Abstract
Chlamydia trachomatis infections can lead to severe chronic complications, including trachoma, ectopic pregnancy, and infertility. The only effective approach to disease control is vaccination. The goal of this work was to identify new potential vaccine candidates through a proteomics approach. We constructed a protein chip array (Antigen Discovery, Inc.) by expressing the open reading frames (ORFs) from C. trachomatis mouse pneumonitis (MoPn) genomic and plasmid DNA and tested it with serum samples from MoPn-immunized mice. Two groups of BALB/c female mice were immunized either intranasally or intravaginally with live elementary bodies (EB). Another two groups were immunized by a combination of the intramuscular and subcutaneous routes with UV-treated EB (UV-EB), using either CpG and Montanide as adjuvants to favor a Th1 response or alum to elicit a Th2 response. Serum samples collected at regular intervals postimmunization were tested in the proteome array. The microarray included the expression products of 909 proteins from a total of 921 ORFs of the Chlamydia MoPn genome and plasmid. A total of 185 immunodominant proteins elicited an early and sustained antibody response in the mice immunized with live EB, and of these, 71 were also recognized by the sera from mice immunized with UV-EB. The reactive antigens included some proteins that were previously described as immunogenic, such as the major outer membrane protein, OmpB, Hsp60, and IncA and proteins from the type III secretion system. In addition, we identified in mice several new immunogens, including 75 hypothetical proteins. In summary, we have identified a new group of immunodominant chlamydial proteins that can be tested for their ability to induce protection.
Chlamydia trachomatis is the most common sexually transmitted bacterial pathogen in the world. It is estimated that 4 to 5 million chlamydial infections occur yearly in the United States (6, 34, 53). Most of the genital chlamydial infections remain localized, but a significant number of females develop long-term sequelae, including infertility, ectopic pregnancy, and chronic abdominal pain (67). Although chlamydial infections can be treated with antibiotics, most of the infections are asymptomatic and, as a result, go untreated (34). Furthermore, even in symptomatic cases, if early treatment is not implemented, long-term sequelae may develop (56). Therefore, a vaccine is the most efficient way to control this pathogen (13).
Efforts to vaccinate against Chlamydia-induced trachoma were carried out in humans and nonhuman primates using whole inactivated and viable organisms (20, 21, 53, 54). Although these vaccine trials showed protection, it was serovar specific and short-lived. In addition, in some poorly protected individuals, reexposure to C. trachomatis resulted in a more severe ocular disease than that observed in nonvaccinated controls (20, 21). Based on these findings, it was proposed that a subunit vaccine was needed in order to avoid the harmful effects of the preparations containing the whole organism.
In the last 3 decades the main focus has shifted to developing vaccines that protect against chlamydial genital infections. The pathogenesis of chlamydial infections and testing of vaccine candidates has been studied mostly in mice using the mouse pneumonitis (MoPn) isolate (3, 12, 38). This is one of the best animal models to test vaccine candidates because infection with Chlamydia MoPn closely resembles a genital infection in humans (11, 44, 61). So far, the native major outer membrane protein (nMOMP) appears to be the most effective antigen. Mice vaccinated with nMOMP showed significant protection against respiratory and genital challenges (41, 46). Furthermore, in monkeys, nMOMP elicited protection against an ocular challenge (26). However, the protection obtained with nMOMP is, in part, dependent on the structural conformation of this protein, as immunizations with recombinant (rMOMP) are not as effective as vaccinations with the nMOMP (59). Also, producing nMOMP for human vaccination is unlikely because extraction of the trimeric nMOMP cannot be scaled up for manufacturing at a reasonable cost. Therefore, in order to develop a successful Chlamydia vaccine, novel antigens that can both offer broad protection against all human chlamydial serovars and easily be produced as recombinant proteins or synthetic peptides must be identified.
Several research groups have used samples from infected patients and immunized animals to identify novel vaccine candidates. For example, Coler et al. (7), used pooled sera and peripheral blood mononuclear cells (PBMC) from C. trachomatis-infected patients to screen a λ CT L-2 genomic library and identified eight immunodominant antigens, among which MOMP was found to induce the most robust protection. Sharma et al. (55) tested 15 serum samples from females with acute C. trachomatis urogenital infection to probe 156 chlamydial fusion proteins and identified seven reactive chlamydial antigens that included CPAF (chlamydial proteasome/protease-like activity factor), a protective antigen. Recently, Wang et al. (66) used a proteome array consisting of 908 open reading frames (ORFs) of C. trachomatis serovar D, and a total of 719 proteins were recognized by one or more antisera from 99 women with urogenital Chlamydia infections. Molina et al. (35) utilized sera from immunized mice and an array with 225 MoPn proteins to identify seven immunodominant antigens. Karunakaran et al. (27) used affinity chromatography and tandem mass spectrometry to identify major histocompatibility complex class II (MHC-II) (I-Ab) Chlamydia peptides from bone marrow-derived murine dendritic cells (DC) infected with MoPn. Mice vaccinated with DC transfected with peptides from PmpG-1 and PmpE/PmpF-2, identified by Karunakaran et al. (27), were found to be protected against genital and respiratory challenges (69). Here, we used a proteome microarray containing ∼99% of the ORFs (comprising the ORFome) of C. trachomatis MoPn to test sera from immunized mice. Using this approach, we were able to identify a new set of antigens from Chlamydia that can be further tested for their ability to induce protection.
MATERIALS AND METHODS
Preparation and titration of stocks of C. trachomatis MoPn.
The mouse C. trachomatis serovar (MoPn strain Nigg II), also called Chlamydia muridarum, was purchased from the American Type Culture Collection (Manassas, VA) and was grown in McCoy cells. Eagle's minimal essential medium was supplemented with 5% fetal bovine serum (EMEM-FBS) and 1 μg/ml of cycloheximide. Purification of elementary bodies (EB) was done as described by Caldwell et al. (4). The EB stock was stored at −70°C in SPG buffer (0.2 M sucrose, 0.02 M sodium phosphate, pH 7.2, and 5 mM glutamic acid). The number of inclusion forming units (IFU) of the stock was determined by titration on HeLa 229 cells.
Immunization of mice.
Three-week-old female BALB/c (H-2d) mice were purchased from Charles River Laboratories (Wilmington, MA). Groups of 12 mice were immunized as follows. For intranasal (i.n.) immunization, BALB/c mice received 104 IFU of MoPn (42, 43). For intravaginal (i.vag.) immunization mice received 105 IFU/mouse (39). The animals immunized intravaginally were treated with 2.0 mg of medroxy-progesterone acetate (Greenstone, Peapack, NJ) subcutaneously (s.c.) 7 days before inoculation (39, 62). EB were inactivated by exposure to a UV transilluminator box (UV-EB) emitting at a wavelength of 302 nm (Spectroline, Westbury, NY) for 10 min as previously described (45). For the combined intramuscular (i.m.) and s.c. (i.m.+s.c.) routes, the mice were vaccinated with 106 IFU of UV-EB per mouse three times 2 weeks apart (48). To elicit a Th1 response, one of the groups immunized by the i.m.+s.c. routes was vaccinated using UV-EB with CpG oligodeoxynucleotide (ODN) 1826 (10 μg/mouse/immunization; Coley Pharmaceutical, Ottawa, Canada) and Montanide ISA 720 (Seppic, Inc., Fairfield, NJ) (22, 33). The Montanide was mixed at a final 70:30 (vol/vol) ratio of the final preparation (41, 46). To induce a Th2 response, a second group was immunized i.m.+s.c. using alum (250 μg/mouse/immunization; 0.3% aluminum hydroxide solution; Alhydrogel 85; Superfos, Denmark) as the adjuvant (23, 30, 47, 48). As a negative-control group, mice were immunized with ovalbumin and the three combined adjuvants. Another control group was not immunized. Blood was collected from the periorbital plexus and terminally by heart puncture. In order to be able to perform all the serological tests with the same sample, the sera from each group of mice were pooled. The experiment was repeated once. All animal protocols were approved by the University of California, Irvine (Irvine, CA), IACUC.
Cloning and microarray production.
To generate the protein microarray of the 921 ORFs of the Chlamydia MoPn, 20-bp gene-specific sequence primers were synthesized. The custom primers included an adapter sequence to allow for the homologous recombination of the PCR product on the linearized T7 expression vector. Large genes (>6,000 bp; TC0437, TC0438, and TC0439) were amplified in smaller segments with a 250-bp overlap in each segment. For assessing expression, the T7 vector incorporated a 5′ polyhistidine (His) epitope and a 3′ hemagglutinin (HA) epitope on each protein (8, 9). Following cloning, DNA sequence verification was performed for each gene. The plasmids were expressed for 5 h using in vitro transcription/translation reaction kits according to the manufacturer's instructions (RTS 100 kit; Roche, Indianapolis, IN). The expressed Chlamydia proteins were printed onto Oncyte film slides (Grace BioLabs, Bend, OR) using a GeneMachines OmniGrid 100 microarray printer (DigiLab, Inc., Holliston, MA).
Microarray probing and verification.
