Abstract
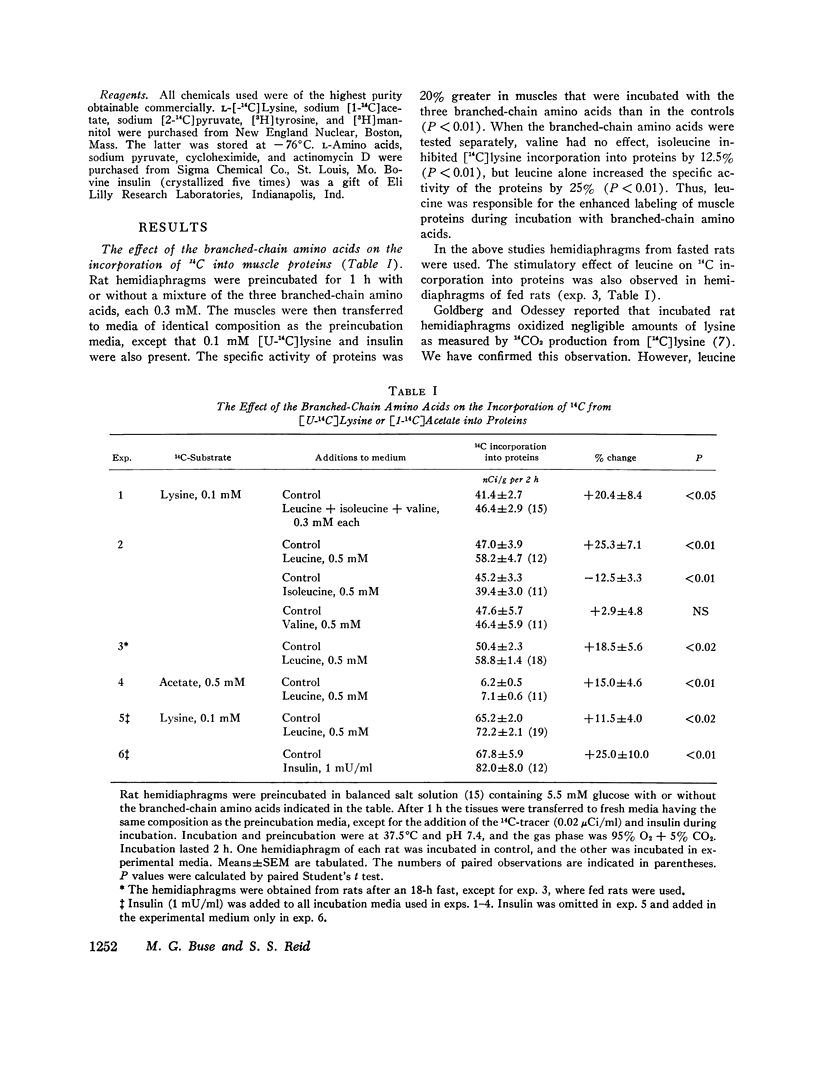
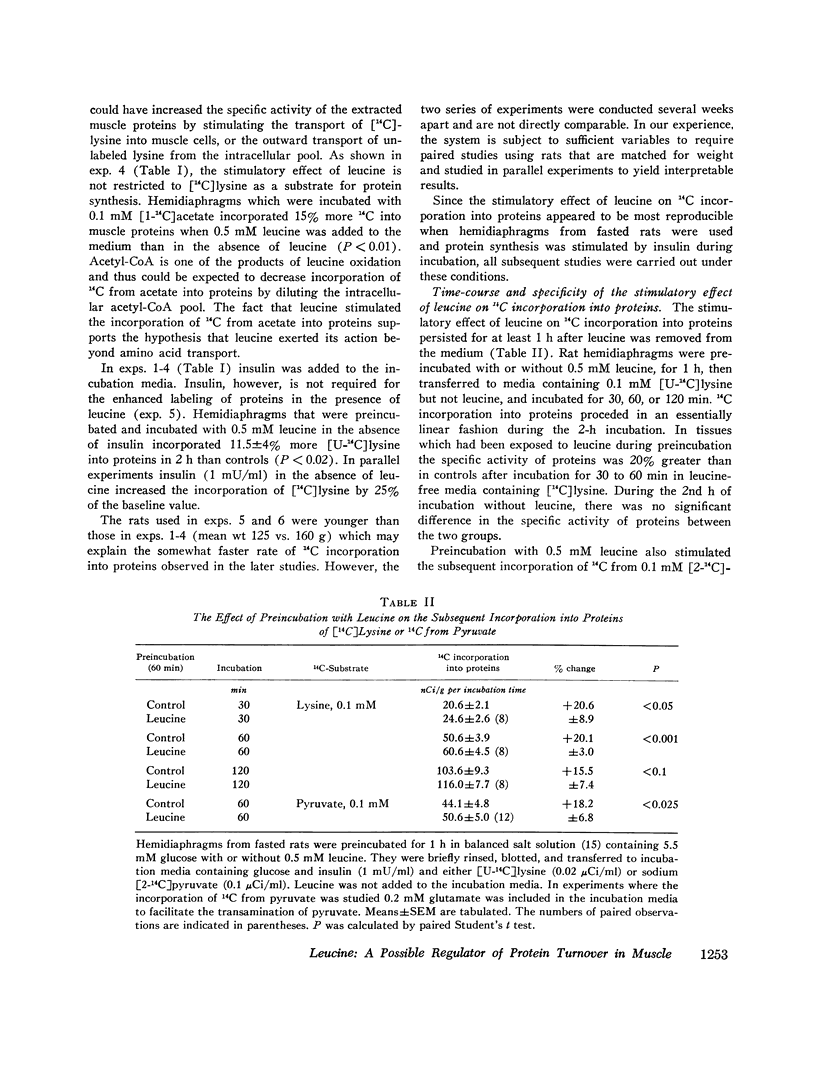
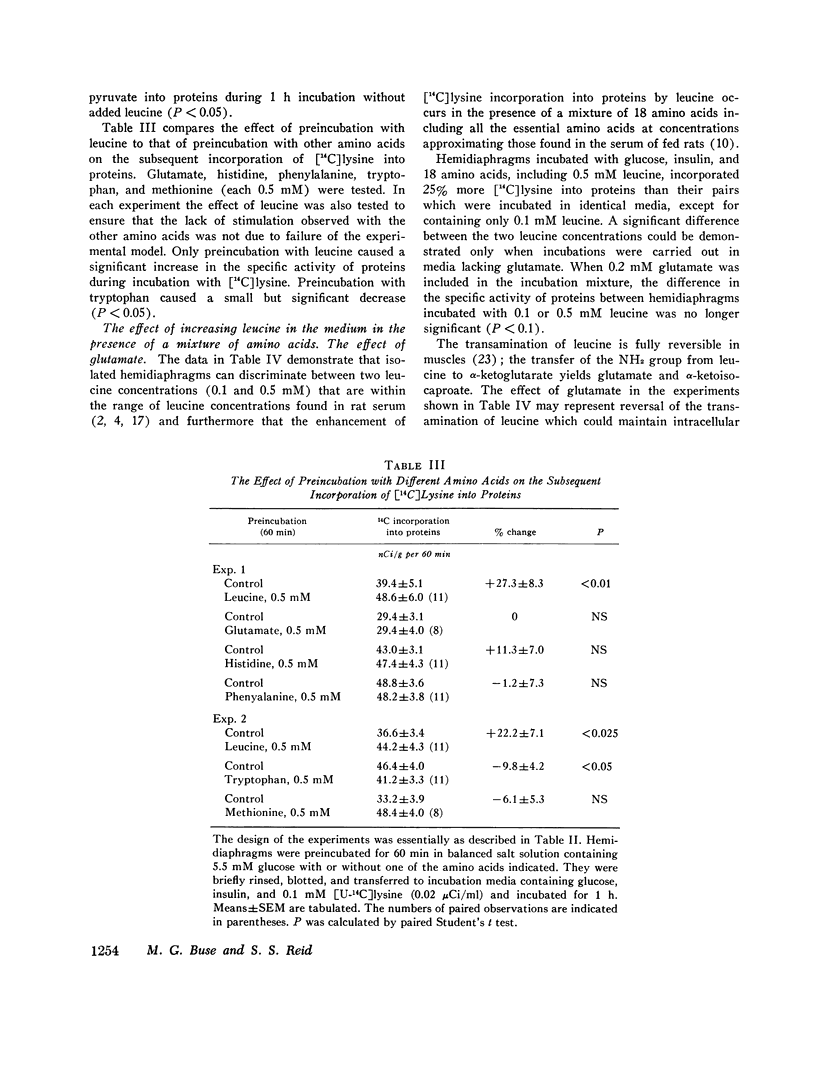
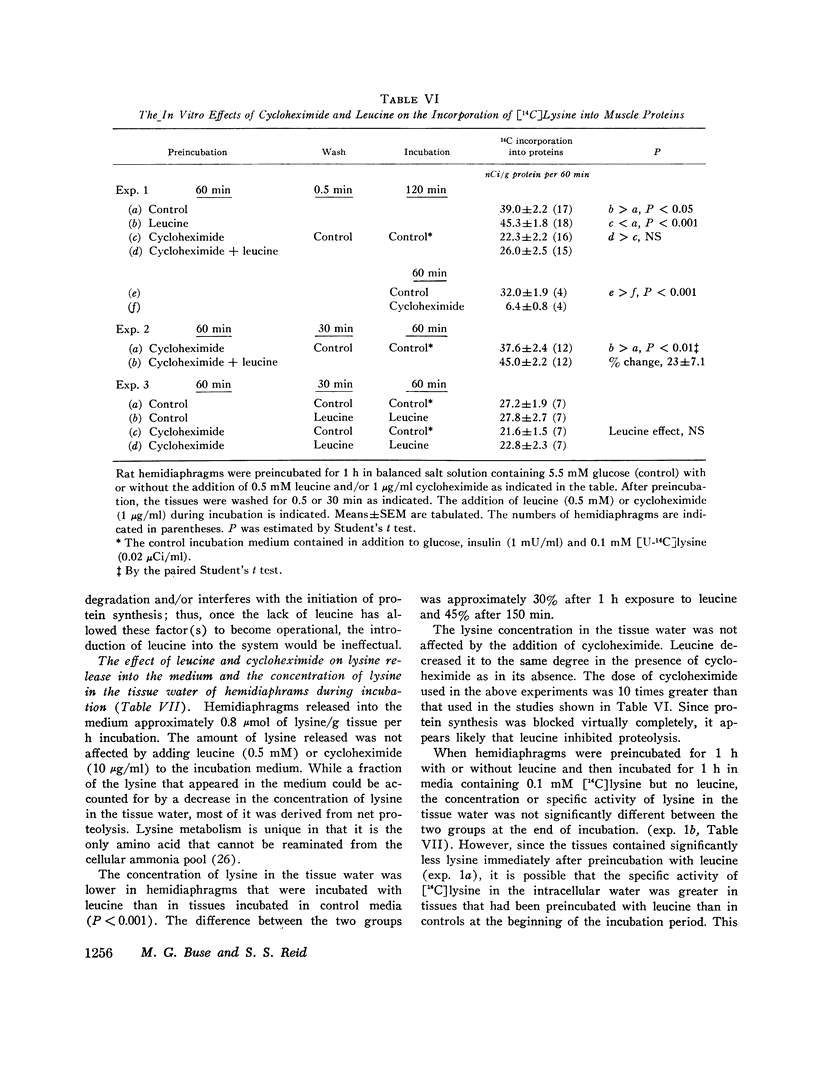
Incorporation of radiolabeled precursors into muscle proteins was studied in isolated rat hemidiaphragms. A mixture of three branched-chain amino acids (0.3 mM each) added to media containing glucose stimulated the incorporation of [14C]lysine into proteins. When tested separately, valine was ineffective, isoleucine was inhibitory, but 0.5 mM leucine increased the specific activity of muscle proteins during incubation with [14C]lysine or [14C]acetate in hemidiaphragms from fed or fasted rats incubated with or without insulin. Preincubation with 0.5 mM leucine increased the specific activity of muscle proteins during a subsequent 30- or 60-min incubation with [14C]lysine or [14C]pyruvate without leucine. Preincubation with other amino acids (glutamate, histidine, methionine, phenylalanine, or tryptophan) did not exert this effect. When hemidiaphragms were incubated with a mixture of amino acids at concentrations found in rat serum and a [14C]lysine tracer, the specific activity of muscle proteins increased when leucine in the medium was raised from 0.1 to 0.5 mM. Experiments with actinomycin D and cycloheximide suggested that neither RNA synthesis nor protein synthesis are required for the initiation of the leucine effect. Leucine was not effective when added after 1 h preincubation without leucine. The concentration of lysine in the tissue water of diaphragms decreased during incubation with 0.5 mM leucine in the presence or absence of cycloheximide, suggesting that leucine inhibited protein degradation. During incubation with [3h]tyrosine (0.35 mM) the addition of 0.5 mM leucine increased the specific activity of muscle proteins, while the specific activity of intracellular tyrosine remained constant and its concentration decreased, suggesting that leucine also promoted