strains can also become homozygous for whole chromosomes by loss of one of the two homologs and duplication of the other one (13, 21). While LOH decreases the genetic repertoire of a strain, it can also result in new phenotypes and generate strains with capacities that are absent from their heterozygous progenitors. The most striking example is LOH at the mating type locus, which generates MTLa/MTLa or MTLα/MTLα strains that, due to the absence of the a1-α2 repressor, can switch to the mating-competent opaque cell type (28, 32). Other examples are clinical isolates that, after acquiring a resistance mutation in one allele of a gene during antimycotic treatment, have become homozygous for the mutated allele, which further increases their resistance (7, 8, 9, 15, 18, 29, 33, 51).
C. albicans possesses a gene family encoding secreted aspartic proteases (Saps). These enzymes are thought to contribute to the virulence of the fungus by degrading tissue barriers, destroying host defense proteins, or providing nutrients (34). The individual SAP genes are differentially expressed at various stages and in different types of experimental infections and may therefore have specific functions (46). In vitro, expression of the SAP2 gene is induced in medium containing proteins as the sole nitrogen source and C. albicans sap2Δ mutants cannot grow under these conditions (20, 47, 49). Induction of the SAP2 promoter depends on the transcription factor Stp1, which is proteolytically activated in the presence of micromolar concentrations of extracellular amino acids that may be generated by basal Sap activity and signal the availability of proteins in the environment (31). Interestingly, in strain SC5314, the two alleles of the SAP2 gene are not functionally equivalent and heterozygous mutants lacking one or the other allele display a striking phenotypic difference. While deletion of the SAP2-1 allele does not affect the growth of the cells, inactivation of the SAP2-2 allele abolishes the ability of the mutants to utilize proteins as the sole nitrogen source (47, 49). This phenotypic difference is not caused by the different activities of the encoded proteins, as expression of either SAP2 allele from a constitutive promoter rescues the growth defect of a sap2Δ-null mutant. Instead, basal expression of the SAP2-2 allele, but not the SAP2-1 allele, provides the proteolytic degradation products that are required for full SAP2 induction. In the absence of the SAP2-2 allele, the SAP2-1 promoter is not induced unless another source of extracellular proteolytic activity is provided, e.g., by adding protease-containing supernatant from a wild-type strain to the culture medium. In contrast, induction of the SAP2-2 promoter does not require the presence of a functional SAP2-1 allele. The difference in the regulation of the SAP2-1 and SAP2-2 alleles is reflected by sequence differences in their promoter regions (47).
A C. albicans triple mutant in which the SAP4, SAP5, and SAP6 genes were inactivated by targeted gene deletion (strain DSY459) was also found to have a severe growth defect in medium containing protein as the sole nitrogen source, and the authors suggested that one of the encoded proteases is required for SAP2 expression (40). However, no such growth defect was observed in independently generated sap4Δ sap5Δ sap6Δ mutants constructed in the same strain background, which expressed SAP2 at wild-type levels (27). In the present work, we unravel the reason for this discrepancy and show that the growth defect of the sap4Δ sap5Δ sap6Δ triple mutant DSY459 is not caused by the absence of the SAP4, SAP5, and SAP6 genes but by a different genomic alteration, namely, LOH for chromosome R coupled with loss of the SAP2-2 allele.
MATERIALS AND METHODS
Strains and growth conditions.
The C. albicans strains used in this study are listed in Table 1. All strains were stored as frozen stocks with 15% glycerol at −80°C and subcultured on YPD agar plates (10 g yeast extract, 20 g peptone, 20 g glucose; 20 g agar per liter) at 30°C. Strains were routinely grown in YPD liquid medium at 30°C in a shaking incubator. To test for growth of the strains on bovine serum albumin (BSA) as the sole nitrogen source, YPD overnight cultures were diluted 10−3 in YCB-BSA (23.4 g yeast carbon base, 4 g BSA per liter, pH 4.0) and grown at 30°C.
Table 1.
C. albicans strains used in this study
| Strain(s) | Parent | Genotype or description | Reference |
|---|---|---|---|
| SC5314 | Wild-type parental strain | 16 | |
| DSY459 | SC5314 | ura3Δ::imm434/ura3Δ::imm434 | 40 |
| sap4Δ::hisG/sap4Δ::hisG | |||
| sap5Δ::hisG/sap5Δ::hisG-URA3-hisG | |||
| sap6Δ::hisG/sap6Δ::hisG | |||
| SAP456MS4A and SAP456MS4B | SC5314 | sap4Δ::FRT/sap4Δ::FRT | 27 |
| sap5Δ::FRT/sap5Δ::FRT | |||
| sap6Δ::FRT/sap6Δ::FRT | |||
| SAP2MS2A | SC5314 | sap2-1Δ::FRT/SAP2-2 | 49 |
| SAP2MS2B | SC5314 | SAP2-1/sap2-2Δ::FRT | 49 |
| SAP2MS4A | SAP2MS2A | sap2-1Δ::FRT/sap2-2Δ::FRT | 49 |
| SAP2MS4B | SAP2MS2B | sap2-1Δ::FRT/sap2-2Δ::FRT | 49 |
| YJB10698 | SC5314 | Homozygous for chromosome R (with SAP2-1); gal1::URA3/gal1::URA3 | 26 |
Plasmid constructions.
The previously described plasmid pSAP2-1K (47) contains the coding region of the SAP2-1 allele under the control of the ACT1 promoter. Replacement of a SalI-PstI fragment containing the URA3 marker by an XhoI-PstI fragment containing the C. albicans-adapted SAT1 (caSAT1) selection marker (38) resulted in pSAP2ex5. The SAP2-2 coding region and 4.3 kb of its upstream region were amplified from genomic DNA of strain SAP2MS2A (sap2-1Δ/SAP2-2) with the primers SAP2P10 and SAP2ex2 (Table 2), digested at the introduced ApaI site and at a KpnI site at nucleotide (nt) +830 of the SAP2 coding region, and ligated together with a KpnI-PstI fragment from pSAP2ex5 containing the C-terminal part of the SAP2 coding region, the ACT1 transcription termination sequence, and the caSAT1 marker into ApaI/PstI-digested pSAP2KS2 (49), thereby generating pSAP2KS4. The SAP2-1 allele and its upstream region were amplified with the same primers from genomic DNA of strain SAP2MS2B (SAP2-1/sap2-2Δ) and cloned in an identical fashion to obtain pSAP2KS5. The complete sequences of the SAP2 promoter regions contained in plasmids pSAP2KS4 and pSAP2KS5 were determined using the primers listed in Table 2.