Mouse serum samples were diluted 1:100 with 1× protein array blocking buffer (Whatman, Piscataway, NJ) containing 10% Escherichia coli lysate (McLab, San Francisco, CA) and incubated at room temperature for 30 min. The microarrays were rehydrated in 1× protein array blocking buffer for 30 min and probed with the diluted serum samples for 2 h at room temperature with constant agitation. The slides were then washed three times with Tris-buffered saline (TBS) containing 0.05% Tween 20 (TTBS) and incubated with biotin-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). After three washes with TTBS, the bound secondary antibodies were detected using streptavidin-conjugated Sensilight P3 (Columbia Biosciences, Columbia, MD), diluted according to the manufacturer's recommendations. The slides were washed three times with TTBS and three times with TBS, followed by a final wash with ultrapure water. The slides were air dried by centrifugation and scanned in a ScanArray Express HT microarray scanner (Perkin Elmer, Waltham, MA), and the fluorescence signal was quantified using QuantArray software (Perkin Elmer, Waltham, MA). All samples were tested in triplicate.
Expression of each individual gene was verified by using a monoclonal antibody (MAb) to the His (Sigma-Aldrich, St. Louis, MO) and HA (Roche, Indianapolis, IN) tags. All antigen-specific signal intensities were first corrected for background noise by using QuantArray software (Perkin Elmer, Waltham, MA). Second, a statistical variance stabilizing normalization (VSN) was applied (24). Then, we corrected for background signal by subtracting the no-DNA control plus 2 standard deviations. Next, the signal of each antigen at the day before immunization was removed from that specific antigen signal postvaccination. The antigen signal from the ovalbumin-immunized group was then subtracted from the antigen signal of groups immunized with UV-EB while the signal from the control group with no immunization was removed from the groups immunized with live EB for the same time point.
Statistical analysis.
A two-tailed unpaired Student's t test, a Mann-Whitney U test, and a Fisher's exact test were used to determine the significance of differences between samples collected from the groups of mice using the SigmaStat software program.
The statistical analysis of the microarray data was performed as previously described (8, 35). Briefly, the results were calibrated and transformed using the variance stabilizing normalization package in the R statistical environment (http://cran.r-project.org/) (24). The Bayes-regularized t test Cyber-T (http://cybert.ics.uci.edu/) was used to perform differential reactivity analysis (1). P values were adjusted using the Bonferroni procedure for controlling the family-wise error rate.
Computational prediction of transmembrane domains utilized the TMHMM, version 2.0, software (36) (http://www.cbs.dtu.dk/services/TMHMM/); signal peptide prediction used SignalP, version 3.0, software (2) (http://www.cbs.dtu.dk/services/SignalP/); cellular location prediction utilized PSORTb, version 2.04, software (19) (http://www.psort.org/psortb/); and predicted isoelectric point was determined using Swiss Institute of Bioinformatics pI/MW software (http://ca.expasy.org/tools/pi_tool.html). The COG (clusters of orthologous groups) information utilized can be found at the NCBI website (http://www.ncbi.nlm.nih.gov/sutils/coxik.cgi?gi=409). Enrichment statistical analysis was performed in the R environment using Fisher's exact test. Segmented ORFs were considered seroreactive if any segment was identified as seroreactive.
RESULTS
Construction and verification of the Chlamydia MoPn proteome microarray.
The microarray included the expression products of 909 proteins, from a total of 921 ORFs, of the Chlamydia MoPn genome and plasmid, as well as the appropriate positive and negative controls (mouse IgG and no-DNA rapid translation system [RTS] reaction product). ORFs TC0437, TC0438, and TC0439, due to their size (>3,000 bp), were expressed in three fragments each with overlapping sequences on both ends. Table S1 in the supplemental material shows the 12 genes that we were not able to clone. Each of the 909 expressed proteins and controls was printed three times in each microarray. Antibodies that recognize the N-terminal poly(His) tag and the C-terminal HA tag were used to assess protein expression as previously described (35). Poly(His) and HA staining was done in technical quadruplicates, i.e., four microarrays for poly(His) and four microarrays for HA. The microarrays were scanned with a PerkinElmer ProscanArray HT dual-laser microarray scanner, and the intensity for each spot was quantified using the ProscanArray software package. Antigens with mean signal intensities greater than the average control value plus 2 standard deviations were positive for the detection of the tag. Tag detection was used as a measure of protein expression. Of the 909 ORFs arrayed, 908 stained positive for the N-terminal poly(His) tag, and 888 stained positive for the C-terminal HA tag. A total of 887 ORFs, representing 96.3% (887 out of 921) of the MoPn genome and plasmid, demonstrated positive signal for both His and HA tags. The ORFs not fully expressed, as determined by the lack of reactivity of the MAb to the His and HA tags, are shown in Table S1. All cloned ORFs were probed with mouse sera since it is possible that the lack of detection of the His or HA tag is due to the blocking of the MAb binding site by the expressed protein.
To verify the consistency of data, microarray chips were probed using three specified serum samples, as previously described (8). Briefly, the same sera and protocol were repeated on three sequential days, using fresh batches of reagents each time. Each serum sample tested was probed on three microarrays on each of the 3 days, and the probing process was repeated by different operators each time. The microarrays were scanned, and the signal intensity of each antigen was normalized. The average signal was calculated from the technical triplicate data points, one from each array for each antigen, using the data obtained from the quantification of the microarrays that were probed on the same day. Scatter plots and distribution correlations were used to compare the data sets obtained from probing events performed on three different days. The three different comparisons, day 1 versus day 2, day 1 versus day 3, and day 2 versus day 3, showed R2 values greater than 0.9, indicating very high correlation between the data sets collected from the different probing events (data not shown). The distribution of the correlations was analyzed to validate the reproducibility of the data by making correlation matrices for all the data. The intraday correlations for each sample were calculated. The mean value for the intraday correlations was 0.9703557 for the three samples. The mean value for interday correlation was 0.8899541 for the three samples. All the interday correlations were above 0.95, except for the correlation involving one sample on day 2.
Identification of immunodominant antigens.
Female BALB/c mice were immunized one time with live EB using either the i.n. or the i.vag. route. Two additional groups of mice were immunized three times, 2 weeks apart, with UV-EB using the i.m.+s.c. routes. One of the UV-EB preparations was formulated with CpG plus Montanide as adjuvants to elicit a Th1 response, and another group combined UV-EB and alum to favor a Th2 response. We also included two negative-control groups, one immunized with ovalbumin and all the adjuvants and a second control group that was not immunized.
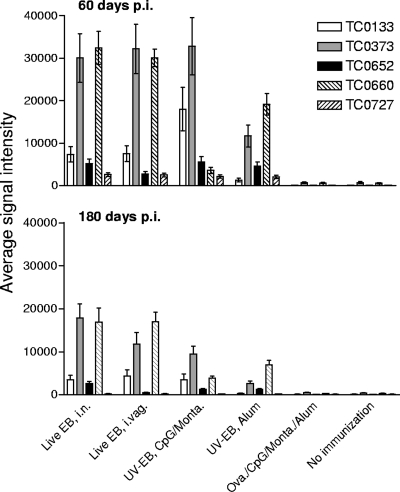
To monitor and compare the antibody responses of all the vaccination groups, we collected serum samples biweekly up to 180 days postimmunization (p.i.). We defined immunologically reactive antigens using three technical criteria: the antigen must have a mean signal intensity that is greater than 2 standard deviations above the average control value, the antigen must have a mean signal intensity that is greater than 2 standard deviations above the mean of the preimmunization sera, and the antigen must have a Cyber-T Bonferroni corrected P value that is less than 0.05 between preimmunization and postimmunization sera. As an example, Fig. 1 shows the mean signal intensities and standard deviations of five dominant antigens that met these criteria at days 60 and 180 p.i.
FIG. 1.
Mean signal intensities and standard deviations of five selected antigens at 60 and 180 days postimmunization.
In order to identify immunodominant antigens that elicit a rapid antibody response, we selected the ORFs that gave a positive signal for at least three consecutive time points during the first 60 days of the p.i. period. Using these criteria, we identified 170 ORFs that gave a positive signal with the sera from the animals immunized i.n. with live EB and 139 ORFs using the sera from the mice immunized i.vag. (Table 1). Of these antigens, 104 were recognized by sera from both groups of mice. Sera from the mice immunized with UV-EB/CpG/Montanide or UV-EB/alum both reacted with 130 ORFs each, and an additional 25 antigens were common to both groups.
TABLE 1.
Number of positive antigens per immunization group
| Group and immunization regimen | No. of positive antigens |
No. of selected immunodominant antigens | |
|---|---|---|---|
| At 60 days p.i. | At 180 days p.i. | ||
| Live EB groups | |||
| Both routes | 104 | 247 | 145 |
| i.n. route | 66 | 88 | 11 |
| i.vag. route | 35 | 98 | 29 |
| Total | 205 | 433 | 185 |
| UV-EB groups | |||
| Both UV-EB groups | 25 | 86 | 28 |
| UV-EB with CpG/Montanide | 105 | 47 | 13 |
| UV-EB with alum | 105 | 76 | 30 |
| Total | 235 | 209 | 71 |
To ensure that the immune response to each ORF was long-lasting, we further selected the immunodominant antigens by choosing those that gave a positive signal for at least three consecutive time points from 61 to 180 days p.i. (Table 1). Using these criteria, we identified 433 antigens from the live-EB-immunized groups and 247 from the UV-EB immunized groups. Since live EB elicit stronger humoral and cell-mediated immune responses than the UV-EB, we used the live EB data to prioritize the selected immunodominant antigens. Table 2 shows the selected antigens that elicited antibodies following immunization with live EB at 60 and 180 d p.i. and the corresponding results obtained with the sera from the mice immunized with UV-EB.