protein synthesis. The concentration of leucine in muscle cells or a compartment thereof may play a role in regulating the turnover of muscle proteins and influence the transition to negative nitrogen balance during fasting, uncontrolled diabetes, and the posttraumatic state. Leucine may play a pivotal role in the protein-sparing effect of amino aicds.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Interrelationships between level of amino acids in plasma and tissues during starvation. Am J Physiol. 1971 Sep;221(3):829–838. doi: 10.1152/ajplegacy.1971.221.3.829. [DOI] [PubMed] [Google Scholar]
- Bergström J., Fürst P., Norée L. O., Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974 Jun;36(6):693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- Blackburn G. L., Flatt J. P., Clowes G. H., Jr, O'Donnell T. F., Hensle T. E. Protein sparing therapy during periods of starvation with sepsis of trauma. Ann Surg. 1973 May;177(5):588–594. doi: 10.1097/00000658-197305000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Drier C., Buse J. F. The effect of epinephrine, glucagon, and the nutritional state on the oxidation of branched chain amino acids and pyruvate by isolated hearts and diaphragms of the rat. J Biol Chem. 1973 Jan 25;248(2):697–706. [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Friderici K. H., Buse J. F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972 Dec 25;247(24):8085–8096. [PubMed] [Google Scholar]
- Buse M. G., Buse J. Effect of free fatty acids and insulin on protein synthesis and amino acid metabolism of isolated rat diaphragms. Diabetes. 1967 Nov;16(11):753–764. doi: 10.2337/diab.16.11.753. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Jursinic S., Reid S. S. Regulation of branched-chain amino acid oxidation in isolated muscles, nerves and aortas of rats. Biochem J. 1975 Jun;148(3):363–374. doi: 10.1042/bj1480363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsten A., Hallgren B., Jagenburg R., Svanborg A., Werkö L. Amino acids and free fatty acids in plasma in diabetes. I. The effect of insulin on the arterial levels. Acta Med Scand. 