Abstract
Salmonella enterica serovar Typhimurium is an intracellular pathogen and a main cause of food-borne illness. In this study, a quantitative PCR (qPCR)-based competitive index (CI) method was developed to simultaneously compare the growth of multiple Salmonella strains. This method was applied to a mixture of 17 Salmonella mutants lacking regulator genes, and their survival ratios were compared based on expression of natural resistance-associated macrophage protein 1 (Nramp1). Nramp1, as a major host innate immune component, controls the intracellular replication of pathogens. Deletion strains containing unique DNA barcodes in place of regulator genes were mixed with the parental control, and the bacteria were inoculated into congenic mice differing only at Nramp1. Most of the deletion strains were outcompeted by wild-type bacteria in either mouse strain, and the lack of Nramp1 didn't increase the tested strain/parent control replication ratios. When the same collection of mutants was tested in congenic mouse-derived primary macrophages, a major Nramp1-expressing cell type, six strains (ΔhimD, ΔphoP/phoQ, ΔrpoE, ΔrpoS, ΔompR/envZ, and Δhfq strains) grew better in Nramp1−/− than in Nramp1+/+ macrophages, suggesting that these six regulators may play roles in overcoming Nramp1-mediated bactericidal activity in primary macrophages. The discrepancy in survival of macrophages and that of mice suggests either that there are differences in macrophage populations or that other cell types expressing Nramp1 control Salmonella proliferation in the host. The method described allows competitive infection analysis to be carried out on complex mixtures of bacteria and provides high reproducibility from independent biological replicates.
Salmonella enterica serovar Typhimurium, hereafter S. Typhimurium, causes gastroenteritis and a self-limiting disease in humans but a typhoid fever-like systemic disease in mice. S. Typhimurium has been studied as a model for typhoid fever because the host range of S. enterica serovar Typhi is limited to humans. Salmonella has been equipped with a plethora of virulence factors to resist hostile host intracellular milieus. Genes that encode virulence factors are widely distributed around the entire chromosome of pathogenic Salmonella, and their expression is tightly controlled by at least 20 different regulators that sense environmental cues during infection. In a previous study, we identified 17 of 83 regulator genes tested to be required for systemic infection in mice (61). Salmonella strains deleted in those 17 regulator genes were significantly attenuated in virulence in BALB/c mice (61). The virulence phenotype can be influenced by several parameters, including the route of administration, the inoculation dose, the organs examined, and the genotype of the host animal (25, 34, 53, 54). The BALB/c strain used in the previous virulence study lacks a functional Nramp1 protein, a major host innate immune component, and cannot control Salmonella replication, thereby succumbing to low infectious doses (27, 43). We reasoned that the Salmonella regulator mutants that were attenuated in BALB/c mice might exhibit different phenotypes in the presence of Nramp1.
Nramp1 (also known as Slc11a1), a highly hydrophobic protein with 12 transmembrane domains, is expressed in cells of myeloid origin and is localized mainly to the phagosomal membrane of macrophages, neutrophils, and dendritic cells (11, 22, 55). This protein is required for resistance against taxonomically unrelated pathogens, including Mycobacterium, Salmonella, and Leishmania (28, 58, 60, 64). The mechanism by which Nramp1 restrains pathogens from proliferating within host tissue cells is likely to be linked to its role as an iron and manganese antiporter, because these are essential nutrients promoting the growth of microorganisms (17, 28, 64). Besides depletion of divalent metals from the phagosomal space, Nramp1 has been reported to exert a variety of other functions. Nramp1 increases major histocompatibility complex (MHC) class II expression and antigen presentation (33, 51) and induces rapid proinflammatory responses such as upregulation of gamma interferon (IFN-γ), interleukin 1β (IL-1β), tumor necrosis factor alpha (TNF-α), and keratinocyte chemoattractant (KC) (31, 32, 46, 55, 56). As well as inducing higher production of cytokines and chemokines, Nramp1 facilitates the formation of reactive oxygen and nitrogen species as an antimicrobial defense mechanism (2, 5, 21). Expression of Nramp1 in macrophages also increases expression of Salmonella pathogenicity island 2 (SPI-2)-associated virulence genes, providing increased bacterial defenses to counteract host immunity (63).
To better understand the interaction between Salmonella and host innate immune responses mediated by Nramp1, we compared replication of a variety of Salmonella regulator mutants in mice with or without Nramp1, as well as primary macrophages derived from these same mice. A traditional method to compare growth between wild-type and mutant strains has been the competitive index (CI) assay (3, 18, 52). The conventional CI test is performed by infecting animals or cells with a mixture of mutants and wild-type bacteria that can be distinguished based on specific phenotypic differences. The number of each strain is enumerated in the input inoculum and in the output organ to compare persistence between test strains and the wild-type strain. The competitive index has become a standard for measuring virulence because it is more sensitive than the 50% lethal dose (LD50) assay and less prone to animal-to-animal differences. However, it has several disadvantages, including the excessive animal usage and the limited selection markers between the strains tested. The phenotypic traits, such as antibiotic resistance and metabolic characteristic, must be able to distinguish parent from mutant bacteria without influencing bacterial virulence (3, 18, 52). In this study, we developed a novel competitive index method using DNA barcode-tagged mutant strains, thus enabling us to determine the survival rates of numerous mutants in a single experiment by quantitative PCR (qPCR). Using the qPCR-based competitive index method (CIqPCR), we identified six Salmonella regulator mutants whose growth was more attenuated than that of the wild type in response to Nramp1 in primary macrophages.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All Salmonella strains used in this study are Salmonella enterica serovar Typhimurium 14028s and its isogenic derivatives. Mutant strains with deletions in regulator genes were constructed using modified pKD13 (pKD13-mod) plasmids (pKD13; GenBank accession no. AY048744), which were designed to replace genes of interest with 135-nucleotide (nt) barcode sequences following homologous recombination. Linearized PCR products amplified from a pKD13-mod plasmid contain a kan cassette in the middle and a 40-nt sequence at each terminus. The 40-nt termini are homologous to a gene of interest and facilitate homologous recombination at the correct chromosomal location (15). Replacement of a target gene with a kan cassette was confirmed by PCR. Prior to elimination of the kan cassette, the mutant allele was transferred to a “clean” genetic background by using P22 transduction, and the location of the kan cassette in the transductant was verified by PCR. Expression of FLP recombinase in trans removes the kan cassette via site-specific recombination (15), resulting in in-frame, nonpolar deletions of the target genes. The sequences inserted in place of a target gene are shown in Fig. 1. Regulator genes deleted in this study are listed in Table S1 in the supplemental material.
FIG. 1.