TABLE 2.
Immunodominant Chlamydia antigens recognized by sera from BALB/c mice immunized with live EB and UV-EB
| Protein identifier | Antibody response to the indicated immunization regimen |
Description | Size (aa)a | |||
|---|---|---|---|---|---|---|
| Live EB i.n. | Live EB i.vag. | UV-EB with CpG/Montanide | UV-EB with alum | |||
| TC0001 | + | + | − | − | Delta-aminolevulinic acid dehydratase | 333 |
| TC0004 | − | + | + | − | Transcription elongation protein | 714 |
| TC0012 | + | + | − | − | DNA topoisomerase I | 865 |
| TC0018 | + | + | − | − | 5-Formyltetrahydrofolate cyclo-ligase, putative | 178 |
| TC0031 | + | + | − | + | DNA gyrase, subunit A | 490 |
| TC0035 | + | + | − | − | Conserved hypothetical protein | 824 |
| TC0040 | + | + | + | − | Type III secretion cytoplasm ATPase, SctN | 442 |
| TC0043 | + | + | − | − | Type III secretion translocase, SctQ | 373 |
| TC0045 | + | + | − | − | Type III secretion protein, SctC | 672 |
| TC0047 | + | + | + | − | Conserved hypothetical protein | 177 |
| TC0052 | + | + | + | + | Major outer membrane protein | 387 |
| TC0063 | + | + | − | − | Conserved hypothetical protein | 224 |
| TC0066 | + | + | − | − | Hypothetical protein | 334 |
| TC0077 | + | + | − | − | Poly(A) polymerase family protein | 410 |
| TC0078 | − | + | − | − | ATP-dependent Clp protease, ATP-binding regulatory subunit, ClpX | 419 |
| TC0079 | + | + | − | − | ATP-dependent Clp protease, proteolytic subunit | 203 |
| TC0084 | + | + | + | − | Conserved hypothetical protein | 769 |
| TC0093 | + | + | − | − | NifU-related protein | 260 |
| TC0104 | + | + | − | − | Bifunctional 3,4-dihydroxy-2-butanone 4-phosphate synthase/GTP cyclohydrolase II protein, RibA/rib | 424 |
| TC0114 | + | + | − | + | Hypothetical protein | 122 |
| TC0116 | + | + | − | − | NADH:ubiquinone oxidoreductase, beta subunit, putative | 431 |
| TC0117 | + | + | − | + | Conserved hypothetical protein | 114 |
| TC0133 | + | + | + | + | Translation elongation factor P | 190 |
| TC0136 | + | + | − | + | 60-kDa chaperonin, putative | 513 |
| TC0137 | + | + | − | − | UDP-N-acetylmuramoylalanyl-d-glutamyl-2,6-diaminopimelate-d-alanyl-d-alanyl ligase MurF, putative | 452 |
| TC0140 | + | + | + | + | Conserved hypothetical protein | 243 |
| TC0144 | + | - | − | − | Conserved hypothetical protein | 137 |
| TC0149 | − | + | − | − | Conserved hypothetical protein | 564 |
| TC0151 | + | − | − | − | 3-Oxoacyl-(acyl carrier protein) synthase II, FabF | 418 |
| TC0153 | + | + | + | − | Inorganic pyrophosphatase, putative | 209 |
| TC0160 | − | + | − | − | Conserved hypothetical protein | 250 |
| TC0163 | + | + | + | + | Lysyl-tRNA synthetase | 524 |
| TC0166 | + | + | − | − | Conserved hypothetical protein | 350 |
| TC0177 | + | + | − | − | Conserved hypothetical protein | 164 |
| TC0178 | + | + | − | − | Glycyl-tRNA synthetase, tetrameric type, alpha/beta subunits | 1003 |
| TC0181 | + | + | − | − | Glycogen synthase | 474 |
| TC0188 | + | + | − | + | Hypothetical protein | 47 |
| TC0189 | + | + | − | + | Conserved hypothetical protein | 450 |
| TC0190 | + | + | + | - | Metalloprotease, insulinase family | 939 |
| TC0194 | + | + | − | + | Conserved hypothetical protein | 146 |
| TC0199 | + | + | − | − | Hypothetical protein | 215 |
| TC0210 | + | + | − | + | Serine protease, HtrA/DegQ/DegS family | 497 |
| TC0212 | − | + | − | − | Conserved hypothetical protein | 425 |
| TC0224 | + | + | − | − | Phenylalanyl-tRNA synthetase | 341 |
| TC0230 | + | + | + | + | Polyribonucleotide nucleotidyltransferase | 693 |
| TC0232 | + | + | − | − | Cytidine/deoxycytidylate deaminase family protein | 157 |
| TC0234 | + | + | − | − | Conserved hypothetical protein | 234 |
| TC0236 | + | + | − | − | Conserved hypothetical protein | 170 |
| TC0244 | + | + | + | + | Fumarate hydratase | 463 |
| TC0248 | + | + | − | − | Conserved hypothetical protein | 601 |
| TC0250 | + | + | − | − | Conserved hypothetical protein | 497 |
| TC0251 | + | + | + | − | Conserved hypothetical protein | 508 |
| TC0253 | + | − | + | + | Conserved hypothetical protein | 172 |
| TC0256 | − | + | − | − | Conserved hypothetical protein | 328 |
| TC0258 | + | + | − | − | Hypothetical protein | 335 |
| TC0265 | + | + | − | − | Hypothetical protein | 84 |
| TC0268 | + | + | + | + | Hypothetical protein | 614 |
| TC0269 | + | + | − | − | Conserved hypothetical protein | 89 |
| TC0279 | + | + | − | − | Conserved hypothetical protein | 418 |
| TC0285 | + | + | − | + | Conserved hypothetical protein | 446 |
| TC0290 | − | + | − | − | Conserved hypothetical protein | 247 |
| TC0300 | + | + | − | − | Conserved hypothetical protein | 100 |
| TC0309 | + | + | − | − | dCTP deaminase | 190 |
| TC0311 | + | + | − | − | Conserved hypothetical protein | 286 |
| TC0319 | + | + | − | − | Hypothetical protein | 434 |
| TC0323 | + | + | − | − | Conserved hypothetical protein | 148 |
| TC0328 | + | + | − | − | Conserved hypothetical protein | 374 |
| TC0331 | + | + | − | − | RNA polymerase sigma factor, sigma-70 family | 253 |
| TC0335 | + | + | − | − | ADP, ATP carrier protein | 529 |
| TC0336 | + | + | + | − | Conserved hypothetical protein | 158 |
| TC0344 | + | + | − | − | Conserved hypothetical protein | 619 |
| TC0347 | + | + | − | − | DNA polymerase III, beta subunit | 366 |
| TC0351 | + | + | − | + | Conserved hypothetical protein | 157 |
| TC0352 | + | + | − | + | Hypothetical protein | 133 |
| TC0363 | + | + | − | + | Type III secretion chaperone, SycE | 146 |
| TC0372 | + | + | − | + | N utilization substance protein A | 434 |
| TC0373 | + | + | + | + | Ribosomal protein S1 | 536 |
| TC0381 | + | + | − | − | Hypothetical protein | 650 |
| TC0386 | + | + | + | + | 60-kDa chaperonin | 544 |
| TC0387 | + | + | + | + | 10-kDa chaperonin | 102 |
| TC0388 | + | + | − | − | Oligoendopeptidase F | 608 |
| TC0389 | + | + | + | − | ATP-dependent Clp protease, subunit B | 867 |
| TC0390 | + | + | + | + | Conserved hypothetical protein | 491 |
| TC0392 | + | + | − | − | Hypothetical protein | 143 |
| TC0396 | + | + | − | − | Inclusion membrane protein | 276 |
| TC0399 | + | + | + | − | Acetyl-coenzyme A carboxyl carrier | 163 |
| TC0410 | + | + | − | − | Conserved hypothetical protein | 266 |
| TC0420 | + | + | − | − | Conserved hypothetical protein | 279 |
| TC0422 | + | + | − | − | Conserved hypothetical protein | 614 |
| TC0425 | + | + | − | + | Monooxygenase related protein | 506 |
| TC0437 | + | + | − | − | Adherence factor | 3,255 |
| TC0438 | + | + | + | + | Adherence factor | 3,335 |
| TC0439 | + | + | + | + | Adherence factor | 3,225 |
| TC0446 | + | + | − | − | Peptide ABC transporter, periplasmic peptide binding protein | 527 |
| TC0448 | − | + | − | − | Conserved hypothetical protein | 135 |