1966 Mar;179(3):361–370. doi: 10.1111/j.0954-6820.1966.tb05471.x. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E., Ohman J. L., Cahill C. F., Jr Plasma amino acid levels in diabetic ketoacidosis. Diabetes. 1970 Oct;19(10):727–728. doi: 10.2337/diab.19.10.727. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Protein turnover and amino acid metabolism in the regulation of gluconeogenesis. Fed Proc. 1974 Apr;33(4):1092–1097. [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Howell E. M., Li J. B., Martel S. B., Prouty W. F. Physiological significance of protein degradation in animal and bacterial cells. Fed Proc. 1974 Apr;33(4):1112–1120. [PubMed] [Google Scholar]
- Goldberg A. L., Odessey R. Oxidation of amino acids by diaphragms from fed and fasted rats. Am J Physiol. 1972 Dec;223(6):1384–1391. doi: 10.1152/ajplegacy.1972.223.6.1384. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. Relationship between intracellular amino acids and protein synthesis in the extensor digitorum longus muscle of rats. Biochem J. 1969 Sep;114(2):171–178. doi: 10.1042/bj1140171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton P., Allison S. P., Littlejohn S., Lloyd J. Insulin and glucose to reduce catabolic response to injury in burned patients. Lancet. 1971 Apr 17;1(7703):767–769. doi: 10.1016/s0140-6736(71)91213-x. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Korner A. Influence of amino acid supply on ribosomes and protein synthesis of perfused rat liver. Biochem J. 1969 Mar;111(5):703–712. doi: 10.1042/bj1110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Rannels D. E., Munger B. L., Morgan H. E. Insulin in the regulation of protein turnover in heart and skeletal muscle. Fed Proc. 1974 Apr;33(4):1098–1104. [PubMed] [Google Scholar]
