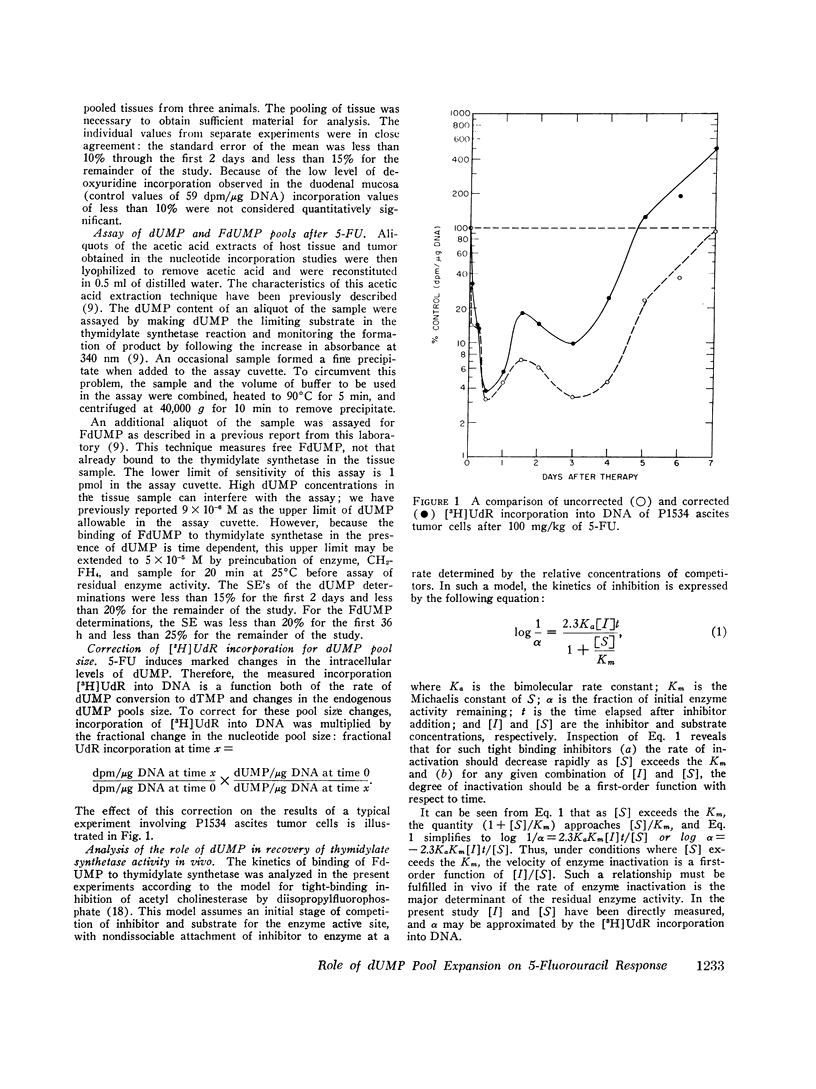
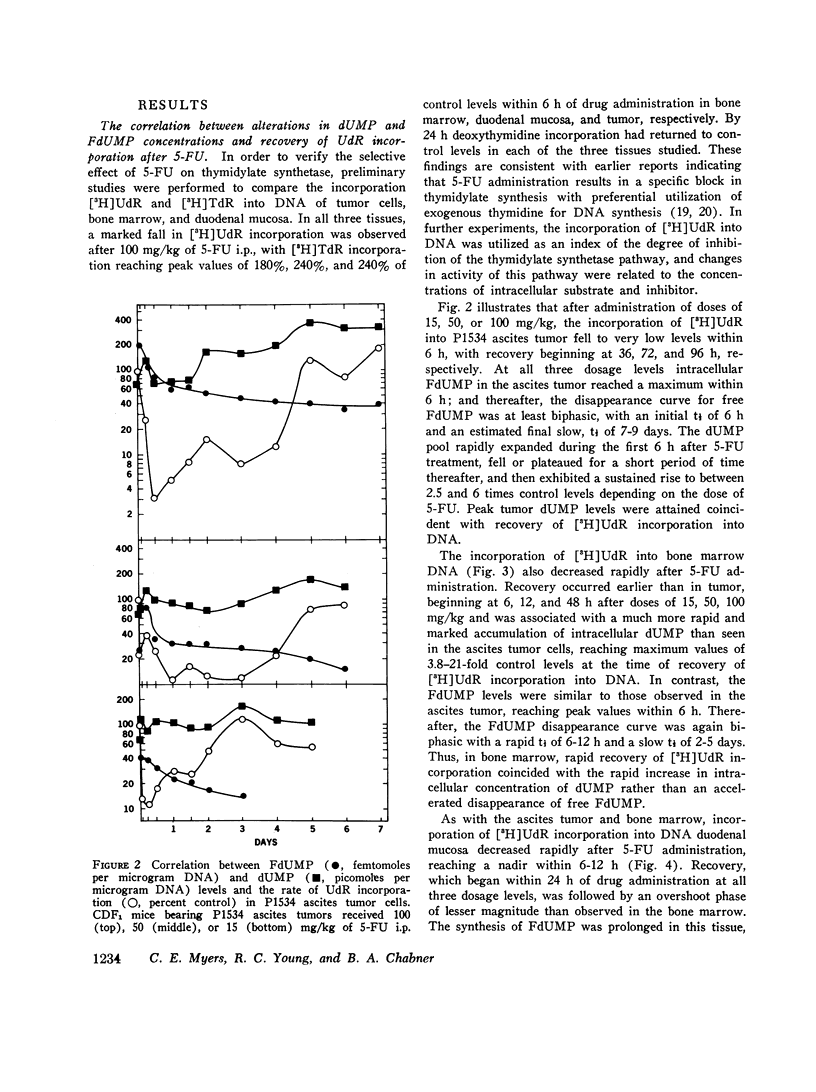
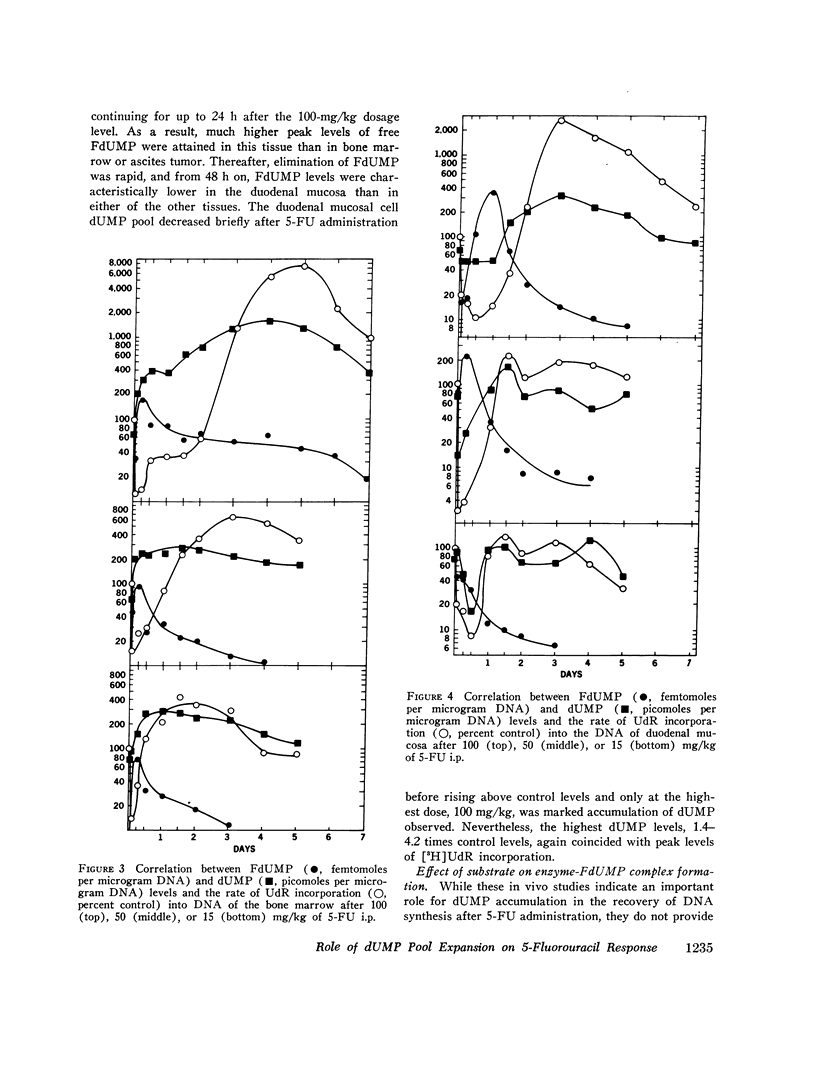
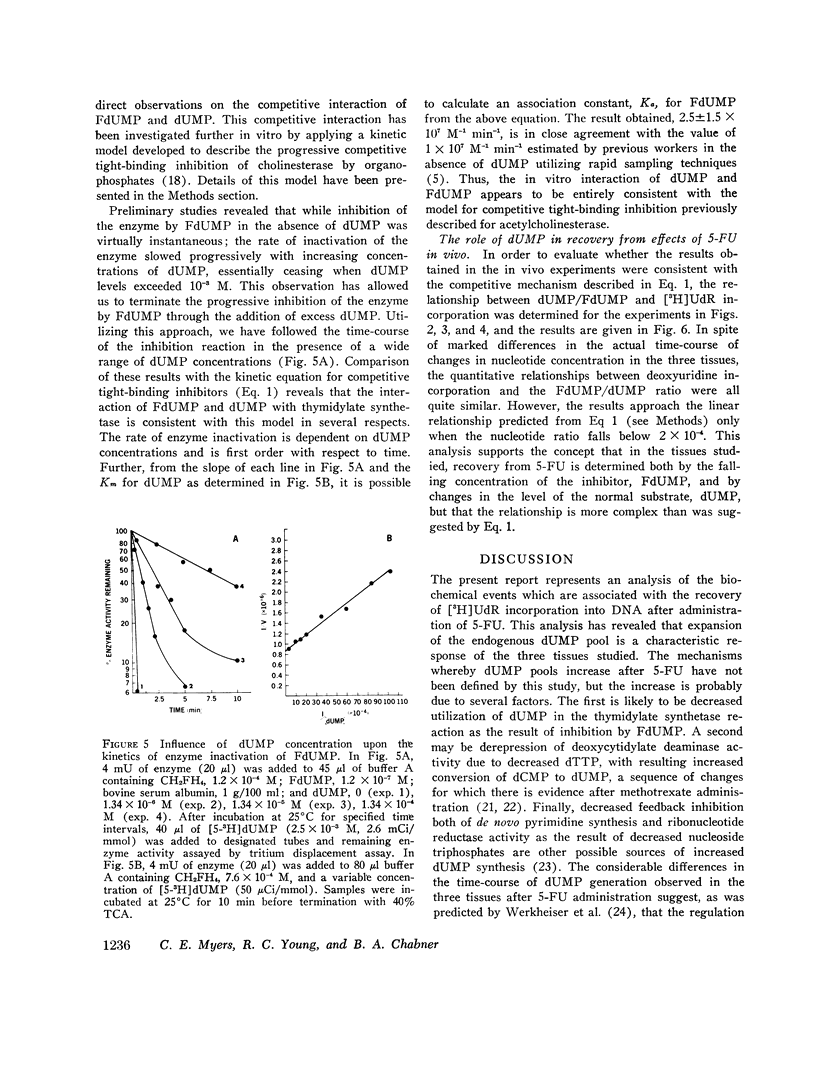
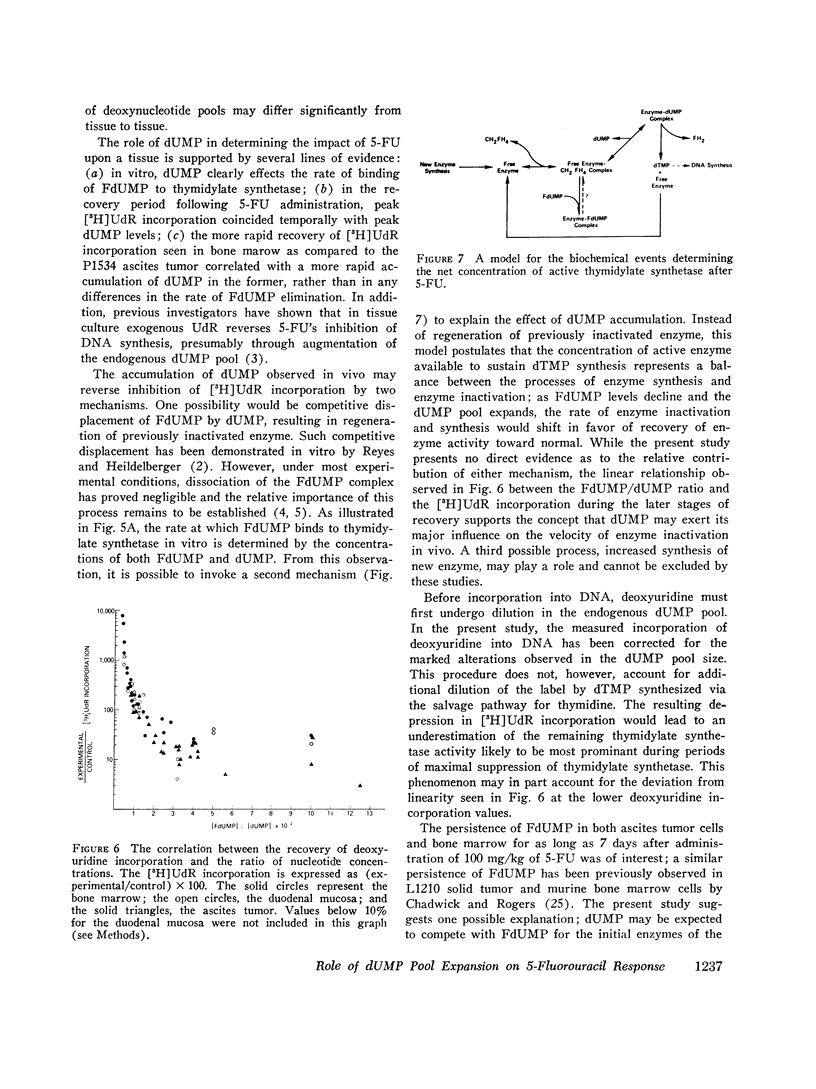
Abstract
5-Fluorodeoxyuridine monophosphate (FdUMP), the active metabolite of 5-fluorouracil (5-FU), is a tight-binding inhibitor of thymidylate synthetase, the enzyme which converts dUMP to TMP. Newly developed assays for FdUMP and dUMP were utilized to assess the competitive roles played by these nucleotides in determining the inhibition of TMP synthesis in mice bearing the P1534 ascites tumor. After 5-FU administration, levels of FdUMP reached a dose-dependent peak within 6 h in the ascites tumor and in bone marrow, and declined thereafter in a biphasic manner with an initial t 1/2 of 6 h and a final t 1/2 of 7-9 days. In duodenal mucosa, FdUMP levels were 1.8-2-fold higher than in the other tissues, but elimination was much more rapid. Simultaneous with the fall in FdUMP a progressive accumulation of the competitive substrate dUMP was observed in each tissue after 5-FU; and peak dUMP levels coincided with recovery of thymidylate synthesis, as determined by the incorporation of [3H]deoxyuridine into DNA. In vitro experiments with partially purifed thymidylate synthetase revealed and initial competitive interaction of dUMP and FdUMP, which, at high concentrations of dUMP was capable of markedly slowing the rate of irreversible inactivation of enzyme by FdUMP. These studies were found to be quantitatively consistent with a two-phase model of enzyme inactivation involving an initial competition between dUMP and FdUMP, with subsequent irreversible inactivation of enzyme by covalent linkage to the inhibitor. Recovery of thymidylate synthesis after 5-FU appears to result from both a fall in intracellular levels of inhibitor and a progressive accumulation of the competitive substrate dUMP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOSCH L., HARBERS E., HEIDELBERGER C. Studies on fluorinated pyrimidines. V. Effects on nucleic acid metabolism in vitro. Cancer Res. 1958 Apr;18(3):335–343. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner B. A., Young R. C. Threshold methotrexate concentration for in vivo inhibition of DNA synthesis in normal and tumorous target tissues. J Clin Invest. 1973 Aug;52(8):1804–1811. doi: 10.1172/JCI107362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick M., Rogers W. I. The physiological disposition of 5-fluorouracil in mice bearing solid L1210 lymphocytic leukemia. Cancer Res. 1972 May;32(5):1045–1056. [PubMed] [Google Scholar]
- Crusberg T. C., Leary R., Kisliuk R. L. Properties of thymidylate synthetase from dichloromethotrexate-resistant Lactobacillus casei. J Biol Chem. 1970 Oct 25;245(20):5292–5296. [PubMed] [Google Scholar]
- Fridland A. Effect of methotrexate on deoxyribonucleotide pools and DNA synthesis in human lymphocytic cells. Cancer Res. 1974 Aug;34(8):1883–1888. [PubMed] [Google Scholar]
- Fridland A., Langenbach R. J., Heidelberger C. Purification of thymidylate synthetase from Ehrlich ascites carcinoma cells. J Biol Chem. 1971 Dec 10;246(23):7110–7114. [PubMed] [Google Scholar]
- Kawai M., Hillcoat B. L. Nonenzymatic exchange of tritium from (5-3H) deoxyuridylate in the thymidylate synthetase assay. Anal Biochem. 1974 Apr;58(2):404–413. doi: 10.1016/0003-2697(74)90209-7. [DOI] [PubMed] [Google Scholar]
- Klímek M. Thymidine incoporation into mammalian strain L-cells after the action of 5-fluorodoxyuridine and the effect of uracil. Neoplasma. 1965;12(5):465–468. [PubMed] [Google Scholar]