Table 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| ACT38a | 5′-ATATGGGCCCTGCAGACATTTTATGATGGAATGAATGGG-3′ |
| BOI2-1a | 5′-CCAAAAACATTTACTCAATTAATAACCA-3′ |
| BOI2-2a,b | 5′-ACTAGTGATACTTGCATATTGGGAATTT-3′ |
| CRZ2-1longa,b | 5′-GTTAATCATTATCTCGAGAATGTTATCAACCATGTCTAATTTGCC-3′ |
| CRZ2-2longa | 5′-GTTAATCATTATAGATCTATTTATTAGATTGTAATAATTTTTTAA-3′ |
| FGR32-1a | 5′-GATGCATTTGCAGATTTAATTTCAATCT-3′ |
| FGR32-2a,b | 5′-CAAGATCTAATTTTGTCTTCAATCTTGT-3′ |
| SAP2ex2a | 5′-ACCCCGGATCCTTAGGTCAAGGCAGAAATACTGGAAGC-3′ |
| SAP2P7b | 5′-GGGGTCTAGAAAGTGAAACGGGTAATATTG-3′ |
| SAP2P8b | 5′-ATAATCTAGAAAAGTTCAAGGTGTTTAATGC-3′ |
| SAP2P10a | 5′-TGGTGGGCCCGCTGATGCTCCCCGACGG-3′ |
| SAP2P12b | 5′-CTCTACAGTTGGACTCATATGGC-3′ |
| SAP2P13b | 5′-GGCTGGGGAACGATCGTAATTCTGTAGTGAAGCC-3′ |
| SAP2P16a | 5′-GCATTCAAATTACCTCGAGCATTATTATTGTC-3′ |
| SAP2P18a | 5′-GCTCTATAGGGCGTTGCTGGGCATGTGGTGGGGC-3′ |
| SAP2P20a | 5′-GTGGTACAAAACTTACAAACAATAATTATGAGAAC-3′ |
| SAP2P22b | 5′-CCATCTAATTTCTTCATACGGTCGTTCAATTC-3′ |
| SAP2P23b | 5′-GCATGTACATGTCTTTGACCGCCTGAATCTCG-3′ |
| SAP2P26a | 5′-ACATTTGATGTGAGTGTGTCAAAATAATGTGTC-3′ |
| TRK1-1a,b | 5′-TGTTAGACTTTACTACTTTGAGCGTCAT-3′ |
| TRK1-2a,b | 5′-TGAGATATCACTGGTTTCAGAATCTATC-3′ |
| ZCF11-1a | 5′-ATATGTCGACAATGAAGATTAAACAGGAAAACATAACAA-3′ |
| ZCF11-2a,b | 5′-ATATAGATCTCATAGTATTGGTAAAAAGTTCCCCAC-3′ |
| 1007-1a,b | 5′-ATATGTCGACAATGGTGTTAATTGTAGTTGATGTAC-3′ |
| 1007-2a | 5′-ATATAGATCTTACTCTGAAAGTCCTTCGTCTTCTTC-3′ |
Used for gene or probe amplification.
Used for sequencing.
C. albicans transformation.
C. albicans strains DSY459 and SC5314 were transformed by electroporation (24) with the gel-purified ApaI-SacI fragments from plasmids pSAP2KS4 and pSAP2KS5, respectively. Selection of nourseothricin-resistant transformants was performed on YPD agar plates containing 200 μg/ml nourseothricin as described previously (38).
Isolation of genomic DNA and Southern hybridization.
Genomic DNA from C. albicans strains was isolated as described previously (38). DNA was digested with the appropriate restriction enzymes, separated on a 1% agarose gel and, after ethidium bromide staining, transferred by vacuum blotting onto a nylon membrane, and fixed by UV cross-linking. Southern hybridization with enhanced chemiluminescence-labeled probes was performed with an Amersham ECL Direct nucleic acid labeling and detection system (GE Healthcare, Braunschweig, Germany) according to the instructions of the manufacturer. An XbaI-EcoRV fragment from pSAP2G1, an ApaI-SalI fragment from pOPT1G22, and a SacI-SacII fragment from pOPT4M2 (37) were used as SAP2-, OPT1-, and OPT4-specific probes to investigate LOH events in strain DSY459. A SAP2 upstream fragment, which was amplified with the primers SAP2P16 and SAP2P26, and a SAP2 downstream fragment (the XbaI-SacI fragment from pSAP2KS4) were used as probes to verify integration of the inserts from pSAP2KS4 and pSAP2KS5 in transformants of strains DSY459 and SC5314, respectively.
Genomic analysis of C. albicans strains.
To analyze strains SC5314 and DSY459 for the presence of allelic polymorphisms on chromosome R, the complete or partial coding sequences of the genes listed in Table 3 were amplified by PCR and informative regions sequenced with the primers described in Table 2. To determine the extent of SAP2-2 promoter sequences in transformants of strain DSY459, the integrated SAP2-2 allele was amplified from selected clones with primers SAP2P18 and ACT38 and the SAP2 promoter region sequenced with the SAP2-specific primers listed in Table 2. The replacement of SAP2-2 sequences by SAP2-1 sequences in transformants of strain SC5314 was analyzed in the same way.
Table 3.