Scar sequences in deletion strains. The 135-nt scar sequences, replacing coding sequences of a target gene, are shown. The scar site or FLP recognition target (FRT) site left after FLP-mediated excision of the kan cassette is highlighted in dark gray. Barcode sequences inserted via SacI and AvrII are composed of 24 random nucleotides and are outlined in the schematic diagram. N, any nucleotide with either a purine or pyrimidine base; V, any nucleotide except thymine-based nucleotides. Sequences recognized by primers scarF and scarR in nested PCR are underlined and indicated by priming sites 4 and 1, respectively. T7 promoter sequences are shown between square brackets.
Isolation and culture of bone marrow-derived macrophages from mouse.
Bone marrow was extracted from the femurs of 5-week-old female 129SvJ mice as previously described (14) and incubated for 7 days in Dulbecco's modified Eagle's medium (DMEM; Invitrogen), which was complemented with 10% fetal bovine serum (Invitrogen) and 20% L929 cell supernatant containing macrophage colony-stimulating factor. Bone marrow-derived macrophages (BMDM) were counted and 2 × 106 cells were seeded in each well of a 6-well plate 1 day before infection. BMDM were incubated in DMEM containing 10% fetal bovine serum at 37°C with 5% CO2 overnight.
CIqPCR in macrophages.
Salmonella strains were grown individually in Luria-Bertani (LB) broth overnight and equivalent amounts of each strain were mixed as an inoculum. A mix of bacteria strains was opsonized with 10% mouse serum (Innovative Research) for 20 min prior to infection (8). Bacterial cells were added to BMDM monolayers at an input multiplicity of infection (MOI) of 10, and infection was initiated by centrifugation at 1,000 × g for 5 min. In order to estimate the threshold cycle (CT) value of each strain in the input, the same dose of bacteria mix, which was inoculated into a single macrophage well, was washed with distilled water and used as a template in nested PCR (2 × 107 CFU/PCR). The resulting PCR products were used as template DNAs in quantitative PCR after a serial dilution. Following 30 min of incubation at 37°C with 5% CO2, the medium was replaced with DMEM containing 100 μg/ml gentamicin, and cells were incubated for 1 h to remove extracellular bacteria. After treatment with 100 μg/ml gentamicin, BMDM were washed with PBS twice and overlaid with DMEM containing 20 μg/ml gentamicin for the remainder of the experiment. In order to enumerate intracellular bacteria at appropriate time points after infection, BMDM were lysed with 1% Triton X-100 for 10 min after several PBS washes, and the lysate was spun to collect bacterial cells at 4,500 × g for 5 min. A pellet of bacterial mix from each well was washed three times with PBS and resuspended in 30 μl of distilled water. A bacterial mix from a single well of a 6-well plate was regarded as an individual output sample, and the cells in suspension from a single well (30 μl) were used as a template in nested PCR. Serially diluted nested PCR products were subjected to quantitative PCR to measure the CT value of each strain in the output samples.
In a nested PCR of input and output samples, two primers recognizing priming sites 4 and 1 (see Fig. 1), scarF (5′-ATTCCGGGGATCCGTCGACCT-3′) and scarR (5′-GTGTAGGCTGGAGCTGCTCC-3′), respectively, were used to amplify 24-nt barcode sequences from a variety of deletion strains. Nested PCR was performed by one cycle of 95°C for 10 min, 30 cycles of 95°C for 30 s, 50°C for 30 s, and 72°C for 30 s, and one cycle of 72°C for 5 min. Serially diluted nested PCR products were used as template DNAs in quantitative PCR using primer scarF and primers specific to the barcode sequences. Duplex DNA products resulting from quantitative PCR were detected with SYBR green reagent (Applied Biosystems) by using a StepOnePlus real-time PCR instrument (Applied Biosystems). Real-time PCR was carried out by 50 cycles of 95°C for 15 s and 60°C for 1 min, following 95°C for 10 min. In order to normalize CT values, the amplification efficiency of each barcode primer during PCR was calculated from the standard curve of each strain by using serially diluted template DNAs. Mutant strain/wild-type strain survival ratios were quantified using PCR amplification efficiency (E) and CT values of strains in input and output qPCR analyses by using the CIqPCR formula below (42, 62). At threshold, Qwt × 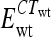 = Qmutant ×
= Qmutant × 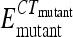 (where Q is template DNA quantity and wt is wild type).
(where Q is template DNA quantity and wt is wild type).
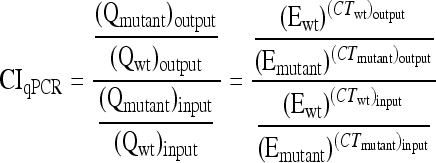 |
(1) |
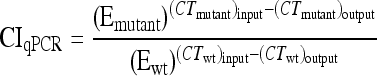 |
(2) |
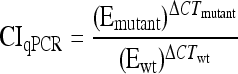 |
(3) |
CIqPCR in mouse.
Salmonella strains were grown individually in LB medium overnight as described above and mixed equivalently in phosphate-buffered saline (PBS) based on optical density at 600 nm (OD600) values. A bacterial mixture was washed with PBS and diluted to 105 CFU/ml. Female 4- to 5-week-old Nramp1+/+ 129SvJ (Jackson Laboratory) mice and congenic Nramp1−/− 129SvJ (57, 58) mice were intraperitoneally (i.p.) infected with 100 μl of the mixed Salmonella strains at a final dose of 104 CFU/mouse. The number of injected bacteria was confirmed by plating diluted inoculum on LB agar plates. A portion of the inoculation mixture, corresponding to 2 × 108 CFU, was resuspended in distilled water and used in nested PCR to evaluate CT values of the strains used as the input. At desired time points after infection, mice were euthanized to compare bacterial persistence between strains. The liver and spleen were homogenized and plated on LB agar to isolate intracellular Salmonella. Colonies were scraped and collected in a tube with PBS. In order to assess CT values in the output, a mixture of bacterial cells corresponding to 2 × 108 CFU was resuspended in distilled water and used as the template for nested PCR (2 × 108 CFU/PCR). Barcode DNAs amplified from nested PCRs of input and output samples served as template DNAs in the qPCR step, as above.
As an alternative, total DNAs were isolated from the spleen or liver homogenates using GeneElute bacterial genomic DNA kit (NA2110, Sigma) and used as templates in nested PCR instead of isolating bacterial colonies on agar plates. The CIqPCR values were comparable between two methods, although interestingly a larger deviation was observed between specimens when a small piece of liver was applied, as reviewed by Mastroeni et al. (38).
Phenotype-based conventional CI.