- KIPNIS D. M., REISS E., HELMREICH E. Functional heterogeneity of the intracellular amino acid pool in mammalian cells. Biochim Biophys Acta. 1961 Aug 19;51:519–524. doi: 10.1016/0006-3002(61)90608-4. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy K., Gopalan C. Effect of isoleucine on skin and electroencephalogram in pellagra. Lancet. 1971 Nov 27;2(7735):1167–1169. doi: 10.1016/s0140-6736(71)90486-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. L., Kenney F. T. Regulation of tyrosine- -ketoglutarate transaminase in rat liver. J Biol Chem. 1971 Dec 25;246(24):7595–7601. [PubMed] [Google Scholar]
- Li J. B., Fulks R. M., Goldberg A. L. Evidence that the intracellular pool of tyrosine serves as precursor for protein synthesis in muscle. J Biol Chem. 1973 Oct 25;248(20):7272–7275. [PubMed] [Google Scholar]
- MANCHESTER K. L., KRAHL M. E. Effect of insulin on the incorporation of C14 from C14-labeled carboxylic acids and bicarbonate into the protein of isolated rat diaphragm. J Biol Chem. 1959 Nov;234:2938–2942. [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- Meikle A. W., Klain G. J. Effect of fasting and fasting-refeeding on conversion of leucine into CO 2 and lipids in rats. Am J Physiol. 1972 May;222(5):1246–1250. doi: 10.1152/ajplegacy.1972.222.5.1246. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- Morgan H. E., Jefferson L. S., Wolpert E. B., Rannels D. E. Regulation of protein synthesis in heart muscle. II. Effect of amino acid levels and insulin on ribosomal aggregation. J Biol Chem. 1971 Apr 10;246(7):2163–2170. [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A. Continuous scintillation counting of carbon-14 and tritium in effluent of the automatic amino acid analyzer. Anal Biochem. 1962 Dec;4:444–458. doi: 10.1016/0003-2697(62)90126-4. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., Hjalmarson A. C., Morgan H. E. Effects of noncarbohydrate substrates on protein synthesis in muscle. Am J Physiol. 1974 Mar;226(3):528–539. doi: 10.1152/ajplegacy.1974.226.3.528. [DOI] [PubMed] [Google Scholar]