Loss of allelic polymorphisms on chromosome R in strain DSY459a
| Gene | ORF no. | Location on chromosome R (nt) | Location of allelic polymorphism in strain: |
|
|---|---|---|---|---|
| SC5314 | DSY459 | |||
| BOI2 | orf19.3230 | 295246 to 291728 | 2682 C/T | 2682 C |
| 2731 A/G | 2731 G | |||
| 2943 C/T | 2943 T | |||
| ZCF11 | orf19.2423 | 697519 to 695735 | 1035 C/T | 1035 T |
| 1038 C/G | 1038 C | |||
| 1041 A/G | 1041 G | |||
| 1521 A/G | 1521 A | |||
| 1689 G/T | 1689 T | |||
| orf19.1007 | 1136506 to 1135448 | 291 C/T | 291 C | |
| 477 A/G | 477 A | |||
| 555 C/T | 555 C | |||
| 885 C/T | 885 C | |||
| CRZ2 | orf19.2356 | 1525254 to 1523701 | 76 A/G | 76 G |
| 84 C/T | 84 T | |||
| 168 C/G | 168 C | |||
| 172 A/G | 172 G | |||
| 191 C/T | 191 T | |||
| 193 A/G | 193 G | |||
| 258 C/T | 258 C | |||
| 447 A/G | 447 G | |||
| 450 A/G | 450 A | |||
| TRK1 | orf19.600 | 1741134 to 1737964 | 465 A/G | 465 G |
| 563 C/T | 563 C | |||
| 801 A/G | 801 G | |||
| 989 A/G | 989 G | |||
| 1097 A/G | 1097 A | |||
| 1131 C/T | 1131 C | |||
| FGR32 | orf19.593 | 1755545 to 1753224 | 1327 A/G | 1327 G |
| 1351 C/T | 1351 C | |||
| 1579 A/G | 1579 A | |||
| 1771 C/T | 1771 T | |||
| 1882 C/T | 1882 C | |||
| 1920 A/G | 1920 G | |||
| 1938 G/T | 1938 G | |||
Information on the positions of the genes on chromosome R was obtained from the Candida genome database (http://www.candidagenome.org). The locations of the polymorphic sites are with respect to the start codons of the respective genes. The centromere is located between TRK1 and FGR32, from nt 1743190 to 1747664.
RESULTS
Growth of different C. albicans sap mutants on BSA as a nitrogen source.
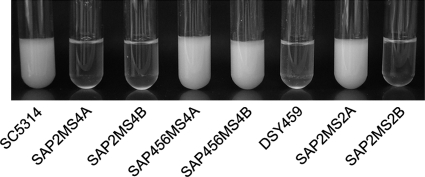
Differences in the phenotypes of C. albicans mutants lacking a specific gene may be caused by the genetic background, unspecific genetic alterations that occurred during strain construction, or variations in the experimental procedures used in different laboratories for phenotypic analysis. The different sap4Δ sap5Δ sap6Δ triple mutants constructed in previous studies (27, 40) were all derived from strain SC5314. Strain DSY459 was generated from a ura3Δ derivative of SC5314 by the Ura-blaster protocol (11), whereas the two independent mutants SAP456MS4A and SAP456MS4B constructed in our lab were generated directly from strain SC5314 using the SAT1-flipping strategy (38). To exclude the possibility that the reported differences in the abilities of these mutants to grow in medium containing BSA as the sole nitrogen source were caused by variations in medium composition or other growth conditions, we directly compared the growth of the various sap4Δ sap5Δ sap6Δ mutants in YCB-BSA medium. The wild-type strain SC5314, two homozygous sap2Δ mutants, and heterozygous mutants lacking the SAP2-1 or the SAP2-2 allele were included for comparison. This experiment confirmed the reported growth defect of strain DSY459, which grew as poorly as the strains lacking SAP2-2 or both SAP2 alleles, whereas strains SAP456MS4A and SAP456MS4B grew as well as the wild-type strain SC5314 and the mutant in which the SAP2-1 allele was deleted (Fig. 1). The phenotypic difference between the independently generated sap4Δ sap5Δ sap6Δ mutants indicated that the growth defect of strain DSY459 is not caused by the absence of the SAP4, SAP5, and SAP6 genes and instead might be the consequence of a different genomic alteration that happened during the construction of this strain.
Fig. 1.
Growth of different C. albicans sapΔ mutants on BSA as the sole nitrogen source. YPD overnight cultures of the strains were diluted 10−3 in YCB-BSA medium and grown for 4 days at 30°C. SC5314 (wild type), SAP2MS4A and SAP2MS4B (sap2-1Δ/sap2-2Δ), SAP456MS4A and SAP456MS4B (sap4Δ/sap4Δ sap5Δ/sap5Δ sap6Δ/sap6Δ), DSY459 (sap4Δ/sap4Δ sap5Δ/sap5Δ sap6Δ/sap6Δ), SAP2MS2A (sap2-1Δ/SAP2-2), and SAP2MS2B (SAP2-1/sap2-2Δ) are shown.
C. albicans strain DSY459 is homozygous for the SAP2-1 allele.
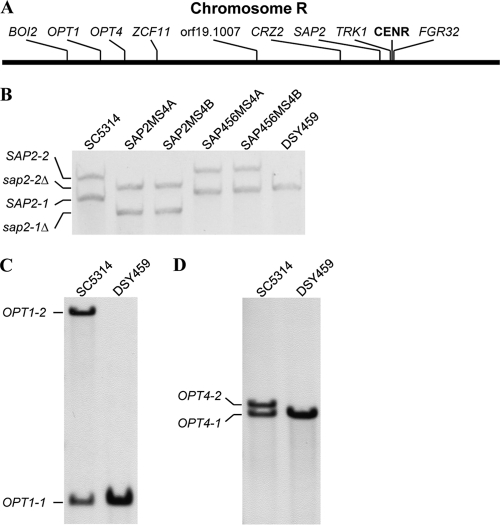
As the SAP2 gene, in particular, the SAP2-2 allele, is known to be important for the growth of C. albicans in YCB-BSA medium, we investigated the genomic structure of the SAP2 locus in strain DSY459. The two SAP2 alleles in strain SC5314 can be distinguished by a ClaI restriction site polymorphism in the SAP2 upstream region, and the allele containing the polymorphic ClaI site was arbitrarily designated SAP2-1 in a previous study (47). Southern hybridization analysis with a SAP2-specific probe showed that in contrast to the wild type and strains SAP456MS4A and SAP456MS4B, which exhibited two hybridizing ClaI fragments of the expected sizes, strain DSY459 contained only the smaller fragment, indicating that DSY459 had become homozygous for the SAP2-1 allele (Fig. 2B).