Bacterial strains were cultivated individually overnight in LB prior to infection. Each strain was washed and diluted in PBS at 2 × 105 CFU/ml. The test strain was mixed with a reference strain (MA6054) at a ratio of 1:1, and 100 μl (104 CFU/mouse) of the mixed cells was used to infect Nramp1+/+ 129SvJ mice intraperitoneally. The inoculum, used in infection, was diluted in PBS and spread on plates to enumerate the injected dose of bacteria. The reference strain, MA6054, produces an arabinose-inducible β-galactosidase and can be distinguished from test strains on LB agar containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg/ml) and arabinose (1 mM) (26). Infected mice were sacrificed at days 2, 5, and 7 postinfection to isolate the spleens. The spleens were homogenized by mechanical disruption, and the suspensions were plated on LB agar plates with X-Gal and arabinose. The conventional competitive index was then calculated as [number of test strains/number of reference strains]output/[number of test strains/number of reference strains]input (3, 18, 52).
Ethics statement.
Mouse experiments were approved under the protocol of Oregon Health & Science University Institutional Animal Care and Use Committee (OHSU IACUC; no. A085/2008) and performed in accordance with the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health to minimize animal suffering.
RESULTS
Strategy to tag deletion mutants with 24-nt barcodes.
Homologous sequence-mediated recombination methods have been widely used to delete genes of interest in eukaryotes and prokaryotes (12, 15, 16). Using the bacteriophage λ Red (γ, β, exo) recombinase system established by Datsenko and Wanner, a target Salmonella gene is replaced with a linear PCR fragment consisting of an antibiotic resistance gene flanked with 40-nt sequences identical to the 5′ and 3′ ends of the gene to be deleted (15, 16). Once antibiotic-resistant clones are obtained, the antibiotic marker gene is eliminated by FLP recombinase, leaving a scar sequence containing a single FLP recombinase target (FRT). We modified plasmid pKD13, which is one of the PCR template plasmids used in the original λ Red recombination method, for use with our method (15). Two restriction enzyme recognition sites (SacI and AvrII) were added between priming site 1 and the FRT site. Then, double-stranded DNAs containing a T7 promoter and a random 24-nt sequence synthesized without T in the first position of each codon were cloned between the SacI and AvrII sites. More than 150 synthetic DNAs were cloned in the pKD13 derivative plasmid and sequenced to ensure that the insert did not have stop codons or frameshift mutations. Finally, 102 modified pKD13 (pKD13-mod) plasmids harboring unique barcodes were constructed and tested in qPCRs using primers specific to the inserted barcodes. A pool containing equivalent DNA from each pKD13-mod was analyzed in qPCR to examine the efficiency of the barcodes during PCR. Of the 102 pKD13-mod derivatives containing 24-nt barcodes, 88 were found to perform well in test qPCRs, producing comparable CT values at a threshold level. Thereafter, these 88 pKD13-mod plasmids were employed as templates in the construction of deletion strains. The allelic replacement strategy was to replace all codons between the translational initiation codon and the last seven codons with a 135-nt sequence containing a DNA barcode and T7 promoter. The final sequence following FLP-mediated recombination encodes a 45-amino-acid sequence without stop codons that is in frame and therefore not likely to be polar upon expression of downstream genes in the same operon. The T7 promoter upstream of the barcode sequences can be used to identify strains using in vitro transcription from the T7 promoter. Small labeled RNAs produced via AvrII digestion followed by in vitro transcription can be applied to microarray hybridization for quantification of strains. An additional advantage of this approach was the ability to leave the last 7 amino acids of the deleted gene to avoid the frequent problem of overlapped gene coding sequences (B. L. Wanner, personal communication). The scar sequence remaining after FLP-mediated excision of the kan cassette is shown in Fig. 1. Using the pKD13-mod library with a variety of barcodes, genes of interest were deleted by λ Red recombination and replaced with a 135-nt sequence, including a unique barcode. For the parental control in competitive infection studies, we inserted a barcode in the pseudogene STM0314, which did not affect Salmonella virulence (see Fig. S1 in the supplemental material).
Calculation of the competitive index utilizing quantitative PCR in mixed populations.
Quantitative reverse transcription-PCR (qRT-PCR) has been a strong tool for measuring transcription levels and expression changes of genes of interest (40, 51, 61). We applied qRT-PCR to a mixed bacterial population to enumerate each bacterial strain and compare growth and survival between strains during a single infection. Each mutant strain was distinguished via a 24-nt barcode with a specific primer in the mixed population. The quantity of PCR products is theoretically proportional to the quantity of initial template DNAs under the exponential phase of increase, when PCR reagents are not limited (24). The amounts of PCR products are deducible from the initial template quantities and amplification efficiencies of barcode primers and are equivalent between strains at a threshold level. If the PCR efficiency is ideal, the amount of PCR product will double for each cycle during the exponential phase of PCR amplification (37). However, due to differences in primer specificity among the barcode sequences, the amount of PCR products will not be increased twice every cycle (29, 44). Therefore, it was necessary to take account of the PCR efficiency of each barcode primer in enumeration by using RT-PCR (44, 62). The relative ratio of a mutant strain to the wild-type strain was determined from the efficiency-calibrated mathematical model, which has been broadly used for relative quantification in RT-PCR (42, 62). To calibrate CT values between strains, the amplification efficiency (E) of each barcode primer was determined using the slope of the standard curve of each strain, as demonstrated in Fig. S2 in the supplemental material (9, 42). As shown in the equation in Materials and Methods, a competitive index formula using qPCR (CIqPCR) was computed based on PCR amplification efficiency and CT values of strains in input and output samples.
In order to rule out the possibility of cross-reactivity between different barcode primers, a bacterial mixture composed of equivalent amounts of 8 barcode-tagged strains was subjected to qPCR-based relative quantification using 8 cognate barcode primers, specific to the 8 mixed strains and 8 unrelated primers, chosen at random but not specific to the 8 test strains (see Fig. S3 in the supplemental material). The unrelated barcodes showed a ΔCT value (reference barcode CT − test barcode CT) of less than −15 cycles (−25.6 ≤ ΔCT ≤ −15.2), whereas barcodes used in the mixed strains exhibited CT values similar to that of the reference barcode (see Fig. S3 in the supplemental material), indicating that these 8 unrelated barcodes gave a signal that was essentially undetectable compared to the signal from relevant barcodes.
Description of the CIqPCR method.