- Ryan N. T., George B. C., Odessey R., Egdahl R. H. Effect of hemorrhagic shock, fasting, and corticosterone administration on leucine oxidation and incorporation into protein by skeletal muscle. Metabolism. 1974 Oct;23(10):901–904. doi: 10.1016/0026-0495(74)90038-9. [DOI] [PubMed] [Google Scholar]
- Sapir D. G., Owen O. E., Pozefsky T., Walser M. Nitrogen sparing induced by a mixture of essential amino acids given chiefly as their keto-analogues during prolonged starvation in obese subjects. J Clin Invest. 1974 Oct;54(4):974–980. doi: 10.1172/JCI107838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff R., Wool I. G. Accumulation of amino acids in muscle of perfused rat heart. Effect of insulin. Biochem J. 1965 Oct;97(1):257–271. doi: 10.1042/bj0970257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff R., Wool I. G. Effect of diabetes on the concentration of amino acids in plasma and heart muscle of rats. Biochem J. 1966 Apr;99(1):173–178. doi: 10.1042/bj0990173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAALKES T. P., UDENFRIEND S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957 Nov;50(5):733–736. [PubMed] [Google Scholar]
- Walser M., Coulter A. W., Dighe S., Crantz F. R. The effect of keto-analogues of essential amino acids in severe chronic uremia. J Clin Invest. 1973 Mar;52(3):678–690. doi: 10.1172/JCI107229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser M., Lund P., Ruderman N. B., Coulter A. W. Synthesis of essential amino acids from their alpha-keto analogues by perfused rat liver and muscle. J Clin Invest. 1973 Nov;52(11):2865–2877. doi: 10.1172/JCI107483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Aikawa T., Matsutaka H., Okuda T., Ishikawa E. Interorganal relationships of amino acid metabolism in fed rats. Am J Physiol. 1974 Jun;226(6):1428–1433. doi: 10.1152/ajplegacy.1974.226.6.1428. [DOI] [PubMed] [Google Scholar]


