Abstract
The human gastric pathogen Helicobacter pylori modifies host cholesterol via glycosylation and incorporates the glycosylated cholesterol into its membrane; however, the benefits of cholesterol to H. pylori are largely unknown. We speculated that cholesterol in the H. pylori membrane might alter the susceptibility of these organisms to membrane-disrupting antibacterial compounds. To test this hypothesis, H. pylori strains were cultured in Ham's F-12 chemically defined medium in the presence or absence of cholesterol. The two cultures were subjected to overnight incubations with serial 2-fold dilutions of 10 bile salts and four ceragenins, which are novel bile salt derivatives that mimic membrane-disrupting activity of antimicrobial peptides. H. pylori cultured with cholesterol was substantially more resistant to seven of the bile salts and three ceragenins than H. pylori cultured without cholesterol. In most cases, these cholesterol-dependent differences ranged from 2 to 7 orders of magnitude; this magnitude depended on concentration of the agent. Cholesterol is modified by glycosylation using Cgt, a cholesteryl glycosyltransferase. Surprisingly, a cgt knockout strain still maintained cholesterol-dependent resistance to bile salts and ceragenins, indicating that cholesterol modification was not involved in resistance. We then tested whether three putative, paralogous inner membrane efflux pumps, HefC, HefF, or HefI, played a role. While HefF and HefI appeared unimportant, HefC was shown to play a critical role in the resistance to bile salts and ceragenins by multiple methods in multiple strain backgrounds. Thus, both cholesterol and the putative bile salt efflux pump HefC play important roles in H. pylori resistance to bile salts and ceragenins.
Helicobacter pylori is a major public health problem worldwide, infecting about 50% of humans. The bacterium causes gastritis and both gastric and duodenal ulcers. The ability of H. pylori to survive in the harsh gastric environment is due to a myriad of factors, such as resistance to acid and oxidative stress and the ability to regulate and express its adherence factors. One area that has received little attention is the ability of this organism to combat host bile salts.
A unique and interesting feature of H. pylori is that it steals host cholesterol, modifies it by glycosylation, and incorporates the glycosylated cholesterol into its membrane (1, 16-18, 20, 21). No other bacterial pathogen is known to glycosylate cholesterol. Because H. pylori encounters host cholesterol in vivo, it is important to understand why and how H. pylori utilizes host cholesterol. This can currently be studied only in vitro, where specific chemically defined media containing or lacking cholesterol can be used (40, 41). H. pylori glycosylation of cholesterol occurs via an alpha linkage at the hydroxyl moiety of cholesterol and is mediated by the enzyme cholesteryl glycosyltransferase (Cgt), encoded by cgt (hp0421) (46). Recent evidence has shown that this cholesterol incorporation and modification contribute to the pathogenicity of the organism in mice (46). Our group recently demonstrated that H. pylori grown in the absence of cholesterol fails to colonize gerbils (19), underscoring the importance of cholesterol in the pathogenesis of this gastric pathogen. We hypothesized that one important role of cholesterol in vivo is to help H. pylori resist bile salts.
Bile salts, synthesized by the host downstream of cholesterol biosynthesis, aid in digestion of dietary lipids through their detergent-like properties and have bactericidal activity against most bacteria. H. pylori encounters bile salts in vivo via two mechanisms. First, H. pylori comes in contact with bile salts in the duodenum, where H. pylori can cause ulcers. Second, reflux of bile from the intestine into the stomach normally occurs following a meal (38). Bile salt concentrations of ∼2 mM (∼1,000 μg/ml) are typically found in human bile (34). A few studies have investigated the bactericidal effects of bile salts on H. pylori using rich medium containing serum or blood (which contains cholesterol) (14, 15, 22, 33), but no study has yet investigated the effects in a chemically defined, cholesterol-free medium, nor has there been a comprehensive comparison among the many bile salt derivatives found in vivo.
Bile salt resistance mechanisms have been studied in enteric bacteria, but little is known about bile salt resistance in H. pylori. For example, in Escherichia coli, the AcrB inner membrane protein, a member of the resistance nodulation division (RND) family, is an efflux pump involved in bile salt resistance (11, 32, 36).