Fig. 2.
Analysis of allelic polymorphisms on chromosome R of strain SC5314 and mutant derivatives. (A) Location of analyzed polymorphic genes on chromosome R. The position of the centromere (CENR) is also shown. (B) Southern hybridization of ClaI-digested genomic DNA of the indicated strains with a SAP2-specific probe. The identities of the hybridizing fragments are given. SC5314 (wild type), SAP2MS4A and SAP2MS4B (sap2-1Δ/sap2-2Δ), SAP456MS4A and SAP456MS4B (sap4Δ/sap4Δ sap5Δ/sap5Δ sap6Δ/sap6Δ), and DSY459 (sap4Δ/sap4Δ sap5Δ/sap5Δ sap6Δ/sap6Δ) are shown. (C and D) Southern hybridization analysis of HindIII (C)- and EcoRI (D)-digested genomic DNA of strains SC5314 and DSY459 with OPT1- and OPT4-specific probes, respectively. The fragments corresponding to the polymorphic OPT1 and OPT4 alleles of strain SC5314 are indicated.
Loss of heterozygosity for chromosome R in strain DSY459.
SAP2 is located on the left arm of chromosome R, about 50 kb from the centromere (Fig. 2A). To investigate the extent of LOH on this chromosome in strain DSY459, we first performed Southern hybridization analyses with probes for two other polymorphic genes that are located in a different region on the left arm of chromosome R (Fig. 2A). The OPT1 and OPT4 alleles of strain SC5314 can be distinguished by HindIII and EcoRI restriction site polymorphisms, respectively (37, 38). Figure 2C and D show that strain DSY459 contained only the OPT1-1 and OPT4-1 alleles. We then tested DSY459 for LOH in additional regions along chromosome R by amplifying and sequencing known polymorphic open reading frames (ORFs) (22; our unpublished data). The results of this analysis, which are summarized in Table 3, demonstrate that DSY459 had become homozygous for all polymorphic positions investigated. Two genes, TRK1 and FGR32, are located on both sides and very close to the centromere of chromosome R, which makes it unlikely that homozygosity for all analyzed genes is the result of multiple recombination events on both arms of this chromosome (Fig. 2A). Rather, the results strongly indicate that DSY459 has completely lost one of the two chromosome R homologs and has become homozygous (or monosomic, but see below) for the other homolog.
Reintroduction of the SAP2-2 allele restores growth of the sap4Δ sap5Δ sap6Δ mutant.
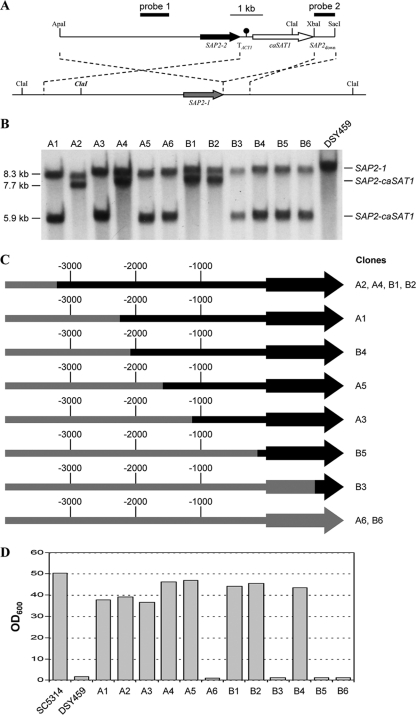
The importance of the SAP2-2 allele for the ability of strain SC5314 to grow in a medium containing BSA as the sole nitrogen source suggested that the loss of this allele might be the true cause of the growth defect of strain DSY459 under these conditions. To test this hypothesis, we reintroduced the SAP2-2 allele into strain DSY459 using a construct designed for integration at the SAP2 locus (Fig. 3A). Southern hybridization analysis of 12 clones from two independent sets of transformants with probes from the SAP2 upstream and downstream regions (Fig. 3A and BAnd data not shown) demonstrated that all transformants had correctly integrated the construct at the SAP2 locus. In eight of the transformants (clones A1, A3, A5, A6, B3, B4, B5, and B6), the new fragment hybridizing with the SAP2 upstream probe had a size of 5.9 kb, as expected when the polymorphic ClaI site in the upstream region of the replaced SAP2-1 copy was retained. The other four transformants (clones A2, A4, B1, and B2) had a new hybridizing fragment of 7.7 kb, demonstrating that the polymorphic ClaI site was lost and that at least 3.2 kb of SAP2-1 promoter sequences had been replaced by the corresponding region of the SAP2-2 allele. The retention of the wild-type fragment after integration of the construct at the SAP2 locus in all 12 transformants also argued that DSY459 contained two copies of chromosome R and that LOH for markers on this chromosome was caused by homozygosity instead of monosomy.
Fig. 3.