- Langenbach R. J., Danenberg P. V., Heidelberger C. Thymidylate synthetase: mechanism of inhibition by 5-fluoro-2'-deoxyuridylate. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1565–1571. doi: 10.1016/0006-291x(72)90892-3. [DOI] [PubMed] [Google Scholar]
- Momparler R. L. Inhibition of cytotoxic action of 1- -D-arabinofuranosylcytosine on S-phase HeLa cells by 5-fluorodeoxyuridine. Cancer Res. 1973 Jul;33(7):1754–1758. [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- Morrison J. F. Kinetics of the reversible inhibition of enzyme-catalysed reactions by tight-binding inhibitors. Biochim Biophys Acta. 1969;185(2):269–286. doi: 10.1016/0005-2744(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Myers C. E., Young R. C., Johns D. G., Chabner B. A. Assay of 5-fluorodeoxyuridine 5'-monophosphate deoxyuridine 5'-monophosphate pools following 5-fluorouracil. Cancer Res. 1974 Oct;34(10):2682–2688. [PubMed] [Google Scholar]
- Reyes P., Heidelberger C. Fluorinated pyrimidines. XXVI. Mammalian thymidylate synthetase: its mechanism of action and inhibition by fluorinated nucleotides. Mol Pharmacol. 1965 Jul;1(1):14–30. [PubMed] [Google Scholar]