Ceragenins are a novel class of synthetic antimicrobial agents that were developed as nonpeptide mimics of antimicrobial peptides (AMPs) (12, 29). They are derived from a common bile salt, and comparisons of ceragenin and AMP mechanisms of bactericidal activities suggest that they share a mechanism of action (10). AMPs, ceragenins, and bile salts all selectively target bacterial membranes. Unlike bile salts, ceragenins are positively charged due to amine groups and can be easily synthesized in large amounts (10). Potency of the ceragenins varies from compound to compound, based on side groups added, solubility, and degree of charge (with greater positive charge correlating with greater antimicrobial activity). Importantly, ceragenins can kill bacterial pathogens that are resistant to multiple antibiotics, including methicillin-resistant Staphylococcus aureus (9) and multidrug-resistant Pseudomonas aeruginosa (8). They are also acid stable and water soluble. There is currently nothing known about the effects of ceragenins on H. pylori grown in chemically defined medium although ceragenins are acid stable and could be a potential therapeutic in vivo. There is a pressing need to investigate new compounds for anti-Helicobacter activity, given the predilection of H. pylori to develop resistance to antibiotics, such as metronidazole (13).
In the current study, we used chemically defined medium (40, 41) to address two important questions. (i) What are the mechanisms of resistance to bile salts and ceragenins? (ii) Does cholesterol impact this susceptibility? We provide evidence that cholesterol increases resistance of H. pylori to bile salts and ceragenins. Moreover, the putative efflux pump homologous to AcrB of E. coli, HefC, plays a critical role in the resistance of H. pylori to bile salts and ceragenin 11.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
H. pylori wild-type (WT) strains 26695 (K. Eaton, University of Michigan), G27 (N. Salama, Fred Hutchison Cancer Center), SS1 (A. Lee, University of New South Wales), and AH244 (R. Alm, AstraZeneca, Boston, MA) were routinely passaged every other day on Campylobacter blood agar (CBA) containing 10% defibrinated sheep blood at 37°C in a humidified microaerobic atmosphere of 5% O2, 10% CO2, and 85% N2. When needed, either kanamycin or chloramphenicol was added at a final concentration of 7.5 μg/ml or 15 μg/ml, or both antibiotics were used. H. pylori was recovered from plates and inoculated into 5 to 7 ml of Ham's F-12 in a T25 cm2 tissue culture flask containing 1 mg/ml bovine serum albumin (F-12-BSA) (A7906; Sigma-Aldrich). After 16 to 18 h of growth, the bacteria were split to two flasks containing F-12-BSA, with cholesterol (50 μg/ml) added to one flask and solvent control added to the other. Due to insolubility of cholesterol in aqueous solutions, cholesterol was prepared in either ethanol or chloroform. The ethanol-solubilized cholesterol (5 mg/ml of stock) was diluted into a 10× working stock of BSA (final concentration in 10× stock, 10 mg/ml BSA and 500 μg/ml cholesterol) to create a suspension. The chloroform-solubilized cholesterol (50 μg/ml final concentration in cultures) was directly added to the tissue culture plates, and the chloroform was allowed to evaporate prior to addition of medium or H. pylori. Solvent controls did not show adverse effects on H. pylori viability. Cholesterol introduced by either method showed similar results (data not shown).
hef and cgt mutants.
The hefC, hefF, and hefI mutants of strain AH244 (all kanamycin resistant) were kindly provided by Richard Alm (AstraZeneca, Boston, MA) and are deletions with a kanamycin cassette inserted (4). The hefC, hefF, and hefI mutants of strain G27 were constructed by transforming wild-type G27 with chromosomal DNA from the corresponding AH244 hef mutants and were confirmed by PCR (Table 1). The hefC mutants of 26695 and SS1 were constructed by transforming wild-type 26695 and SS1 with chromosomal DNA from the hefC mutant of G27 hefC and AH244 hefC, respectively, and confirmed by PCR.
TABLE 1.