Replacement of one of the SAP2-1 copies of strain DSY459 by the SAP2-2 allele restores growth on proteins as the sole nitrogen source. (A) Structures of the ApaI-SacI fragment from plasmid pSAP2KS4 (top), which was used for transformation, and the homozygous SAP2-1 locus in strain DSY459 (bottom). The SAP2-1 and SAP2-2 coding regions are symbolized by the gray and black arrows, respectively, the transcription termination sequence from the ACT1 gene (TACT1) by the black circle, and the caSAT1 selection marker by the white arrow. SAP2down, SAP2 downstream fragment. Relevant restriction sites are indicated. The polymorphic ClaI site, which is present only in the SAP2-1 allele, is highlighted in bold and italics. The probes used for Southern hybridization analysis of the transformants are indicated by black bars. The dashed lines indicate the regions of homology along which the crossover can occur during integration of the construct. (B) Southern hybridization of ClaI-digested genomic DNA of strain DSY459 and two independent sets of transformants (clones A1 to A6 and B1 to B6) with SAP2-specific probe 1. The fragments corresponding to the parental SAP2-1 copies and the integrated SAP2-caSAT1 construct as well as their sizes are indicated. (C) Extent of SAP2-2 sequences in the replaced SAP2-1 copy of various transformants of strain DSY459, as determined by direct sequencing. SAP2-2 sequences are shown in black and SAP2-1 sequences in gray. The promoter region is indicated by thick lines, and arrows represent the SAP2 coding region. Note that the exact site of crossover was not determined for clones A2, A4, B1, and B2 and the indicated SAP2-2 region is the minimum in all four clones (at least to the replaced polymorphic ClaI site). (D) Growth of strains SC5314 and DSY459 and transformants in YCB-BSA medium. YPD overnight cultures of the strains were diluted 10−3 in YCB-BSA medium, and the optical densities of the cultures at 600 nm (OD600) were determined after 3 days of growth at 30°C.
To determine to which extent SAP2-1 sequences had been replaced by SAP2-2 sequences in the transformants that retained the polymorphic ClaI site after integration, the integrated SAP2 copy was reamplified with specific primers and sequenced. This analysis showed that clones A1, A3, A5, B4, and B5 contained between 134 bp and 2.2 kb of SAP2-2-specific upstream sequences, whereas clones A6, B3, and B6 had retained the complete SAP2-1 upstream sequence (Fig. 3C).
We then tested the ability of the transformants to grow in YCB-BSA medium. As can be seen in Fig. 3D, all clones that had integrated the SAP2-2 allele with at least 1.1 kb of its upstream region (A1, A2, A3, A4, A5, B1, B2, and B4) could grow in YCB-BSA medium; i.e., reintroduction of the lost SAP2-2 allele restored growth of the sap4Δ sap5Δ sap6Δ mutant. The four clones in which the SAP2-1 promoter was completely or almost completely retained after integration of the replacement construct (A6, B3, B5, and B6) displayed the same growth defect as the parental strain DSY459. These results demonstrate that loss of the SAP2-2 allele due to LOH for chromosome R, and not deletion of the SAP4, SAP5, and SAP6 genes, explains the inability of strain DSY459 to utilize proteins as the sole nitrogen source.
Homozygosity for chromosome R with the SAP2-1 allele in strain SC5314 results in a growth defect in YCB-BSA medium.
It was previously shown that deletion of the SAP2-2 allele in strain SC5314 results in a growth defect in YCB-BSA medium (Fig. 1) (27, 47, 49). As these mutants contain only one copy of the SAP2-1 allele, it seemed possible that a reduced gene dosage may be an additional prerequisite for the growth defect of strains containing only the SAP2-1 allele. On the other hand, strain DSY459, which has become homozygous for chromosome R, contains two copies of the SAP2-1 allele, but in this case, the additional absence of the SAP4, SAP5, and SAP6 genes might have impacted the ability of the mutant to grow in YCB-BSA medium. We therefore wished to determine whether homozygosity for chromosome R containing the SAP2-1 allele is sufficient to cause a growth defect when proteins are the only available nitrogen source. Strain YJB10698 (also known as AF3976) is a derivative of strain SC5314 that had become homozygous for chromosome R after an in vivo passage and reisolation from the kidneys of an intravenously infected mouse (12, 26). Southern hybridization analysis showed that strain YJB10698, like DSY459, contained only the smaller ClaI fragment hybridizing with a SAP2-specific probe (data not shown); i.e., the strain had become homozygous for chromosome R containing the SAP2-1 allele. Figure 4 shows that strain YJB10698 displays a growth defect in YCB-BSA medium similar to that of strain SAP2MS2B, in which the SAP2-2 allele has been deleted. Therefore, homozygosity for chromosome R with the SAP2-1 allele appears to be sufficient to result in a growth defect on proteins as the sole nitrogen source.
Fig. 4.

Homozygosity for chromosome R containing the SAP2-1 allele causes a growth defect in YCB-BSA medium. YPD overnight cultures of the strains were diluted 10−3 in YCB-BSA medium and grown for 4 days at 30°C. SC5314 (SAP2-1/SAP2-2), YJB10698 (SAP2-1/SAP2-1), SAP2MS2A (sap2-1Δ/SAP2-2), and SAP2MS2B (/SAP2-1/sap2-2Δ) are shown.
Homozygosity for the SAP2-1 allele in strain SC5314 causes a growth defect in YCB-BSA medium.