| TC0449 | + | + | + | − | Conserved hypothetical protein | 237 |
| TC0458 | − | + | − | − | Glucosamine-6-phosphate isomerase, putative | 256 |
| TC0473 | + | + | − | + | Peptide ABC transporter, permease protein | 287 |
| TC0478 | + | − | − | + | Conserved hypothetical protein | 272 |
| TC0479 | − | + | − | + | Pyrophosphate-fructose 6 phosphotransferase | 548 |
| TC0491 | − | + | − | − | Stationary-phase survival protein, SurE | 291 |
| TC0500 | + | + | − | − | Hypothetical protein | 214 |
| TC0503 | + | + | − | − | Inclusion membrane protein B | 123 |
| TC0504 | + | + | − | − | Hypothetical protein | 177 |
| TC0512 | + | + | − | − | Outer membrane protein, putative | 792 |
| TC0519 | + | + | − | − | Glycogen phosphorylase | 813 |
| TC0521 | + | + | + | + | Chromosomal replication initiator protein, DnaA | 456 |
| TC0522 | + | + | − | − | Inner membrane protein, putative | 787 |
| TC0527 | + | + | − | − | Conserved hypothetical protein | 412 |
| TC0536 | − | + | − | − | Acetyl-coenzyme A carboxylase carboxyl transferase, alpha subunit | 324 |
| TC0539 | + | + | − | − | N-Acetylmuramoyl-l-alanine amidase, putative | 268 |
| TC0551 | + | + | + | + | NADH:ubiquinone oxidoreductase, gamma subunit, putative | 318 |
| TC0557 | + | + | − | − | Phospholipase D family protein | 474 |
| TC0561 | + | + | − | − | Conserved hypothetical protein | 558 |
| TC0574 | + | + | − | − | Hypothetical protein | 127 |
| TC0582 | + | + | − | + | ATP synthase, subunit A | 591 |
| TC0588 | + | + | − | − | DNA-directed RNA polymerase, beta subunit | 1,396 |
| TC0589 | + | + | − | − | DNA-directed RNA polymerase | 1,252 |
| TC0590 | + | + | − | − | Ribosomal protein L7/L12 | 130 |
| TC0596 | + | − | − | − | Translation elongation factor Tu | 399 |
| TC0619 | + | + | − | − | DnaJ protein | 392 |
| TC0623 | + | + | − | + | Protease, Lon family | 819 |
| TC0629 | − | + | − | + | Conserved hypothetical protein | 566 |
| TC0631 | − | + | + | + | Conserved hypothetical protein | 102 |
| TC0632 | + | + | − | − | Polypeptide deformylase | 181 |
| TC0638 | − | + | − | − | BioY family protein | 196 |
| TC0652 | + | + | + | + | Conserved hypothetical protein | 195 |
| TC0660 | + | + | + | + | Amino acid ABC transporter | 259 |
| TC0661 | + | + | − | + | Phospho-2-dehydro-3-deoxyheptonate aldolase, putative | 279 |
| TC0664 | + | + | − | − | HIT family protein | 126 |
| TC0675 | + | − | + | − | DnaK protein | 658 |
| TC0677 | + | + | − | + | Conserved hypothetical protein | 254 |
| TC0680 | + | + | + | + | 2-Oxo acid dehydrogenase, E2 component, lipoamide acyltransferase | 410 |
| TC0688 | − | + | − | − | Signal peptidase II | 167 |
| TC0691 | + | + | − | + | Poly(A) polymerase family protein | 425 |
| TC0701 | + | + | − | − | Ribosomal protein L21, RplU | 107 |
| TC0705 | + | + | − | − | Conserved hypothetical protein | 161 |
| TC0706 | − | + | + | + | Hemolysin, putative | 374 |
| TC0709 | + | + | + | + | Hypothetical protein | 71 |
| TC0721 | + | + | − | − | Translation elongation factor | 694 |
| TC0725 | + | + | − | − | Tail specific protease precursor, putative | 649 |
| TC0726 | + | + | − | − | Sulfur-rich protein | 152 |
| TC0727 | + | + | + | + | 60-kDa outer membrane protein | 554 |
| TC0728 | − | + | − | − | Cysteine-rich outer membrane protein 3 | 88 |
| TC0733 | − | + | − | − | SecDF protein, putative | 1,400 |
| TC0738 | + | + | − | + | 1-Acyl-sn-glycerol-3-phosphate acyltransferase, putative | 216 |
| TC0740 | − | + | − | − | UDP-N-acetylglucosamine 1-carboxyvinyltransferase | 442 |
| TC0746 | − | + | − | − | Conserved hypothetical protein | 329 |
| TC0755 | + | + | − | − | Conserved hypothetical protein | 234 |
| TC0756 | − | + | − | + | Conserved hypothetical protein | 209 |
| TC0759 | + | + | − | − | Conserved hypothetical protein | 300 |
| TC0760 | + | − | − | − | Phenylalanyl-tRNA synthetase | 790 |
| TC0763 | + | + | − | − | Peptide ABC transporter, permease protein, putative | 578 |
| TC0773 | + | + | − | − | Amino acid ABC transporter | 263 |
| TC0781 | + | + | + | + | Protease IV, putative | 332 |
| TC0787 | + | − | − | − | Nucleoside 2P kinase | 141 |
| TC0788 | + | − | − | − | Holliday junction DNA helicase, RuvA | 200 |
| TC0790 | + | + | + | + | Conserved hypothetical protein | 185 |
| TC0794 | + | + | − | − | DNA-directed RNA polymerase | 394 |
| TC0797 | − | + | − | − | Preprotein translocase, SecY subunit | 457 |
| TC0799 | + | + | − | − | Ribosomal protein S5 | 165 |
| TC0802 | + | + | − | − | Ribosomal protein S8 | 133 |
| TC0816 | + | + | − | − | Conserved hypothetical protein | 293 |
| TC0817 | − | + | − | − | Methionyl-tRNA formyltransferase | 316 |
| TC0819 | + | − | + | + | 3R-hydroxymyristol-acyl carrier | 153 |
| TC0822 | + | + | − | − | Cytosolic acyl-coenzyme A thioester hydrolase family protein | 159 |
| TC0828 | + | + | − | + | Peptidyl-prolyl cis-trans isomerase, Mip | 261 |
| TC0833 | + | + | − | + | Uracil phosphoribosyltransferase | 303 |
| TC0835 | − | + | − | − | Conserved hypothetical protein | 318 |
| TC0836 | + | + | − | − | Conserved hypothetical protein | 199 |
| TC0838 | − | + | − | + | Conserved hypothetical protein | 140 |
| TC0848 | + | + | + | - | Type III secretion protein SctJ | 328 |
| TC0850 | + | + | + | + | Type III secretion translocase, SctL | 235 |
| TC0854 | + | + | − | − | Conserved hypothetical protein | 147 |
| TC0855 | − | + | − | − | Conserved hypothetical protein | 330 |
| TC0861 | + | + | + | + | General secretion pathway protein D | 759 |
| TC0884 | + | + | − | − | Thiol:disulfide interchange protein, DsbD, putative | 692 |
| TC0892 | + | − | − | − | Antioxidant, AhpC/Tsa family | 195 |
| TC0901 | − | + | − | − | Conserved hypothetical protein | 243 |
| TC0903 | + | + | − | + | 2-Amino-4-hydroxy-6-hydroxymethyldihydropterin pyrophosphokinase/dihydropteroate synthase | 450 |
| TC0909 | + | + | − | + | Conserved hypothetical protein | 875 |
| TC0913 | + | + | − | − | Conserved hypothetical protein | 548 |
| TC0919 | + | + | − | − | Transcriptional regulator | 227 |
| TCA05 | + | + | − | − | Virulence protein pGP4-D | 102 |
| TCA07 | − | + | − | − | Virulence protein pGP6-D | 246 |
aa, amino acids.
Of the 185 selected immunodominant antigens, a total of 145 antigens were recognized by sera from both groups of mice immunized with live EB. Eleven antigens elicited antibodies only in the mice immunized i.n., and 29 were positive only in the group infected i.vag. The sera from mice immunized with UV-EB gave a positive signal with a total of 71 ORFs that were also positive with the live-EB-immunized groups. Of these, 28 antigens were common to both UV-EB groups, 13 were unique to the animals immunized with UV-EB/CpG/Montanide, and 30 were unique to the group vaccinated with UV-EB/alum.
Assignment of cell function.