Primers used in this study
| Primer function and designation(s) (name)a | Primer target | Sequence 5′-3′b | Product size (bp) for mutant (WT) |
|---|---|---|---|
| Construction of hefI::cat::hefI | |||
| DM701 (Cat-F) | cat 5′ end | GATATAGATTGAAAAGTGGAT | 742 |
| DM702 (Cat-R) | cat 3′ end | TTATCAGTGCGACAAACTGGG | |
| DM861 (HefI-F5) | hefI 5′ end | GGGCGATAAAAAGCGTTCGTTTAG | (797) |
| DM904 (HefI-R5) | hefI 5′ end with cat appended | ATCCACTTTTCAATCTATATCATAAGTGTC AGCGTGATAGC | |
| DM864 (HefI-F3) | hefI 3′ end with cat appended | CCCAGTTTGTCGCACTGATAAGGGAGAACG GACATGCTCTT | (1,162) |
| DM862 (HefI-R3) | hefI 3′ end | TCATGTTTGATTGCTTTTAATTCCCGC | |
| DM861/DM702 | Fusion of 5′ end ofhefItocat | 1,539c | |
| DM862/DM701 | Fusion of 3′ end of hefI to cat | 1,904c | |
| DM861/DM862 | Fusion of 5′ end of hefI::catto cat::hefI3′ end | 2,701c | |
| DM917 (HefI-F) | hefI 5′ end | CGGTTAGGGGGATTTCTAGC | 2,156c,d |
| DM918 (HefI-R) | hefI 3′ end | GCCACGCTCATGTTAAATCC | |
| Confirmation of hefC mutant | |||
| DM825 (HefC-F) | hefC 5′ end | AATTTCTTCGGTGCGTTTTG | 2,045 (3,087) |
| DM827 (HefC-R) | hefC 3′ end | TCGGTCTTAGACGGGTTTTG | |
| DM772 (Kan-F) | aphA3 | CGCCGTATGTAAGGATTTCAG | 1,084 |
| DM773 (Kan-R) | aphA3 | CCCAATCAGGCTTGATCCCCAG | |
| DM825/DM773 | 1,398 | ||
| DM827/DM772 | 1,723 | ||
| Confirmation of hefF mutant | |||
| DM881 (HefF-F) | hefF | GATATTTCGCCCACTCAAG | 3,466 (2,816) |
| DM882 (HefF-R) | hefF | CCCACGCTATGAGAAAATAAT | |
| DM881/DM773 | 1,546 | ||
| DM882/DM772 | 3,004 | ||
| DM772/DM773 | 1,084 | ||
| Confirmation of hefI mutant | |||
| DM883 (HefI-F) | hefI | GCCTCTTTGTGGGCGATAAA | 3,721 (2,959) |
| DM886 (HefI-R) | hefI | CAACGCTGCTTATCATGCCC | |
| DM883/DM773 | 2,334 | ||
| DM886/DM772 | 2,471 | ||
| DM773/DM773 | 1,084 |
In the primer names, F preceded by a hyphen indicates forward, and R preceded by a hyphen indicates reverse.
All primers were developed in the present study. Underlining indicates the cat sequence.
PCR fusion product.
Final amplification of hefI::cat::hefI.
The hefF hefI double mutant was constructed by using the hefF mutant G27 and constructing an hefI::cat::hefI PCR fusion according to the method of Chalker et al. (7), with modifications (19). A total of 1,086 bp was deleted from hefI to create an in-frame deletion. The template for hefI amplification was chromosomal DNA from wild-type strain 26695, whose genome has been sequenced (42). The hefI gene from strain 26695 is 95% identical to that of strain G27, which also has been sequenced (2), and we reasoned that this high degree of homology would be sufficient for homologous recombination. Briefly, the 5′ and 3′ ends of hefI were separately amplified by PCR to include overhangs of the cat cassette (Table 1). The nonpolar cat cassette was amplified from a pBS-cat derivative (26). The hefI::cat::hefI final fusion product (2,156 bp) was transformed into the G27 hefF mutant by natural transformation; doubly resistant (kanamycin and chloramphenicol) transformants were passaged once, and then four were chosen for PCR confirmation. One confirmed double mutant was used for the experiments.
The 26695 cgt mutant was described previously (19).
Chemicals.
Chemicals were from Sigma-Aldrich or Research Organics, except where noted. All master stocks were made in sterile water and filter sterilized except as noted. Ceragenins CSA-11, CSA-13, CSA-54, and CSA-90 were chemically synthesized and dissolved in water, as described previously (10). Lower-concentration working stocks were prepared by dilution of master stocks in water as necessary.