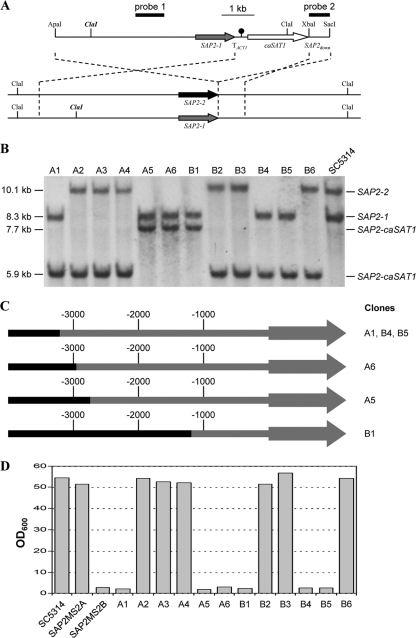
The results noted above did not exclude the possibility that LOH for additional genes located on chromosome R was required for the growth defect of strains that are homozygous for the SAP2-1 allele. To unambiguously demonstrate that homozygosity for the SAP2-1 allele was sufficient to cause the growth defect in YCB-BSA medium, we replaced the SAP2-2 allele and its regulatory region in strain SC5314 by the corresponding sequences of the SAP2-1 allele (Fig. 5A). Southern hybridization analysis of 12 clones from two independent sets of transformants with probes from the SAP2 upstream and downstream regions (Fig. 5BAnd data not shown) demonstrated that all transformants had correctly integrated the construct at the SAP2 locus. In six of the transformants (clones A2, A3, A4, B2, B3, and B6), the integration had occurred in the SAP2-1 allele and, as expected, all these transformants grew as well as the parental strain SC5314 in YCB-BSA medium (Fig. 5B and D). The other six transformants (clones A1, A5, A6, B1, B4, and B5) had integrated the construct into the SAP2-2 allele (Fig. 5B). In three of the transformants (A1, B4, and B5), the polymorphic ClaI site had been introduced, as is evident from the appearance of a new hybridizing fragment of 5.9 kb, demonstrating that at least 3.2 kb of SAP2-2 promoter sequences had been replaced by the corresponding region of the SAP2-1 allele. The remaining three transformants (A5, A6, and B1) contained a new hybridizing ClaI fragment of 7.7 kb, indicating that less than 3.2 kb of SAP2-2 promoter sequences had been replaced by SAP2-1 sequences. Sequence analysis of these clones showed that the introduced SAP2-1 allele contained between 1.2 kb and 3 kb of SAP2-1-specific upstream sequences (Fig. 5C). All transformants in which the SAP2-2 allele had been replaced by the SAP2-1 allele, including clone B1, which retained the largest portion of SAP2-2 upstream sequences, displayed a growth defect in YCB-BSA medium (Fig. 5D). These results demonstrate that homozygosity for the SAP2-1 allele and as little as 1.2 kb of its upstream sequence is sufficient to abolish the ability of the cells to resume growth when proteins are the only available nitrogen source.
Fig. 5.
Replacement of the SAP2-2 allele in strain SC5314 by the SAP2-1 allele causes a growth defect in YCB-BSA medium. (A) Structures of the ApaI-SacI fragment from plasmid pSAP2KS5 (top), which was used for transformation, and the wild-type SAP2-1 and SAP2-2 alleles in strain SC5314 (bottom). The SAP2-1 and SAP2-2 coding regions are symbolized by the gray and black arrows, respectively, the transcription termination sequence from the ACT1 gene (TACT1) by the black circle, and the caSAT1 selection marker by the white arrow. Relevant restriction sites are indicated. The polymorphic ClaI site, which is present only in the SAP2-1 allele, is highlighted in bold and italics. The probes used for Southern hybridization analysis of the transformants are indicated by black bars. The dashed lines indicate the regions of homology along which the crossover can occur during integration of the construct. (B) Southern hybridization of ClaI-digested genomic DNA of strain SC5314 and two independent sets of transformants (clones A1 to A6 and B1 to B6) with SAP2-specific probe 1. The fragments corresponding to the parental SAP2 alleles and the integrated SAP2-caSAT1 construct as well as their sizes are indicated. (C) Extent of SAP2-1 promoter sequences in the replaced SAP2-2 allele of various transformants of strain SC5314, as determined by direct sequencing. SAP2-1 sequences are shown in gray and SAP2-2 sequences in black. The promoter region is indicated by thick lines, and arrows represent the SAP2 coding region. Note that the exact site of crossover was not determined for clones A1, B4, and B5 and the indicated SAP2-1 region is the minimum in all three clones (at least to the polymorphic ClaI site). (D) Growth of strain SC5314 and transformants on BSA as the sole nitrogen source. YPD overnight cultures of the strains were diluted 10−3 in YCB-BSA medium, and the optical densities of the cultures were determined after 3 days of growth at 30°C. The heterozygous sap2Δ mutants SAP2MS2A (sap2-1Δ/SAP2-2) and SAP2MS2B (/SAP2-1/sap2-2Δ) were included for comparison.
DISCUSSION
The genomic plasticity of C. albicans is considered an important mechanism of adaptation to alterations in the environment (12, 43). Changes in the copy number of certain chromosomes, e.g., monosomy for chromosome 5, and other chromosomal variations allow assimilation of alternative carbon sources due to derepression of the necessary genes (21, 23, 39). Conversely, amplification of chromosome 5 or its left arm causes azole resistance by increasing the gene dosage of ERG11, encoding the drug target, and TAC1, encoding a transcriptional activator of efflux pumps (42, 44). In recent years, it has become evident that such genomic alterations may also occur with a relatively high frequency during the construction of specific C. albicans mutants. Many genetically engineered strains have been found to exhibit aneuploidies for different chromosomes, which can affect gene expression and produce phenotypes not related to target gene inactivation, including virulence attenuation (1, 4, 5, 41). Such unintended genomic alterations may remain undetected and lead to erroneous conclusions about the function of a gene under study.
LOH, which occurs frequently in clinical C. albicans strains that have acquired mutations conferring azole resistance, has also been observed in C. albicans mutants constructed in the laboratory (14). At least some stocks of strain CAI8, a derivative of strain SC5314, have become homozygous at the mating type locus (MTLa/MTLa) and can switch to the opaque phase and mate as a cells (36). Depletion of the morphogenetic regulator Efg1 in a conditional mutant constructed from strain CAI8 induced the formation of opaque-phase cells (45), suggesting that white-opaque switching can occur in MTL heterozygous strains when Efg1 is absent. It was later shown that the conditional efg1 mutant was also an a strain and that the ability of efg1Δ mutants to switch to the opaque phase depended on MTL homozygosity (53). Here we present an example in which the phenotype of a C. albicans gene deletion mutant is in fact caused by LOH at another unlinked genomic locus. The sap4Δ sap5Δ sap6Δ triple mutant DSY459 has been used to assess the importance of the secreted aspartic proteases Sap4, Sap5, and Sap6 for the virulence of C. albicans in different infection models (3, 6, 10, 25, 40, 50). Our results demonstrate that the reported inability of this mutant to utilize proteins as the sole nitrogen source is not caused by the deletion of the SAP4, SAP5, and SAP6 genes but by the loss of the SAP2-2 allele. Although LOH at the SAP2 locus is unlikely to be responsible for the attenuated virulence of the mutant, the fact that loss of the SAP2-2 allele caused the in vitro growth defect of strain DSY459 and that the strain has become homozygous for the whole chromosome R must be considered when conclusions on the role of SAP4, SAP5, and SAP6 are based on phenotypes obtained with this strain.