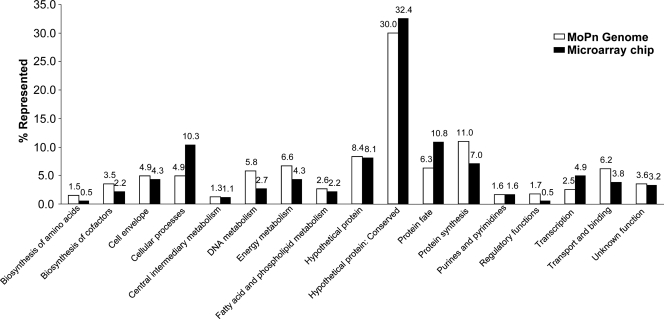
We next classified the immunodominant antigens according to their annotated and computationally predicted features for all 908 expression-confirmed proteins. We used the Comprehensive Microbial Resource (CMR) from the J. Craig Venter Institute (JCVI; http://www.jcvi.org) to assign predicted cellular roles to the antigens selected from the protein microarray. Each protein is assigned to one or more cellular role categories (CRCs). Most of the cellular roles of the selected antigens are in close proportion to the percentages represented in the MoPn genome (Fig. 2). A majority of the antigens that reacted are categorized as hypothetical proteins (75 out of 185 selected ORFs), and 60 of these are classified as conserved hypothetical proteins. These 60 conserved hypothetical proteins account for ∼32% of the immunodominant antigens, whereas the total of conserved hypothetical proteins in the MoPn genome is ∼30%. Similarly, other categories, such as cell envelope, central intermediary metabolism, purines and pyrimidines, and unknown functions, are also in proportion to the total percentages represented in the genome. We identified three CRCs that were significantly not in proportion to the percentage represented in the genome. The results in Table 3 illustrate that seroreactivity is not evenly distributed between different JCVI CRCs, and some categories are more likely to be seroreactive than others. Only proteins from the cellular process, protein fate, and transcription categories have a significantly higher representation in reactive antigen selection than in the proteome, with fold enrichment values of >1.8 and significant P values. These three categories were significantly more likely to be seroreactive for the live EB, whereas only cellular processes and protein fate are significant for UV-EB. Similarly, utilization of the NCBI database of clusters of orthologous groups (COGs) of proteins allowed us to characterize the seroreactive antigens and the COGs that were enriched and underrepresented for seroreactivity in the genome (Table 4). Each COG consists of individual proteins or groups of paralogs from at least three lineages and is comprised of 25 categories of functional definitions. Each protein in the database is assigned to one or more COGs, with a total of 962 COGs assigned to the 908 ORFs on the array. COG O, containing proteins involved in posttranslational modification, protein turnover, and chaperones were more likely to be reactive for both live EB and UV-EB (2.25-fold enrichment with a P value of 4.3 × 10−4 and 3.54-fold enrichment with a P value of 1.0 × 10−4, respectively). Proteins involved in intracellular trafficking and secretion (COG U) were also significantly more likely to be seroreactive for only the live-EB-infected mice (1.70-fold enrichment; P value of 2.6 × 10−2). In contrast, COG L (replication and repair) was significantly underrepresented and contained only six seroreactive proteins, despite the presence of 61 COG L ORFs in our array (0.44-fold enrichment; P value of 1.6 × 10−2). Analysis of individual COGs showed that no category was entirely reactive. For instance, the most predictive COG (COG O) was likely to be reactive in 50% of proteins annotated with this feature (17 seroreactive proteins out of 34). The next most predictive COG (COG U) contained 37.8% seroreactive proteins (14 out of 37).
FIG. 2.
Distribution of predicted roles of the selected antigens in comparison to the whole Chlamydia MoPn genome using the JCVI cellular role categories.
TABLE 3.
Cellular role category enrichment based on JCVI CRCs
| CRC | Total no. of proteins on the array | Immunization with live EB |
Immunization with UV-EB |
||||
|---|---|---|---|---|---|---|---|
| No. of hits | Fold enrichmenta | P value | No. of hits | Fold enrichmenta | P value | ||
| Amino acid biosynthesis | 14 | 1 | 0.34 | 0.32 | 1 | 0.85 | 1.0 |
| Biosynthesis of cofactors | 31 | 4 | 0.62 | 0.37 | 1 | 0.38 | 0.51 |
| Cell envelope | 45 | 11 | 1.17 | 0.57 | 3 | 0.79 | 1.0 |
| Cellular processes | 45 | 17 | 1.80b | 0.0078 | 8 | 2.12b | 0.046 |
| Central intermediary metabolism | 12 | 2 | 0.79 | 1.0 | 1 | 0.99 | 1.0 |
| DNA metabolism | 52 | 5 | 0.46 | 0.36 | 2 | 0.46 | 0.31 |
| Energy metabolism | 61 | 8 | 0.63 | 0.14 | 5 | 0.98 | 1.0 |
| Fatty acid and phospholipid metabolism | 23 | 5 | 1.04 | 1.0 | 3 | 1.56 | 0.43 |
| Hypothetical protein | 75 | 15 | 0.95 | 1.0 | 5 | 0.79 | 0.83 |
| Hypothetical protein: conserved | 275 | 60 | 1.04 | 0.72 | 22 | 0.95 | 0.90 |
| Protein fate | 58 | 27 | 2.22b | 6.4 × 10−6 | 15 | 3.08b | 3.1 × 10−5 |
| Protein synthesis | 97 | 13 | 0.64 | 0.064 | 3 | 0.37 | 0.051 |
| Purines, pyrimidines | 15 | 4 | 1.27 | 0.53 | 1 | 0.79 | 1.0 |
| Regulatory functions | 16 | 1 | 0.30 | 0.22 | 0 | 0.00 | 0.39 |
| Transcription | 23 | 9 | 1.87b | 0.039 | 4 | 2.07 | 0.12 |
| Transport and binding | 55 | 7 | 0.61 | 0.17 | 3 | 0.65 | 0.62 |
| Unknown function | 33 | 6 | 0.87 | 0.83 | 1 | 0.36 | 0.52 |
| Total | 930 | 195 | 78 | ||||
Relative to the percentage represented in the genome.
P value of <0.05.
TABLE 4.
Clusters of orthologous group of proteins enrichment based on NCBI
| NCBI COG classification |
Total no. of proteins on the array | Immunization with live EB |
Immunization with UV-EB |
|||||
|---|---|---|---|---|---|---|---|---|
| Name | Description | No. of hits | Fold enrichmenta | P value | No. of hits | Fold enrichmenta | P value | |
| A | RNA processing and modification | 0 | 0 | 0.00 | 1.0 | 0 | 0.00 | 1.0 |
| B | Chromatin structure and dynamics | 1 | 0 | 0.00 | 1.0 | 0 | 0.00 | 1.0 |
| C | Energy production and conversion | 41 | 11 | 1.21 | 0.45 | 7 | 1.87 | 0.09 |
| D | Cell cycle control, mitosis and meiosis | 10 | 0 | 0.00 | 0.13 | 0 | 0.00 | 0.61 |
| E | Amino acid transport and metabolism | 45 | 7 | 0.70 | 0.36 | 3 | 0.73 | 0.79 |
| F | Nucleotide transport and metabolism | 20 | 5 | 0.90 | 1.0 | 1 | 0.55 | 1.0 |
| G | Carbohydrate transport and metabolism | 33 | 6 | 0.82 | 0.67 | 2 | 0.66 | 0.76 |
| H | Coenzyme transport and metabolism | 38 | 6 | 0.71 | 0.43 | 2 | 0.58 | 0.57 |
| I | Lipid transport and metabolism | 38 | 7 | 0.83 | 0.69 | 3 | 0.86 | 1.0 |
| J | Translation | 103 | 18 | 0.79 | 0.26 | 5 | 0.53 | 0.15 |
| K | Transcription | 23 | 6 | 1.17 | 0.62 | 1 | 0.48 | 0.71 |
| L | Replication, recombination and repair | 61 | 6 | 0.44b | 0.016 | 2 | 0.36 | 0.11 |
| M | Cell wall/membrane biogenesis | 44 | 9 | 0.92 | 0.85 | 1 | 0.25 | 0.17 |
| N | Cell motility | 15 | 5 | 1.50 | 0.34 | 3 | 2.19 | 0.15 |
| O | Posttranslational modification, protein turnover, chaperones | 34 | 17 | 2.25b | 4.3 × 10−4 | 11 | 3.54b | 1.0 × 10−4 |
| P | Inorganic ion transport and metabolism | 25 | 3 | 0.54 | 0.33 | 1 | 0.44 | 0.72 |
| Q | Secondary metabolites biosynthesis, transport and catabolism | 4 | 1 | 1.12 | 1.0 | 0 | 0.00 | 1.0 |
| R | General function prediction only | 64 | 14 | 0.98 | 1.0 | 4 | 0.68 | 0.51 |
| S | Function unknown | 33 | 8 | 1.09 | 0.83 | 4 | 1.33 | 0.53 |
| T | Signal transduction mechanisms | 19 | 5 | 1.18 | 0.59 | 2 | 1.15 | 0.69 |
| U | Intracellular trafficking and secretion | 37 | 14 | 1.70b | 0.026 | 7 | 2.07 | 0.071 |
| V | Defense mechanisms | 2 | 0 | 0.00 | 1.0 | 0 | 0.00 | 1.0 |
| W | Extracellular structures | 0 | 0 | 0.00 | 1.0 | 0 | 0.00 | 1.0 |
| Y | Nuclear structure | 0 | 0 | 0.00 | 1.0 | 0 | 0.00 | 1.0 |
| Z | Cytoskeleton | 0 | 0 | 0.00 | 1.0 | 0 | 0.00 | 1.0 |
| Not in COGs | 272 | 66 | 1.09 | 0.35 | 29 | 1.17 | 0.32 | |
| Total | 962 | 214 | 88 | |||||
Relative to the percentage represented in the genome.