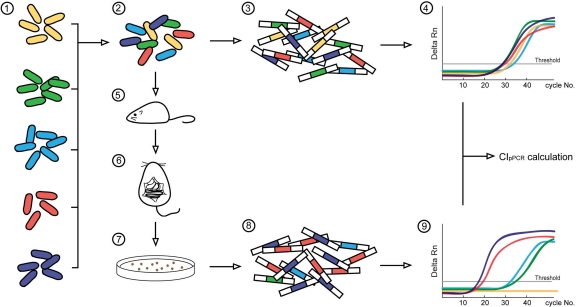
A flowchart of the CIqPCR method in mice is shown in Fig. 2. A reference strain (the ΔSTM0314 strain) and deletion strains, each with a unique barcode, were cultivated individually prior to infection. Strains were mixed equally in the inoculum and 104 CFU of the mixed cells were intraperitoneally administered to each mouse. The inoculum was subjected to qPCR-based quantification to calculate initial CT values (CTinput) for each strain. At day 5 postinfection, the liver and spleen were harvested, homogenized, and plated on agar plates to grow the intracellular bacterial population. Bacterial strains were collected and combined, and the mix was used to measure CToutput values of the deletion strains. CTinput and CToutput values were entered in the CIqPCR equation to assess the competitive indices of the strains tested. As an alternative to plating the spleen and liver homogenates, total DNA was prepared from the infected organs and used as a template in quantitative PCR. Similar results were observed with both methods.
FIG. 2.
Schematic of the qPCR-based CI method in a mouse model. Bacterial strains were cultivated separately overnight (1) and mixed equivalently based on OD600 measurement (2). Mixed cells corresponding to 2 × 108 CFU were used directly in nested PCR to amplify the barcode sequences from the input inoculum (3). Product DNAs from nested PCR were used as templates in the following input qPCR (4). In order to compare survival rates between strains, mice were i.p. injected with 104 CFU consisting of an equal mix of each of the mutant strains and the parent strain (5). The inoculum was verified by plating and counting the number of CFU. The mice were euthanized to isolate Salmonella-infected organs at the desired time point after infection (6). Organs were homogenized and spread on LB agar to grow bacteria (7). Bacterial colonies were scraped and collected the next day. The mixture was diluted in PBS buffer, and 2 × 108 CFU were used as the template for nested PCR (8). Bacterial strains from the output populations were analyzed by qPCR using barcode-specific primers (9). CIqPCR was calculated by comparing CT values between a wild-type strain and a mutant strain in input and output qPCRs, taking into account that the amplification efficiency of individual barcodes ranged from 1.53 to 1.94.
The quantitative PCR was performed using a two-step PCR procedure described in detail in Materials and Methods. In an initial nested PCR step, two outside primers corresponding to either end of the 135-bp scar sequences were used to amplify the barcode sequences. The products, each of which had the same ends but a unique barcode in the middle, were serially diluted and used as templates in a second round of amplification: qPCR in which one of the outside primers and the barcode-specific primer were used. By using nested PCR rather than qRT-PCR directly on the mixture, the specificity was greatly increased, as has been shown in other studies and observed in our laboratory (1, 23, 49).
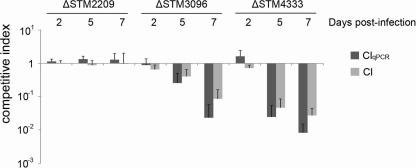
The reliability of the CIqPCR assay was validated by comparison with the traditional CI method (Fig. 3). Four strains, including a reference strain (the ΔSTM0314 strain) and three barcode-labeled strains (ΔSTM2209, ΔSTM3096, and ΔSTM4333 strains), were mixed equivalently, and the bacteria were inoculated intraperitoneally into mice (104 CFU/mouse). Intracellular bacteria residing in the spleens were enumerated using CIqPCR at days 2, 5, and 7 postinfection. In parallel with the CIqPCR assay, each deletion strain was mixed with a reference strain (MA6054 [26]), which has an arabinose-inducible β-galactosidase gene, at a 1:1 ratio, and 104 CFU of the mixed bacteria was inoculated into each mouse. Mice were sacrificed at days 2, 5, and 7 postinfection, and the spleen homogenates were plated on LB agar with X-Gal and arabinose. The conventional CI was calculated using the equation described in Materials and Methods. The CIqPCR assay exhibited results similar to those of the conventional CI assay. ΔSTM3096 and ΔSTM4333 strains showed a tendency to decrease more in the CIqPCR assay than in the traditional CI. This difference may be due to the lower bacterial number for each strain at inoculation in the CIqPCR assay (2.5 × 103 CFU in CIqPCR versus 5 × 103 CFU in CI), which may be cleared faster by the host immune system. However, the differences were not statistically significant (P values were all >0.15).
FIG. 3.
Comparison of CIqPCR with the traditional CI. In the CIqPCR assay, a reference strain (the ΔSTM0314 strain) and three deletion strains (ΔSTM2209, ΔSTM3096, and ΔSTM4333 strains) with barcodes were mixed equivalently, and the mix was used to infect a group of three Nramp1+/+ 129SvJ mice at 104 CFU/mouse. For the traditional CI infection, a mixture containing a reference strain (MA6054) and each single strain at a 1:1 ratio was used to infect a group of three Nramp1+/+ 129SvJ mice at 104 CFU/mouse. Mice were sacrificed at days 2, 5, and 7 postinfection, and the intracellular bacteria residing in the spleens were enumerated using the traditional CI formula or CIqPCR formula as described in Materials and Methods. There was no statistically significant difference between the two methods based on Student's t test.
Survival of Salmonella mutants lacking virulence regulators in mice (Nramp1+/+ and Nramp1−/− 129SvJ).