- Roberts D. An isotopic assay for thymidylate synthetase. Biochemistry. 1966 Nov;5(11):3546–3548. doi: 10.1021/bi00875a022. [DOI] [PubMed] [Google Scholar]
- Rosenoff S. H., Bull J. M., Young R. C. The effect of chemotherapy on the kinetics and proliferative capacity of normal and tumorous tissues in vivo. Blood. 1975 Jan;45(1):107–118. [PubMed] [Google Scholar]
- Santi D. V., McHenry C. S., Sommer H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974 Jan 29;13(3):471–481. doi: 10.1021/bi00700a012. [DOI] [PubMed] [Google Scholar]
- Silber R. Regulatory mechanisms in the human leukocyte. I. The feedback control of deoxycytidylate deaminase. Blood. 1967 Jun;29(6):896–905. [PubMed] [Google Scholar]
- Sommer H., Santi D. V. Purification and amino acid analysis of an active site peptide from thymidylate synthetase containing covalently bound 5-fluoro-2'-deoxyuridylate and methylenetetrahydrofolate. Biochem Biophys Res Commun. 1974 Apr 8;57(3):689–695. doi: 10.1016/0006-291x(74)90601-9. [DOI] [PubMed] [Google Scholar]
- Werkheiser W. C., Grindey G. B., Moran R. G. Mathematical simulation of the interaction of drugs that inhibit deoxyribonucleic acid biosynthesis. Mol Pharmacol. 1973 May;9(3):320–329. [PubMed] [Google Scholar]