P value of <0.05.
In addition to the assigned protein annotation, we also analyzed enrichment based on computationally predicted features (Table 5). Cellular localization was predicted from ORF sequences with pSORTb software. From our entire ORF collection, pSORTb predicted 17 outer membrane proteins, of which 6 were seroreactive in live-EB-infected mice and 4 were reactive in UV-EB-immunized mice. UV-EB-immunized mouse serum, but not live-EB-vaccinated mouse serum, was significantly more likely to be seroreactive to pSORTb-predicted outer membrane proteins, showing significant enrichment with a 3.01-fold enrichment and a P value of 3.7 × 10−2. Similarly, proteins predicted to contain signal peptide were also significantly enriched for both live-EB- and UV-EB-immunized mice (1.50-fold enrichment with a P value of 2.4 × 10−3 and 2.10-fold enrichment with a P value of 3.2 × 10−4, respectively). Conversely, and as expected, proteins predicted not to contain a signal peptide were less likely to be seroreactive for UV-EB- and live-EB-immunized mice. Lastly, mice infected with live EB were more likely to be seroreactive to proteins predicted to contain one or more transmembrane domains and less likely to be seroreactive to proteins lacking a transmembrane domain (1.28-fold enrichment with a P value of 2.0 × 10−2 and 0.91-fold enrichment with a P value of 2.0 × 10−2, respectively).
TABLE 5.
Computationally predicted feature enrichment
| Computational model and parameter or value | Total no. of ORFs on the array | Immunization with live EB |
Immunization with UV-EB |
||||
|---|---|---|---|---|---|---|---|
| No. of hits | Fold enrichmenta | P value | No. of hits | Fold enrichmenta | P value | ||
| TMHMM | |||||||
| 0 | 694 | 129 | 0.91b | 0.020 | 53 | 0.98 | 0.77 |
| ≥1 | 214 | 56 | 1.28b | 0.020 | 18 | 1.08 | 0.77 |
| ≥5 | 69 | 11 | 0.78 | 0.44 | 3 | 0.56 | 0.35 |
| ≥10 | 26 | 5 | 0.94 | 1.0 | 1 | 0.49 | 0.71 |
| SignalP | |||||||
| ≥0.7c | 134 | 41 | 1.50b | 2.4 × 10−3 | 22 | 2.10b | 3.2 × 10−4 |
| <0.7 | 774 | 144 | 0.91b | 2.4 × 10−3 | 49 | 0.81b | 3.2 × 10−4 |
| PSORTb | |||||||
| Cytoplasmic | 404 | 86 | 1.04 | 0.56 | 36 | 1.14 | 0.32 |
| Cytoplasmic membrane | 195 | 38 | 0.96 | 0.76 | 11 | 0.72 | 0.23 |
| Extracellular | 8 | 2 | 1.23 | 0.67 | 0 | 0.00 | 1.0 |
| Outer membrane | 17 | 6 | 1.73 | 0.13 | 4 | 3.01b | 0.037 |
| Periplasmic | 10 | 3 | 1.47 | 0.43 | 2 | 2.56 | 0.18 |
| Unknown | 274 | 50 | 0.90 | 0.32 | 18 | 0.84 | 0.42 |
| Total | 909 | 185 | 71 | ||||
Relative to the percentage represented in the genome.
P value of <0.05.
Contains signal peptide.
DISCUSSION
In this study we have used a proteomic approach to search for novel antigens that can be used to formulate a subunit vaccine against chlamydial infections. Sera from mice immunized with live and nonviable EB were screened in a microarray expressing more than 99% of the ORFome of the Chlamydia MoPn genomic and plasmid DNA. We identified 185 chlamydial immunodominant antigens, and the majority of them have not previously been reported as being immunogenic in mice.
In the past, most of the vaccines were formulated using whole inactivated or attenuated organisms (50). Due to safety concerns, most of the recently implemented vaccines, such as the hepatitis B virus and the human papillomavirus vaccines, have been developed using a limited number of recombinant antigens (25, 60). Formulation of subunit vaccines, however, requires the identification of antigens that can elicit a protective immune response. Due to the relatively large genome size of many pathogenic organisms, this task cannot easily be accomplished. The development of high-throughput methods, the implementation of molecular techniques, and advancements in computational analysis have, in recent years, greatly facilitated this task.
To determine the chlamydial antigens that elicit an antibody response, we immunized mice with live and inactivated EB and screened the sera with a proteome microarray (8). To identify the immunodominant antigens, we used several selection criteria. From our initial screening we selected antigens that mounted an early immune response, i.e., before 60 days p.i., and that were still giving a positive signal at 61 to 180 days p.i. Immunization with live EB using the i.n. route is currently the approach that provides the best protection in mice against a genital challenge (42, 43). Therefore, to prioritize the immunodominant antigens, we used the results obtained with the sera from the animals immunized with live Chlamydia EB.
Our selected antigens include several that have previously been identified as potential vaccine candidates. Some of these antigens have been shown to elicit an immune response and, in some instances, protection in animal models against a chlamydial challenge, including TC0052 (MOMP), TC0066, TC0248 (CPAF), TC0268, TC0420, TC0512, TC0727 (60-kDa cysteine-rich protein [CRP]), and TC0816 (Cap1) (5, 7, 16, 27, 31, 40, 46, 49, 57, 68). Members of the putative type III secretion system of Chlamydia have also been studied for their potential as vaccine candidates since several of these proteins are shared among Gram-negative pathogenic bacteria (3). From the type III secretion system our selection included TC0040 (SctN), TC0043 (SctQ), TC0045 (SctC), TC0363 (SycE), TC0848 (SctJ), and TC0850 (SctL). Several of these antigens have been found to elicit an immune response and in some instances to elicit partial protection (7, 69).
We found that reactivity was not evenly distributed across the proteome, and no individual category was completely seroreactive. As expected, proteins predicted to be on the outer membrane that contain a signal peptide or transmembrane domain are significantly overrepresented in the seroreactive antigen list. Also proteins annotated as being involved in intracellular trafficking (COG U) and posttranslational modification, protein turnover, and chaperones (COG O) are also enriching features. While these results classifying antigenicity by protein localization are consistent with past results and expectations, they provide a quantitative and more informative understanding than previously reported (15, 63, 64). Importantly, the enrichment analysis has shown that no category is entirely reactive, yet some categories contain a disproportional number of seroreactive proteins compared to their representation within the genome. Moreover, annotation of proteins by CRC, COG, and computationally predicted features was capable of identifying features associated with seroreactivity.
The largest group of seroreactive antigens identified in this study is classified as hypothetical proteins. Of the selected 75 hypothetical proteins, 60 are conserved. Most of them are present only among the Chlamydia human serovars and not in other organisms and, therefore, are of particular interest because of their potential to induce cross-protection against multiple human serovars (51, 58). In addition, we identified other antigens, in particular, Hsp10 and Hsp60, that have being characterized for their potential role in inducing pathological changes (28, 37). Selection of these types of proteins is also important since it may help identify the antigens that are involved in the pathogenesis of chlamydial infections.
There is another group of proteins that have been identified as immunogenic by other investigators that were not included in our final selection of dominant antigens. This was the case, for example, with TC0086 (OmpB), TC0263 (PmpG), TC0313, TC0741 (Tarp), and TC0715 (ClpP-1) (7, 17, 18, 32, 52, 65, 68). Some of these proteins, such as TC0741 (Tarp), gave positive signals late, after 60 days p.i., and therefore did not meet our selection criteria. In our opinion, an effective vaccine formulation should include antigens that elicit an early immune response so that the spread of Chlamydia can be halted early on, and tissue damage can be prevented.
There are some limitations with the approach we have used for this study. For example, currently, we cannot quantitate the amount of antigen present in each spot of the microarray (8). By testing for the expression of the poly(His) and the HA tags with MAbs we can confirm, in most instances, that the ORF is expressed partially or in its entirety. However, antibody binding to the poly(His) and HA tags is affected by several factors. For example, the availability of the epitope for binding may be affected by the folding and/or conformation of the expressed ORF. As a result, we cannot make a direct quantitative comparison of the antibody response to the various antigens. We can, however, quantitatively follow the antibody response to a particular antigen since the amount of protein per spot for each antigen is constant from array to array.
Also, as with any other bacterially based system, we cannot control modifications of the expressed proteins, such as glycosylation, phosphorylation, or lipidation. Therefore, if these modifications affect the immunogenicity of a specific epitope, we may or may not be able to detect a particular antigen. Using the same type of microarray as the one utilized in this study, it was shown that sera from immunized humans and animals recognized all known glycosylated proteins of vaccinia virus (10). We can therefore conclude that at least some antibodies to these proteins are directed to domains that are not affected by posttranscriptional modifications. Similarly, conformational epitopes and epitopes dependent on disulfide bridges may or may not form correctly in the protein array utilized for this study. Here, we have shown that several cysteine-rich proteins, such as MOMP and the 60-kDa CRP, were recognized by sera of animals immunized with both live EB and UV-EB, supporting the premise that most antigens elicit antibodies against a wide array of epitopes. On the other hand, certain antigens that have been found to elicit a strong antibody response and protection, such as TCA04 (Pgp3), failed to meet our criteria for positivity (14). In the case of Pgp3, we cannot exclude the possibility that the protein expressed in the microarray lacked the correct conformation. The antibody response to Pgp3 is preferentially directed to conformational epitopes, and this may explain our failure to identify it as an immunodominant antigen (29).