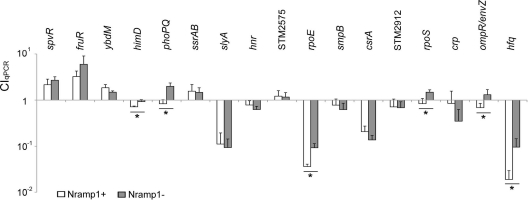
In order to confirm the validity of the CIqPCR method as a high-throughput screening tool, the persistence of 17 mutants lacking regulator genes was compared within Nramp1+/+ and Nramp1−/− mice using quantitative PCR. Our previous work identified 17 regulators required for growth in BALB/c mice by either intragastric (i.g.) or intraperitoneal (i.p.) infection (61). While these regulators were clearly required for systemic infection in BALB/c mice, which is Nramp1−/−, we thought it possible that some of these mutant strains might have a differential effect on survival of 129SvJ Nramp1+/+ and 129SvJ Nramp1−/− mice. The 17 regulators required for survival in Nramp1−/− BALB/c mice are SpvR, FruR, YbdM, HimD, PhoP/PhoQ, SsrA/SsrB, SlyA, Hnr, STM2575, RpoE, SmpB, CsrA, STM2912, RpoS, Crp, OmpR/EnvZ, and Hfq (see Table S1 in the supplemental material). Two genes (STM2575 and STM2912) annotated as regulators but not characterized further were included. Genes encoding these 17 regulators were replaced with unique barcode DNAs listed in Table S2 in the supplemental material. A mixture containing equal amounts (around 556 CFU/strain) of the 17 mutants and the reference strain (the ΔSTM0314 strain) was inoculated i.p. into mouse strain 129SvJ with or without Nramp1 at 104 CFU/mouse. Bacterial cells in the input inoculum and the output splenic homogenates were subjected to a qPCR-based CI test as described above, and the survival rate of each strain was enumerated at day 5 postinfection (Fig. 4). Considering that a CIqPCR value of 1 indicates a comparable growth between the parent strain and a mutant, most of the deletion strains showed growth attenuation in both congenic Nramp1+/+ and Nramp1−/− mice, which is consistent with our single-infection results (see Table S1 in the supplemental material) (61) and the results reported elsewhere by other investigators. Some mutant strains, including ΔspvR, ΔhimD, ΔSTM2912, ΔrpoS, and ΔompR/envZ strains, were outcompeted by wild-type bacteria more strongly in Nramp1−/− mice than in Nramp1+/+mice. The mutant strains with lower CIqPCR values in Nramp1−/− mice are likely to be attenuated in intracellular replication independent of Nramp1, whereas the wild-type reference bacteria proliferate better in Nramp1−/− mice.
FIG. 4.
CIqPCR analysis of 18 strains in Nramp1+/+ and Nramp1−/− mice. Groups of five mice (Nramp1+/+ and Nramp1−/− 129SvJ) were infected with equal mixtures of 17 strains, containing mutations in the genes indicated, as well as the wild-type control. The total number of infecting bacteria was about 104. The spleens were extracted at day 5 postinfection, and persistence levels of Salmonella strains in the spleen were compared via qPCR using the formula shown. CIqPCR values were averaged from the five mice in each group and shown in white (Nramp1+/+) and gray (Nramp1−/−). Strains with CIqPCR values greater than 1 indicate that they outcompeted the wild-type strain; strains with values less than 1 indicate that they were outcompeted by the wild-type strain. A Student t test was applied for statistical analysis of the results, and strains showing significant changes between Nramp1+/+ and Nramp1−/− mice (P < 0.05) are labeled with an asterisk.
Virulence-attributed phenotypes can vary depending on the origin of host cells (7). After i.p. administration into host animals, Salmonella migrates quickly to the filtering organs, the spleen and liver (45, 47). To assess whether the survival and growth of the regulator mutant strains differed in the spleen and liver, the same mixture of 17 deletion strains and a reference strain was injected into Nramp1+/+ or Nramp1−/− mice, and the persistence in the spleen was compared to the persistence in the liver in each mouse strain (see Fig. S4 in the supplemental material). With the exception of 6 strains (ΔphoP/phoQ, ΔssrA/ssrB, ΔslyA, ΔcsrA, ΔSTM2912, and Δhfq strains), the regulator mutants exhibited similar growth profiles between the two organs, suggesting that the spleen and liver provide a similar milieu to the survival of the tested strains. Notably, the ΔssrAB strain had a lower CIqPCR in Nramp1−/− liver than in Nramp1+/+ liver but comparable values in the spleen. Overall, strains (including ΔhimD, ΔslyA, Δhnr, ΔrpoE, ΔcsrA, and Δhfq strains) appeared to be more defective for growth in the liver than in the spleen. The lower CIqPCR in the liver might be attributable to a more restrictive environment in the liver for the growth of some mutants, as observed in the infection with Listeria monocytogenes (10, 13). The more significant attenuation of the ΔslyA strain in the liver has been noted before and is consistent with our observation (35).
Survival of Salmonella mutants lacking virulence regulators in primary bone marrow-derived macrophage from Nramp1+/+ and Nramp1−/− 129SvJ mice.
Comparing the persistence of Salmonella mutants lacking regulators in Nramp1+/+ and Nramp1−/− mice to that of the parent control, we did not find any regulator mutants that survived better in Nramp1−/− than in Nramp1+/+ mice (see Fig. 4). Most of the deletion strains were defective for growth in either mouse strain. After phagocytosis of pathogenic bacteria, Nramp1 is targeted to the membrane of the pathogen-containing phagosome in macrophages, neutrophils, and myeloid-derived dendritic cells (11, 22, 48, 55). In order to further investigate the effects of the 17 regulators on Salmonella survival in the presence of Nramp1, we also performed the assay in a cell culture model of infection. BMDM were prepared from both Nramp1+/+ and Nramp1−/− 129SvJ mice and infected with equal mixtures of the 17 deletion strains and the parent strain at an input MOI of 10 to 1. At 30 min, 6 h, and 18 h after infection, primary macrophages were lysed and the intracellular bacteria were subjected to two-step qPCR to enumerate each deletion strain (Fig. 5; see also Fig. S5 in the supplemental material). CIqPCR values at 18 h postinfection are shown in Fig. 5. Some Salmonella strains, including the ΔhimD, ΔphoP/phoQ, ΔrpoE, ΔrpoS, ΔompR/envZ, and Δhfq strains, survived better (i.e., a higher CIqPCR value) in Nramp1−/− macrophages than in Nramp1+/+ macrophages, indicating that these strains were more sensitive to the effects of Nramp1. Additionally, Salmonella strains not expressing SlyA, RpoE, CsrA, and Hfq were attenuated more than 5-fold compared to the parent strain in both Nramp1+/+ and Nramp1−/− mouse-derived cells, implying significant roles of SlyA, RpoE, CsrA, and Hfq during bacterial survival in macrophages. However, two mutant strains, the ΔspvR and ΔfruR strains, replicated significantly better than a wild-type strain in both Nramp1+/+ and Nramp1−/− macrophages. Different phenotypes between macrophages and animal models may be attributed to the function of other cell types in controlling replication of pathogenic bacteria in the whole animal, as described further in Discussion.
FIG. 5.