The stress exerted upon cells during transformation can induce chromosome duplication as well as subsequent loss of supernumerary chromosomes (4). Such a sequence of events provides a possible explanation for how strain DSY459 became homozygous for chromosome R during multiple rounds of transformation. LOH on chromosome R does not seem to be an uncommon event, as LOH for the whole chromosome R has been reported previously for other SC5314 derivatives (14, 26) and we also have observed LOH at the SAP2 locus during strain construction in our lab (48; our unpublished data). As in the case of strain DSY459, the actual genetic manipulation affected chromosomes other than chromosome R in these latter cases. Similarly, aneuploidies in genetically engineered strains often involve chromosomes other than those that were intentionally manipulated (1). Aneuploidies and LOH for certain chromosomal regions can be detected by two microarray-based technologies, comparative genome hybridization (CGH) and genomewide single nucleotide polymorphism (SNP) analyses (13, 41). However, these methods are not routinely used in most laboratories and would also not detect all possible LOH events or point mutations. Therefore, the possibility of unspecific genomic alterations that are induced during the construction of strains or arise spontaneously during their propagation and storage can never be completely excluded. The construction of several independent mutants and the complementation of mutant phenotypes by reintroduction of deleted genes are important control measures when linking a phenotype to the inactivation of a specific gene.
Apart from acquired drug resistance mutations in ERG11, TAC1, MRR1, and UPC2, there are only a few documented examples of functional differences between the two alleles of a gene in a particular C. albicans strain, like the ALS3, OPT3, PAP1, and SAP2 alleles of strain SC5314 or the CDR2 alleles of other strains (19, 30, 35, 37, 47). Among these, SAP2 is a special case because the functional difference between the two alleles is not related to unique properties of the encoded proteins but is a consequence of their differential regulation. Basal expression of the SAP2-2 allele enables the generation of small amounts of proteolytic degradation products from extracellular proteins, which are required for full induction of both SAP2-1 and SAP2-2 (47).
Allelic differences may be subtle, such that LOH will have only minor or undetectable effects on the phenotype of a strain, but there are also cases in which one allele of a gene is nonfunctional, as described for HIS4 in strain SC5314 (17). In this case, loss of the intact HIS4 copy renders the strain auxotrophic for histidine. As strain SC5314 and its derivatives are used as parental strains for the construction of C. albicans mutants in most laboratories, knowledge about the phenotypes caused by defined LOH events in this strain may alert researchers to the possibility that such an undesired genomic alteration has occurred at the corresponding locus during the construction of a strain. The specific growth defect on proteins due to loss of the SAP2-2 allele is an illustrative example of LOH at a different genomic locus as the true cause of a C. albicans mutant phenotype.
ACKNOWLEDGMENTS
We thank Bernhard Hube for providing C. albicans strain DSY459 and Judy Berman and Anja Forche for the gift of strain YJB10698.
This study was supported by the Deutsche Forschungsgemeinschaft (SFB 630).
Footnotes
Published ahead of print on 19 November 2010.
REFERENCES
- 1. Arbour M., et al. 2009. Widespread occurrence of chromosomal aneuploidy following the routine production of Candida albicans mutants. FEMS Yeast Res. 9:1070–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahn Y. S., et al. 2007. Sensing the environment: lessons from fungi. Nat. Rev. Microbiol. 5:57–69 [DOI] [PubMed] [Google Scholar]
- 3. Borg-von Zepelin M., Beggah S., Boggian K., Sanglard D., Monod M. 1998. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol. Microbiol. 28:543–554 [DOI] [PubMed] [Google Scholar]
- 4. Bouchonville K., Forche A., Tang K. E., Selmecki A., Berman J. 2009. Aneuploid chromosomes are highly unstable during DNA transformation of Candida albicans. Eukaryot. Cell 8:1554–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen X., Magee B. B., Dawson D., Magee P. T., Kumamoto C. A. 2004. Chromosome 1 trisomy compromises the virulence of Candida albicans. Mol. Microbiol. 51:551–565 [DOI] [PubMed] [Google Scholar]
- 6. Correia A., et al. 2010. Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect. Immun. 78:4839–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coste A., et al. 2007. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell 6:1889–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coste A., et al. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 172:2139–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunkel N., Blaß J., Rogers P. D., Morschhäuser J. 2008. Mutations in the multi-drug resistance regulator MRR1, followed by loss of heterozygosity, are the main cause of MDR1 overexpression in fluconazole-resistant Candida albicans strains. Mol. Microbiol. 69:827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felk A., et al. 2002. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect. Immun. 70:3689–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fonzi W. A., Irwin M. Y. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forche A., Magee P. T., Selmecki A., Berman J., May G. 2009. Evolution in Candida albicans populations during a single passage through a mouse host. Genetics 182:799–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forche A., May G., Magee P. T. 2005. Demonstration of loss of heterozygosity by single-nucleotide polymorphism microarray analysis and alterations in strain morphology in Candida albicans strains during infection. Eukaryot. Cell 4:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forche A., Steinbach M., Berman J. 2009. Efficient and rapid identification of Candida albicans allelic status using SNP-RFLP. FEMS Yeast Res. 9:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franz R., et al. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillum A. M., Tsay E. Y., Kirsch D. R. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 17. Gomez-Raja J., Andaluz E., Magee B., Calderone R., Larriba G. 2008. A single SNP, G929T (Gly310Val), determines the presence of a functional and a non-functional allele of HIS4 in Candida albicans SC5314: detection of the non-functional allele in laboratory strains. Fungal Genet. Biol. 45:527–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heilmann C. J., Schneider S., Barker K. S., Rogers P. D., Morschhäuser J. 2010. An A643T mutation in the transcription factor Upc2p causes constitutive ERG11 upregulation and increased fluconazole resistance in Candida albicans. Antimicrob. Agents Chemother. 54:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holmes A. R., et al. 2006. Heterozygosity and functional allelic variation in the Candida albicans efflux pump genes CDR1 and CDR2. Mol. Microbiol. 62:170–186 [DOI] [PubMed] [Google Scholar]