Here, we used inbred mice and, therefore, defining “immunodominance” based on the response of individual animals that, by definition, are immunogenetically identical will not be appropriate. Therefore, we selected immunodominant antigens based on different criteria. We immunized 12 mice per group and repeated the experiment again with another group of 12 mice. We pooled the sera from each group of mice in order to have enough sample to perform all the assays. Therefore, each antigen at each time point was evaluated with the sera from the 12 mice in each group from two independent immunizations. In addition, rather than using more mice and only one type of immunization, we used four different immunization protocols. The selected antigens had to elicit an early antibody response the first 60 days after immunization and sustain these positive signals until 120 to 180 days after immunization. Using these criteria, the majority of antigens selected gave positive signals in two or more of the immunization groups. As shown in Table 2, of the 185 selected immunodominant proteins that elicited an early and sustained antibody response, only 26 gave a positive signal with only one of the four immunization groups. Doing similar experiments in strains of mice with different genetic backgrounds should help to further define the chlamydial immunodominant antigens in this animal model.
In conclusion, we screened the whole Chlamydia ORFome and were able to identify 185 antigens that elicited both an early antibody response and maintained a strong antibody response in mice following immunization with live EB. While some of these antigens had been previously identified, a majority of them are novel and can be further studied for their ability to protect mice against a Chlamydia infection. We chose to work with the mouse model since it is the most frequently utilized to study the pathogenesis of chlamydial infections and to test vaccine candidates. Furthermore, in an animal model, by controlling the experimental conditions, it may be possible to more readily distinguish protective antigens from those that are not protective or may elicit pathogenesis. We realize that our results will have to be confirmed using both human samples from individuals infected with Chlamydia and other animal models (66). Validating the data with other types of samples will also help to reduce the number of antigens that need to be tested.
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grants R41 AI072847 and RO1 AI067888 from the National Institute of Allergy and Infectious Diseases.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 18 October 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Baldi, P., and A. D. Long. 2001. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17:509-519. [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 3.Brunham, R. C., and J. Rey-Ladino. 2005. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 5:149-161. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell, H. D., J. Kromhout, and J. Schachter. 1981. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31:1161-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, H. D., and J. Schachter. 1982. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect. Immun. 35:1024-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2009. Chlamydia screening among sexually active young female enrollees of health plans—United States, 2000-2007. MMWR Morb. Mortal. Wkly. Rep. 58:362-365. [PubMed] [Google Scholar]
- 7.Coler, R. N., A. Bhatia, J. F. Maisonneuve, P. Probst, B. Barth, P. Ovendale, H. Fang, M. Alderson, Y. Lobet, J. Cohen, P. Mettens, and S. G. Reed. 2009. Identification and characterization of novel recombinant vaccine antigens for immunization against genital Chlamydia trachomatis. FEMS Immunol. Med. Microbiol. 55:258-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, D. H., X. Liang, J. E. Hernandez, A. Randall, S. Hirst, Y. Mu, K. M. Romero, T. T. Nguyen, M. Kalantari-Dehaghi, S. Crotty, P. Baldi, L. P. Villarreal, and P. L. Felgner. 2005. Profiling the humoral immune response to infection by using proteome microarrays: high-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. U. S. A. 102:547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, D. H., D. M. Molina, J. Wrammert, J. Miller, S. Hirst, Y. Mu, J. Pablo, B. Unal, R. Nakajima-Sasaki, X. Liang, S. Crotty, K. L. Karem, I. K. Damon, R. Ahmed, L. Villarreal, and P. L. Felgner. 2007. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 7:1678-1686. [DOI] [PubMed] [Google Scholar]
- 11.de la Maza, L. M., S. Pal, A. Khamesipour, and E. M. Peterson. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62:2094-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Maza, L. M., and E. M. Peterson. 2002. Vaccines for Chlamydia trachomatis infections. Curr. Opin. Invest. Drugs. 3:980-986. [PubMed] [Google Scholar]
- 13.de la Maza, M. A., and L. M. de la Maza. 1995. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine 13:119-127. [DOI] [PubMed] [Google Scholar]
- 14.Donati, M., V. Sambri, M. Comanducci, K. Di Leo, E. Storni, L. Giacani, G. Ratti, and R. Cevenini. 2003. DNA immunization with pgp3 gene of Chlamydia trachomatis inhibits the spread of chlamydial infection from the lower to the upper genital tract in C3H/HeN mice. Vaccine 21:1089-1093. [DOI] [PubMed] [Google Scholar]
- 15.Felgner, P. L., M. A. Kayala, A. Vigil, C. Burk, R. Nakajima-Sasaki, J. Pablo, D. M. Molina, S. Hirst, J. S. Chew, D. Wang, G. Tan, M. Duffield, R. Yang, J. Neel, N. Chantratita, G. Bancroft, G. Lertmemongkolchai, D. H. Davies, P. Baldi, S. Peacock, and R. W. Titball. 2009. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc. Natl. Acad. Sci. U. S. A. 106:13499-13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fling, S. P., R. A. Sutherland, L. N. Steele, B. Hess, S. E. D'Orazio, J. Maisonneuve, M. F. Lampe, P. Probst, and M. N. Starnbach. 2001. CD8+ T cells recognize an inclusion membrane-associated protein from the vacuolar pathogen Chlamydia trachomatis. Proc. Natl. Acad. Sci. U. S. A. 98:1160-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Follmann, F., A. W. Olsen, K. T. Jensen, P. R. Hansen, P. Andersen, and M. Theisen. 2008. Antigenic profiling of a Chlamydia trachomatis gene-expression library. J. Infect. Dis. 197:897-905. [DOI] [PubMed] [Google Scholar]
- 18.Forsbach-Birk, V., U. Simnacher, K. I. Pfrepper, E. Soutschek, A. O. Kiselev, M. F. Lampe, T. Meyer, E. Straube, and A. Essig. 2010. Identification and evaluation of a combination of chlamydial antigens to support the diagnosis of severe and invasive Chlamydia trachomatis infections. Clin. Microbiol. Infect. 16:1237-1244. [DOI] [PubMed] [Google Scholar]
- 19.Gardy, J. L., M. R. Laird, F. Chen, S. Rey, C. J. Walsh, M. Ester, and F. S. Brinkman. 2005. PSORTb v. 2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21:617-623. [DOI] [PubMed] [Google Scholar]
- 20.Grayston, J. T., and S. P. Wang. 1978. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex. Transm. Dis. 5:73-77. [DOI] [PubMed] [Google Scholar]
- 21.Grayston, T., and S. Wang. 1975. New knowledge of chlamydiae and the diseases they cause. J. Infect. Dis. 132:87-105. [DOI] [PubMed] [Google Scholar]
- 22.Harandi, A. M., and J. Holmgren. 2004. CpG DNA as a potent inducer of mucosal immunity: implications for immunoprophylaxis and immunotherapy of mucosal infections. Curr. Opin. Invest. Drugs 5:141-145. [PubMed] [Google Scholar]
- 23.HogenEsch, H. 2002. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 20(Suppl. 3):S34-S39. [DOI] [PubMed] [Google Scholar]
- 24.Huber, W., A. von Heydebreck, H. Sultmann, A. Poustka, and M. Vingron. 2002. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18(Suppl. 1):S96-S104. [DOI] [PubMed] [Google Scholar]
- 25.Ionescu-Matiu, I., R. C. Kennedy, J. T. Sparrow, A. R. Culwell, Y. Sanchez, J. L. Melnick, and G. R. Dreesman. 1983. Epitopes associated with a synthetic hepatitis B surface antigen peptide. J. Immunol. 130:1947-1952. [PubMed] [Google Scholar]
- 26.Kari, L., W. M. Whitmire, D. D. Crane, N. Reveneau, J. H. Carlson, M. M. Goheen, E. M. Peterson, S. Pal, L. M. de la Maza, and H. D. Caldwell. 2009. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J. Immunol. 182:8063-8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karunakaran, K. P., J. Rey-Ladino, N. Stoynov, K. Berg, C. Shen, X. Jiang, B. R. Gabel, H. Yu, L. J. Foster, and R. C. Brunham. 2008. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J. Immunol. 180:2459-2465. [DOI] [PubMed] [Google Scholar]
- 28.LaVerda, D., L. N. Albanese, P. E. Ruther, S. G. Morrison, R. P. Morrison, K. A. Ault, and G. I. Byrne. 2000. Seroreactivity to Chlamydia trachomatis Hsp10 correlates with severity of human genital tract disease. Infect. Immun. 68:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Z., Y. Zhong, L. Lei, Y. Wu, S. Wang, and G. Zhong. 2008. Antibodies from women urogenitally infected with C. trachomatis predominantly recognized the plasmid protein Pgp3 in a conformation-dependent manner. BMC Microbiol. 8:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindblad, E. B. 2004. Aluminium adjuvants—in retrospect and prospect. Vaccine 22:3658-3668. [DOI] [PubMed] [Google Scholar]