CIqPCR analysis of 18 strains in Nramp1+/+ and Nramp1−/− BMDM. Equal mixtures of the deletion strains indicated above were used to infect Nramp1+/+ and Nramp1−/− bone marrow-derived macrophages that were approximately 50% confluent at an input MOI of 10. Macrophages were lysed at18 h postinfection, and the survival ratios of Salmonella mutants to the reference strain were compared as described in Materials and Methods. CIqPCR values from three independent BMDM infections were averaged, and CIqPCR in Nramp1+/+ (white) and Nramp1−/− (gray) are shown. Strains with CIqPCR values greater than 1 indicate that they outcompeted the wild-type bacteria in survival; strains with values less than 1 indicate that they were outcompeted by the wild type. Mutant strains showing significant differences in CIqPCR values for Nramp1+/+ and Nramp1−/− cells are denoted by an asterisk (P < 0.05 in the Student t test).
DISCUSSION
In this work, we demonstrate the efficacy of a novel qPCR-based CI method to distinguish the effect of Salmonella regulators in the presence or absence of Nramp1. By tagging mutant strains with unique DNA sequences, we were able to evaluate growth of multiple strains by quantitative PCR. Using far fewer mice than what would be required for LD50 experiments, the qPCR-based competitive index method was robust and resulted in reduced variation between animals (average standard deviation of 0.41 among mice and 0.36 among macrophage cultures). As in other high-throughput screening methods, the CIqPCR method required several parameters to be optimized for successful reproducibility in an animal model inoculated with the same pool of mutant strains. Using too high of an inoculum may overwhelm the host immune response, quickly killing the mice and leading to the growth of avirulent mutants that could otherwise be attenuated. However, using too small of an inoculum may result in complete clearance of individual mutants, leading to a spuriously low competitive index. Higher-dose inoculation has been suggested to obtain consistent phenotypic characteristics of mutant strains unless it causes a physical burden irrelevant to phenotypic results (39). We used a mixture of 104 CFU as an inoculum in Nramp1+/+ or Nramp1−/− mice. The larger the number of strains to be tested together, the smaller the inoculum of any single mutant. Consistent results were obtained regardless of the number of tested strains when the number of mixed strains was between 2 and 36 in the inoculum of 104 CFU (data not shown). However, titration of optimal inoculum should be carried out depending on the genotype of the host animal, the route of administration, and the period of infection. Some mutant strains are attenuated for growth in intragastric infection but not in intraperitoneal infection or vice versa (see Table S1 in the supplemental material). Bacterial persistence profiles can also be influenced by the infection period as well. We observed that some Salmonella mutant strains (ΔSTM2281, ΔhilD, and ΔbarA strains) were attenuated at day 7 postinfection but were comparable with a wild-type strain at day 2 postinfection (data not shown). A short infection period may not provide a sufficient time for some mutants to exhibit their phenotype if the deleted genes play roles in long-term systemic infection in the host, such as resistance to the adaptive immune system.
The composition of strains in the mixed population might also affect their survival phenotypes. The possibility of trans complementation has been an issue in previous coinfection experiments, in which one bacterial mutant could complement another during infection of the same mouse. Trans complementation is more likely to occur in high-dose inoculations where multiple bacteria may infect a common host cell. Recently, outer membrane vesicles have been highlighted due to their role in transferring virulence factors between adjacent bacteria and even between host cells (6, 30, 59) (Yoon et al., submitted for publication). Regulator mutants defective in the expression of virulence factors, which are secreted by outer membrane vesicles, might be complemented, at least in principle, by vesicles produced from coinfecting parental bacteria. However, in comparing individual infections to infections with a mixture, we have not yet observed the possibility of trans complementation. The importance of dose, combination and ratio of strains, infection time, site of recovery, and infection route has been previously emphasized in mixed infections using two or more strains (4, 53).
Nramp1 is expressed exclusively in dendritic cells, macrophages, and neutrophils—all cells of myeloid origin (22, 48, 51). Comparing bacterial survival between Nramp1+/+ and Nramp1−/− macrophages, we identified 6 regulators (HimD, PhoP/PhoQ, RpoE, RpoS, OmpR/EnvZ, and Hfq) whose absence more strongly attenuated Salmonella survival in Nramp1+/+ than in Nramp1−/− macrophages. These 6 regulators may regulate virulence factors necessary for resistance to Nramp1-mediated bactericidal activities within macrophages. Salmonella has been reported to increase expression of Salmonella pathogenicity island 2 (SPI-2)-associated virulence genes in response to Nramp1, presumably as a bacterial defense (63). In a previous study, we observed that these 6 regulators coordinately activated SPI-2 genes under acidic minimal media that partially mimic the macrophage intracellular milieu (61). Accordingly, these 6 regulators may have a role in the induction of SPI-2 expression responding to Nramp1.
In the acute mouse infection model, Salmonella cells are rapidly disseminated to the spleen and liver and are present in several cell types (19). However, the survival phenotype within macrophages has been regarded as a barometer to infer virulence in the animal; therefore, we anticipated that there would be little difference between Nramp1+/+/Nramp1−/− mice and primary macrophages derived from the same strains of mice. Interestingly, Salmonella strains with deletions in spvR, himD, phoP/phoQ, ssrA/ssrB, smpB, crp, and ompR/envZ were significantly attenuated in mice at day 5 postinfection (Fig. 4) and at a shorter infection time (less than 4 days [data not shown]) but not in bone marrow-derived macrophages (Fig. 5). This discrepancy might be attributable to several possibilities. The microbicidal activity of macrophages may vary between the sources, as described in reference 36. Buchmeier and Heffron reported that the survival of Salmonella mutant strains is influenced by the origin of macrophages (7). Peritoneal macrophages are more microbicidal than splenic and bone marrow-derived macrophages toward Salmonella (7). Salmonella strains inoculated into the peritoneal cavities of the mice appear to be cleared more efficiently than Salmonella in primary macrophage infections. Differential survival within other tested organs has been observed in infection with a variety of pathogenic bacteria, and thus the target organ has been another parameter in considering the survival ability of a mutant in the host (7, 10, 13, 20). Another possible explanation for the discrepancy in survival between macrophages and mice is the contribution of cell types other than macrophages in controlling bacterial proliferation. Geddes et al. determined the cell types targeted by Salmonella using flow cytometry and found that ∼55% and ∼18% of the infected cells were neutrophils and monocytes, respectively, whereas macrophages containing Salmonella were hardly detected (19). Furthermore, Salmonella was able to replicate intracellularly within neutrophils despite the short half-life and bactericidal properties of these cells. Surprisingly ∼23% of infected splenic cells were B and T cells (19). Dendritic cells play a role in shuttling Salmonella across the intestinal epithelial barrier and are the only cell type capable of stimulating naïve T cells (41, 50). Recently, it was reported that Nramp1 expression is increased in intestinal, splenic, and bone marrow-derived dendritic cells upon Salmonella infection, although the increased expression did not trigger increased clearance of bacteria (55). Based on these diverse locations of Salmonella, the roles of Nramp1 in other phagocytic cells needs to be defined to understand the interaction between Nramp1 and Salmonella. Survival in cells other than macrophages may be important for Salmonella to either manipulate host cellular functions or subvert host immune responses and cause systemic disease.