- 20. Hube B., et al. 1997. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect. Immun. 65:3529–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janbon G., Sherman F., Rustchenko E. 1998. Monosomy of a specific chromosome determines L-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 95:5150–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones T., et al. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 101:7329–7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kabir M. A., Ahmad A., Greenberg J. R., Wang Y. K., Rustchenko E. 2005. Loss and gain of chromosome 5 controls growth of Candida albicans on sorbose due to dispersed redundant negative regulators. Proc. Natl. Acad. Sci. U. S. A. 102:12147–12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Köhler G. A., White T. C., Agabian N. 1997. Overexpression of a cloned IMP dehydrogenase gene of Candida albicans confers resistance to the specific inhibitor mycophenolic acid. J. Bacteriol. 179:2331–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kretschmar M., et al. 1999. Germ tubes and proteinase activity contribute to virulence of Candida albicans in murine peritonitis. Infect. Immun. 67:6637–6642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Legrand M., et al. 2008. Haplotype mapping of a diploid non-meiotic organism using existing and induced aneuploidies. PLoS Genet. 4:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lermann U., Morschhäuser J. 2008. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology 154:3281–3295 [DOI] [PubMed] [Google Scholar]
- 28. Lockhart S. R., et al. 2002. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics 162:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MacCallum D. M., et al. 2010. Genetic dissection of azole resistance mechanisms in Candida albicans and their validation in a mouse model of disseminated infection. Antimicrob. Agents Chemother. 54:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manoharlal R., Gorantala J., Sharma M., Sanglard D., Prasad R. 2010. PAP1 [poly(A) polymerase 1] homozygosity and hyperadenylation are major determinants of increased mRNA stability of CDR1 in azole-resistant clinical isolates of Candida albicans. Microbiology 156:313–326 [DOI] [PubMed] [Google Scholar]
- 31. Martinez P., Ljungdahl P. O. 2005. Divergence of Stp1 and Stp2 transcription factors in Candida albicans places virulence factors required for proper nutrient acquisition under amino acid control. Mol. Cell. Biol. 25:9435–9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller M. G., Johnson A. D. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302 [DOI] [PubMed] [Google Scholar]
- 33. Morschhäuser J., et al. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 3:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naglik J. R., Challacombe S. J., Hube B. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 67:400–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oh S. H., et al. 2005. Functional specificity of Candida albicans Als3p proteins and clade specificity of ALS3 alleles discriminated by the number of copies of the tandem repeat sequence in the central domain. Microbiology 151:673–681 [DOI] [PubMed] [Google Scholar]
- 36. Park Y. N., Morschhäuser J. 2005. Candida albicans MTLα tup1Δ mutants can reversibly switch to mating-competent, filamentous growth forms. Mol. Microbiol. 58:1288–1302 [DOI] [PubMed] [Google Scholar]
- 37. Reuß O., Morschhäuser J. 2006. A family of oligopeptide transporters is required for growth of Candida albicans on proteins. Mol. Microbiol. 60:795–812 [DOI] [PubMed] [Google Scholar]
- 38. Reuß O., Vik A., Kolter R., Morschhäuser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 39. Rustchenko E. P., Howard D. H., Sherman F. 1994. Chromosomal alterations of Candida albicans are associated with the gain and loss of assimilating functions. J. Bacteriol. 176:3231–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanglard D., Hube B., Monod M., Odds F. C., Gow N. A. 1997. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect. Immun. 65:3539–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Selmecki A., Bergmann S., Berman J. 2005. Comparative genome hybridization reveals widespread aneuploidy in Candida albicans laboratory strains. Mol. Microbiol. 55:1553–1565 [DOI] [PubMed] [Google Scholar]
- 42. Selmecki A., Forche A., Berman J. 2006. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313:367–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Selmecki A., Forche A., Berman J. 2010. Genomic plasticity of the human fungal pathogen Candida albicans. Eukaryot. Cell 9:991–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Selmecki A., Gerami-Nejad M., Paulson C., Forche A., Berman J. 2008. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol. 68:624–641 [DOI] [PubMed] [Google Scholar]
- 45. Sonneborn A., Tebarth B., Ernst J. F. 1999. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect. Immun. 67:4655–4660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Staib P., Kretschmar M., Nichterlein T., Hof H., Morschhäuser J. 2000. Differential activation of a Candida albicans virulence gene family during infection. Proc. Natl. Acad. Sci. U. S. A. 97:6102–6107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Staib P., Kretschmar M., Nichterlein T., Hof H., Morschhäuser J. 2002. Host versus in vitro signals and intrastrain allelic differences in the expression of a Candida albicans virulence gene. Mol. Microbiol. 44:1351–1366 [DOI] [PubMed] [Google Scholar]
- 48. Staib P., Kretschmar M., Nichterlein T., Hof H., Morschhäuser J. 2002. Transcriptional regulators Cph1p and Efg1p mediate activation of the Candida albicans virulence gene SAP5 during infection. Infect. Immun. 70:921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Staib P., et al. 2008. Tetracycline-inducible expression of individual secreted aspartic proteases in Candida albicans allows isoenzyme-specific inhibitor screening. Antimicrob. Agents Chemother. 52:146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watts H. J., Cheah F. S., Hube B., Sanglard D., Gow N. A. 1998. Altered adherence in strains of Candida albicans harbouring null mutations in secreted aspartic proteinase genes. FEMS Microbiol. Lett. 159:129–135 [DOI] [PubMed] [Google Scholar]
- 51. White T. C. 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α demethylase in Candida albicans. Antimicrob. Agents Chemother. 41:1488–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White T. C., Marr K. A., Bowden R. A. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zordan R. E., Miller M. G., Galgoczy D. J., Tuch B. B., Johnson A. D. 2007. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 5:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]