- 31.McNeilly, C. L., K. W. Beagley, R. J. Moore, V. Haring, P. Timms, and L. M. Hafner. 2007. Expression library immunization confers partial protection against Chlamydia muridarum genital infection. Vaccine 25:2643-2655. [DOI] [PubMed] [Google Scholar]
- 32.Meoni, E., E. Faenzi, E. Frigimelica, L. Zedda, D. Skibinski, S. Giovinazzi, A. Bonci, R. Petracca, E. Bartolini, G. Galli, M. Agnusdei, F. Nardelli, F. Buricchi, N. Norais, I. Ferlenghi, M. Donati, R. Cevenini, O. Finco, G. Grandi, and R. Grifantini. 2009. CT043, a protective antigen that induces a CD4+ Th1 response during Chlamydia trachomatis infection in mice and humans. Infect. Immun. 77:4168-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles, A. P., H. A. McClellan, K. M. Rausch, D. Zhu, M. D. Whitmore, S. Singh, L. B. Martin, Y. Wu, B. K. Giersing, A. W. Stowers, C. A. Long, and A. Saul. 2005. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 23:2530-2539. [DOI] [PubMed] [Google Scholar]
- 34.Miller, W. C., C. A. Ford, M. Morris, M. S. Handcock, J. L. Schmitz, M. M. Hobbs, M. S. Cohen, K. M. Harris, and J. R. Udry. 2004. Prevalence of chlamydial and gonococcal infections among young adults in the United States. JAMA 291:2229-2236. [DOI] [PubMed] [Google Scholar]
- 35.Molina, D. M., S. Pal, M. A. Kayala, A. Teng, P. J. Kim, P. Baldi, P. L. Felgner, X. Liang, and L. M. de la Maza. 2010. Identification of immunodominant antigens of Chlamydia trachomatis using proteome microarrays. Vaccine 28:3014-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller, S., M. D. Croning, and R. Apweiler. 2001. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17:646-653. [DOI] [PubMed] [Google Scholar]
- 37.Morrison, R. P., R. J. Belland, K. Lyng, and H. D. Caldwell. 1989. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J. Exp. Med. 170:1271-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison, R. P., and H. D. Caldwell. 2002. Immunity to murine chlamydial genital infection. Infect. Immun. 70:2741-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison, R. P., K. Feilzer, and D. B. Tumas. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63:4661-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy, A. K., J. P. Chambers, P. A. Meier, G. Zhong, and B. P. Arulanandam. 2007. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect. Immun. 75:666-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pal, S., H. L. Davis, E. M. Peterson, and L. M. de la Maza. 2002. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect. Immun. 70:4812-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pal, S., T. J. Fielder, E. M. Peterson, and L. M. de la Maza. 1994. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 62:3354-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pal, S., W. Hui, E. M. Peterson, and L. M. de la Maza. 1998. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J. Med. Microbiol. 47:599-605. [DOI] [PubMed] [Google Scholar]
- 44.Pal, S., E. M. Peterson, and L. M. de la Maza. 2004. New murine model for the study of Chlamydia trachomatis genitourinary tract infections in males. Infect. Immun. 72:4210-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal, S., E. M. Peterson, and L. M. de La Maza. 2000. Role of Nramp1 deletion in Chlamydia infection in mice. Infect. Immun. 68:4831-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 73:8153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pal, S., A. P. Schmidt, E. M. Peterson, C. L. Wilson, and L. M. de la Maza. 2006. Role of matrix metalloproteinase-7 in the modulation of a Chlamydia trachomatis infection. Immunology 117:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 1997. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect. Immun. 65:3361-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pal, S., I. Theodor, E. M. Peterson, and L. M. de la Maza. 2001. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69:6240-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plotkin, S. A. 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47:401-409. [DOI] [PubMed] [Google Scholar]
- 51.Read, T. D., G. S. Myers, R. C. Brunham, W. C. Nelson, I. T. Paulsen, J. Heidelberg, E. Holtzapple, H. Khouri, N. B. Federova, H. A. Carty, L. A. Umayam, D. H. Haft, J. Peterson, M. J. Beanan, O. White, S. L. Salzberg, R. C. Hsia, G. McClarty, R. G. Rank, P. M. Bavoil, and C. M. Fraser. 2003. Genome sequence of Chlamydophila caviae (Chlamydia psittaci GPIC): examining the role of niche-specific genes in the evolution of the Chlamydiaceae. Nucleic Acids Res. 31:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Campillo, M., L. Bini, M. Comanducci, R. Raggiaschi, B. Marzocchi, V. Pallini, and G. Ratti. 1999. Identification of immunoreactive proteins of Chlamydia trachomatis by Western blot analysis of a two-dimensional electrophoresis map with patient sera. Electrophoresis 20:2269-2279. [DOI] [PubMed] [Google Scholar]
- 53.Schachter, J. 1999. Infection and disease epidemiology, p. 139-170. In R. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis and immunity. ASM Press, Washington, DC.
- 54.Schachter, J., and C. R. Dawson. 1978. Human chlamydial infections. PSG Publishing Co., Littleton, MA.
- 55.Sharma, J., Y. Zhong, F. Dong, J. M. Piper, G. Wang, and G. Zhong. 2006. Profiling of human antibody responses to Chlamydia trachomatis urogenital tract infection using microplates arrayed with 156 chlamydial fusion proteins. Infect. Immun. 74:1490-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamm, W., and K. Holmes. 1999. Chlamydia trachomatis infections of the adult, p. 407-422. In K. K. Holmes, P. F. Sparling, P.-A. Mardh, et al. (ed.), Sexually transmitted diseases. McGraw-Hill Book Co., New York, NY.
- 57.Starnbach, M. N., W. P. Loomis, P. Ovendale, D. Regan, B. Hess, M. R. Alderson, and S. P. Fling. 2003. An inclusion membrane protein from Chlamydia trachomatis enters the MHC class I pathway and stimulates a CD8+ T cell response. J. Immunol. 171:4742-4749. [DOI] [PubMed] [Google Scholar]
- 58.Stephens, R. S., S. Kalman, C. Lammel, J. Fan, R. Marathe, L. Aravind, W. Mitchell, L. Olinger, R. L. Tatusov, Q. Zhao, E. V. Koonin, and R. W. Davis. 1998. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science 282:754-759. [DOI] [PubMed] [Google Scholar]
- 59.Sun, G., S. Pal, J. Weiland, E. M. Peterson, and L. M. de la Maza. 2009. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine 27:5020-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. U. S. A. 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swenson, C. E., and J. Schachter. 1984. Infertility as a consequence of chlamydial infection of the upper genital tract in female mice. Sex. Transm. Dis. 11:64-67. [DOI] [PubMed] [Google Scholar]
- 62.Tuffrey, M., and D. Taylor-Robinson. 1981. Progesterone as a key factor in the development of a mouse model for genital-tract infection with Chlamydia trachomatis. FEMS Microbiol. Lett. 12:111-115. [Google Scholar]
- 63.Vigil, A., D. H. Davies, and P. L. Felgner. 2010. Defining the humoral immune response to infectious agents using high-density protein microarrays. Future Microbiol. 5:241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vigil, A., R. Ortega, R. Nakajima-Sasaki, J. Pablo, D. M. Molina, C. C. Chao, H. W. Chen, W. M. Ching, and P. L. Felgner. 2010. Genome-wide profiling of humoral immune response to Coxiella burnetii infection by protein microarray. Proteomics 10:2259-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, J., L. Chen, F. Chen, X. Zhang, Y. Zhang, J. Baseman, S. Perdue, I. T. Yeh, R. Shain, M. Holland, R. Bailey, D. Mabey, P. Yu, and G. Zhong. 2009. A chlamydial type III-secreted effector protein (Tarp) is predominantly recognized by antibodies from humans infected with Chlamydia trachomatis and induces protective immunity against upper genital tract pathologies in mice. Vaccine 27:2967-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, J., Y. Zhang, C. Lu, L. Lei, P. Yu, and G. Zhong. 2010. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J. Immunol. 185:1670-1680. [DOI] [PubMed] [Google Scholar]
- 67.Westrom, L., R. Joesoef, G. Reynolds, A. Hagdu, and S. E. Thompson. 1992. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex. Transm. Dis. 19:185-192. [PubMed] [Google Scholar]
- 68.Yu, H., X. Jiang, C. Shen, K. P. Karunakaran, and R. C. Brunham. 2009. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J. Immunol. 182:1602-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu, H., X. Jiang, C. Shen, K. P. Karunakaran, J. Jiang, N. L. Rosin, and R. C. Brunham. 2010. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-γ)/tumor necrosis factor alpha and IFN-γ/interleukin-17 double-positive CD4+ T cells. Infect. Immun. 78:2272-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.