In conclusion, our results demonstrate that the CIqPCR method is a novel technique that can be employed to enumerate multiple strains in mixed pools of bacteria in a short time within the same animal, decreasing animal-to-animal variation. This new approach to investigating the role of bacterial regulators is applicable to other pathogenic bacteria and will help elucidate how virulence is coordinated in relation to specific host factors.
Supplementary Material
Acknowledgments
This work was supported by the NIAID National Center for Research Resources under grant no. 5R01 AI 022933 23 and the U.S. Department of Energy Office of Biological and Environmental Research (DOE/BER) under contract no. DE-AC05-76RLO-1830.
We thank Rebecca Tempel and Jacob Eccles for fruitful discussions.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 1 November 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Aberle, S. W., and E. Puchhammer-Stockl. 2002. Diagnosis of herpesvirus infections of the central nervous system. J. Clin. Virol. 25(Suppl. 1):S79-S85. [DOI] [PubMed] [Google Scholar]
- 2.Barton, C. H., S. H. Whitehead, and J. M. Blackwell. 1995. Nramp transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on oxidative burst and nitric oxide pathways. Mol. Med. 1:267-279. [PMC free article] [PubMed] [Google Scholar]
- 3.Baumler, A. J., R. M. Tsolis, P. J. Valentine, T. A. Ficht, and F. Heffron. 1997. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella typhimurium to cause murine typhoid. Infect. Immun. 65:2254-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beuzon, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 3:1345-1352. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell, J. M., S. Searle, T. Goswami, and E. N. Miller. 2000. Understanding the multiple functions of Nramp1. Microbes Infect. 2:317-321. [DOI] [PubMed] [Google Scholar]
- 6.Bomberger, J. M., D. P. Maceachran, B. A. Coutermarsh, S. Ye, G. A. O'Toole, and B. A. Stanton. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchmeier, N. A., and F. Heffron. 1989. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect. Immun. 57:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier, N. A., and F. Heffron. 1991. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 59:2232-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustin, S. A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25:169-193. [DOI] [PubMed] [Google Scholar]
- 10.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canonne-Hergaux, F., J. Calafat, E. Richer, M. Cellier, S. Grinstein, N. Borregaard, and P. Gros. 2002. Expression and subcellular localization of NRAMP1 in human neutrophil granules. Blood 100:268-275. [DOI] [PubMed] [Google Scholar]
- 12.Chu, A. M., and R. W. Davis. 2008. High-throughput creation of a whole-genome collection of yeast knockout strains. Methods Mol. Biol. 416:205-220. [DOI] [PubMed] [Google Scholar]
- 13.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coombes, B. K., M. E. Wickham, N. F. Brown, S. Lemire, L. Bossi, W. W. Hsiao, F. S. Brinkman, and B. B. Finlay. 2005. Genetic and molecular analysis of GogB, a phage-encoded type III-secreted substrate in Salmonella enterica serovar Typhimurium with autonomous expression from its associated phage. J. Mol. Biol. 348:817-830. [DOI] [PubMed] [Google Scholar]
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellermeier, C. D., A. Janakiraman, and J. M. Slauch. 2002. Construction of targeted single copy lac fusions using lambda red and FLP-mediated site-specific recombination in bacteria. Gene 290:153-161. [DOI] [PubMed] [Google Scholar]
- 17.Forbes, J. R., and P. Gros. 2003. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 102:1884-1892. [DOI] [PubMed] [Google Scholar]
- 18.Freter, R., P. C. O'Brien, and M. S. Macsai. 1981. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect. Immun. 34:234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geddes, K., F. Cruz, and F. Heffron. 2007. Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog. 3:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerichter, C. B. 1960. The dissemination of Salmonella typhi, S. paratyphi A and S. paratyphi B through the organs of the white mouse by oral infection. J. Hyg. (Lond.) 58:307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govoni, G., and P. Gros. 1998. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm. Res. 47:277-284. [DOI] [PubMed] [Google Scholar]
- 22.Gruenheid, S., E. Pinner, M. Desjardins, and P. Gros. 1997. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med. 185:717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafez, H. M., R. Hauck, D. Luschow, and L. McDougald. 2005. Comparison of the specificity and sensitivity of PCR, nested PCR, and real-time PCR for the diagnosis of histomoniasis. Avian Dis. 49:366-370. [DOI] [PubMed] [Google Scholar]
- 24.Heid, C. A., J. Stevens, K. J. Livak, and P. M. Williams. 1996. Real-time quantitative PCR. Genome Res. 6:986-994. [DOI] [PubMed] [Google Scholar]
- 25.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 26.Ho, T. D., N. Figueroa-Bossi, M. Wang, S. Uzzau, L. Bossi, and J. M. Slauch. 2002. Identification of GtgE, a novel virulence factor encoded on the Gifsy-2 bacteriophage of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:5234-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hormaeche, C. E. 1979. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology 37:311-318. [PMC free article] [PubMed] [Google Scholar]
- 28.Huynh, C., D. L. Sacks, and N. W. Andrews. 2006. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J. Exp. Med. 203:2363-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamphuis, W., A. Schneemann, L. M. van Beek, A. B. Smit, P. F. Hoyng, and E. Koya. 2001. Prostanoid receptor gene expression profile in human trabecular meshwork: a quantitative real-time PCR approach. Invest. Ophthalmol. Vis. Sci. 42:3209-3215. [PubMed] [Google Scholar]
- 30.Kesty, N. C., K. M. Mason, M. Reedy, S. E. Miller, and M. J. Kuehn. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23:4538-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalmanach, A. C., and F. Lantier. 1999. Host cytokine response and resistance to Salmonella infection. Microbes Infect. 1:719-726. [DOI] [PubMed] [Google Scholar]
- 32.Lalmanach, A. C., A. Montagne, P. Menanteau, and F. Lantier. 2001. Effect of the mouse Nramp1 genotype on the expression of IFN-gamma gene in early response to Salmonella infection. Microbes Infect. 3:639-644. [DOI] [PubMed] [Google Scholar]
- 33.Lang, T., E. Prina, D. Sibthorpe, and J. M. Blackwell. 1997. Nramp1 transfection transfers Ity/Lsh/Bcg-related pleiotropic effects on macrophage activation: influence on antigen processing and presentation. Infect. Immun. 65:380-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby, S. J., L. G. Adams, T. A. Ficht, C. Allen, H. A. Whitford, N. A. Buchmeier, S. Bossie, and D. G. Guiney. 1997. The spv genes on the Salmonella dublin virulence plasmid are required for severe enteritis and systemic infection in the natural host. Infect. Immun. 65:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libby, S. J., W. Goebel, A. Ludwig, N. Buchmeier, F. Bowe, F. C. Fang, D. G. Guiney, J. G. Songer, and F. Heffron. 1994. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc. Natl. Acad. Sci. U. S. A. 91:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu, G., X. P. Xia, S. L. Gong, and Y. Zhao. 2006. The macrophage heterogeneity: difference between mouse peritoneal exudate and splenic F4/80+ macrophages. J. Cell. Physiol. 209:341-352. [DOI] [PubMed] [Google Scholar]
- 37.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 38.Mastroeni, P., A. Grant, O. Restif, and D. Maskell. 2009. A dynamic view of the spread and intracellular distribution of Salmonella enterica. Nat. Rev. Microbiol. 7:73-80. [DOI] [PubMed] [Google Scholar]
- 39.Meynell, G. G., and B. A. Stocker. 1957. Some hypotheses on the aetiology of fatal infections in partially resistant hosts and their application to mice challenged with Salmonella paratyphi-B or Salmonella typhimurium by intraperitoneal injection. J. Gen. Microbiol. 16:38-58. [DOI] [PubMed] [Google Scholar]
- 40.Mocellin, S., C. R. Rossi, P. Pilati, D. Nitti, and F. M. Marincola. 2003. Quantitative real-time PCR: a powerful ally in cancer research. Trends Mol. Med. 9:189-195. [DOI] [PubMed] [Google Scholar]
- 41.Niess, J. H., S. Brand, X. Gu, L. Landsman, S. Jung, B. A. McCormick, J. M. Vyas, M. Boes, H. L. Ploegh, J. G. Fox, D. R. Littman, and H. C. Reinecker. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307:254-258. [DOI] [PubMed] [Google Scholar]
- 42.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plant, J., and A. A. Glynn. 1974. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature 248:345-347. [DOI] [PubMed] [Google Scholar]
- 44.Ramakers, C., J. M. Ruijter, R. H. Deprez, and A. F. Moorman. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339:62-66. [DOI] [PubMed] [Google Scholar]
- 45.Richter-Dahlfors, A., A. M. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roach, T. I., D. Chatterjee, and J. M. Blackwell. 1994. Induction of early-response genes KC and JE by mycobacterial lipoarabinomannans: regulation of KC expression in murine macrophages by Lsh/Ity/Bcg (candidate Nramp). Infect. Immun. 62:1176-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubin, R. H., and L. Weinstein (ed.). 1977. Salmonellosis: microbiologic, pathologic and clinical features. Stratton Intercontinental Medical Book Corp., New York, NY.
- 48.Searle, S., N. A. Bright, T. I. Roach, P. G. Atkinson, C. H. Barton, R. H. Meloen, and J. M. Blackwell. 1998. Localisation of Nramp1 in macrophages: modulation with activation and infection. J. Cell Sci. 111:2855-2866. [DOI] [PubMed] [Google Scholar]
- 49.Skotnikova, O. I., A. Y. Sobolev, V. V. Demkin, N. P. Nikolaeva, E. Y. Nosova, E. L. Isaeva, A. M. Moroz, and V. I. Litvinov. 2000. Application of nested-PCR technique for the diagnosis of tuberculosis. Bull. Exp. Biol. Med. 129:612-614. [DOI] [PubMed] [Google Scholar]
- 50.Steinman, R. M., and J. Banchereau. 2007. Taking dendritic cells into medicine. Nature 449:419-426. [DOI] [PubMed] [Google Scholar]
- 51.Stober, C. B., S. Brode, J. K. White, J. F. Popoff, and J. M. Blackwell. 2007. Slc11a1, formerly Nramp1, is expressed in dendritic cells and influences major histocompatibility complex class II expression and antigen-presenting cell function. Infect. Immun. 75:5059-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Unsworth, K. E., and D. W. Holden. 2000. Identification and analysis of bacterial virulence genes in vivo. Philos. Trans. R Soc. Lond. B Biol. Sci. 355:613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uzzau, S., P. A. Gulig, B. Paglietti, G. Leori, B. A. Stocker, and S. Rubino. 2000. Role of the Salmonella abortusovis virulence plasmid in the infection of BALB/c mice. FEMS Microbiol. Lett. 188:15-18. [DOI] [PubMed] [Google Scholar]
- 55.Valdez, Y., G. E. Diehl, B. A. Vallance, G. A. Grassl, J. A. Guttman, N. F. Brown, C. M. Rosenberger, D. R. Littman, P. Gros, and B. B. Finlay. 2008. Nramp1 expression by dendritic cells modulates inflammatory responses during Salmonella Typhimurium infection. Cell. Microbiol. 10:1646-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Valdez, Y., G. A. Grassl, J. A. Guttman, B. Coburn, P. Gros, B. A. Vallance, and B. B. Finlay. 2009. Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell. Microbiol. 11:351-362. [DOI] [PubMed] [Google Scholar]
- 57.Vidal, S., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vidal, S. M., D. Malo, K. Vogan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73:469-485. [DOI] [PubMed] [Google Scholar]
- 59.Wai, S. N., B. Lindmark, T. Soderblom, A. Takade, M. Westermark, J. Oscarsson, J. Jass, A. Richter-Dahlfors, Y. Mizunoe, and B. E. Uhlin. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25-35. [DOI] [PubMed] [Google Scholar]
- 60.Wyllie, S., P. Seu, and J. A. Goss. 2002. The natural resistance-associated macrophage protein 1 Slc11a1 (formerly Nramp1) and iron metabolism in macrophages. Microbes Infect. 4:351-359. [DOI] [PubMed] [Google Scholar]
- 61.Yoon, H., J. E. McDermott, S. Porwollik, M. McClelland, and F. Heffron. 2009. Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog. 5:e1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan, J. S., A. Reed, F. Chen, and C. N. J. Stewart. 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaharik, M. L., B. A. Vallance, J. L. Puente, P. Gros, and B. B. Finlay. 2002. Host-pathogen interactions: host resistance factor Nramp1 up-regulates the expression of Salmonella pathogenicity island-2 virulence genes. Proc. Natl. Acad. Sci. U. S. A. 99:15705-15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zwilling, B. S., D. E. Kuhn, L. Wikoff, D. Brown, and W. Lafuse. 1999. Role of iron in Nramp1-mediated inhibition of mycobacterial growth. Infect. Immun. 67:1386-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.