Bacterial viability assays.
After overnight growth of H. pylori in F-12-BSA medium in the presence or absence of cholesterol, bacteria were harvested, washed once with phosphate-buffered saline (PBS),and resuspended in PBS for use as the inoculum.
Bile salts or ceragenins (decreasing at 2-fold increments) were added to 24-well tissue culture plates containing 1 ml of F-12-BSA medium with or without cholesterol (50 μg/ml). Equal numbers of viable bacteria (∼1 × 108/ml in 50 μl) were added to all wells. Wells lacking compounds served as controls. After 16 to 18 h of growth in the presence of bile salts or ceragenins, bacteria (1 ml) were pelleted and resuspended in 1 ml of PBS to avoid continued residual killing by the compound. Tenfold serial dilutions in PBS were plated in duplicate for viable counts on CBA and incubated microaerobically at 37°C for 4 to 5 days.
Disc diffusion assays.
For disc diffusion assays, the medium used was F-12 agar containing BSA (1 mg/ml) and β-cyclodextrin (BCD; 200 μg/ml) either with (50 μg/ml) or without cholesterol (40). In some experiments CBA was used. H. pylori strain G27 was grown overnight in F-12-BSA broth with or without cholesterol and used to spread ∼108 CFU/ml onto the F-12-BSA-BCD agar plates containing or lacking cholesterol. Sterile filter paper discs (6-mm diameter; Becton Dickinson, Sparks, MD) were added, and 5 μl of a 100 mg/ml solution of bile salt (dissolved in sterile water) was added, except where indicated in the figure legends. Water was used as a negative control. Plates were incubated in a microaerobic atmosphere for 5 days, and zones of inhibition were measured (mm diameter).
ATP measurements.
Bacteria were measured for ATP levels using a Cell Titer Glow Kit (Promega). This assay has been validated for use in H. pylori previously (40).
RESULTS
H. pylori grown in the presence of cholesterol is more resistant to most bile salts.
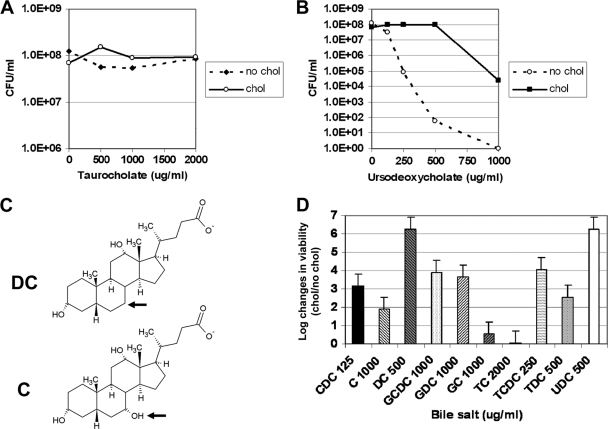
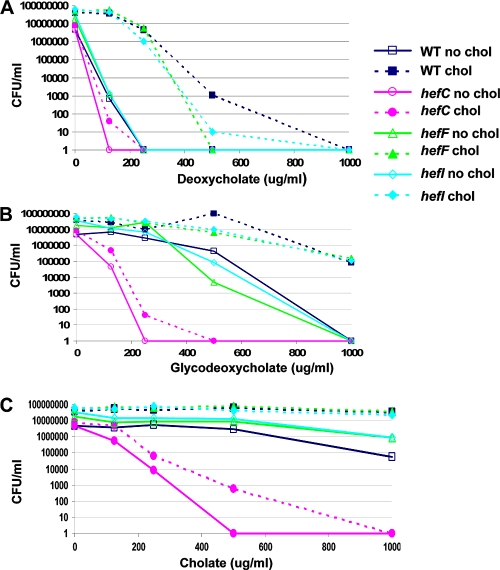
The physiologic significance of cholesterol and its derivatives in the H. pylori membrane is not well understood. We sought to determine whether cholesterol affected resistance of H. pylori to bile salts. To this end, H. pylori was grown for 16 to 18 h in the chemically defined medium F-12-BSA in the presence or absence of cholesterol. Under these conditions, growth rates (∼4-h doubling time) and viability were similar (data not shown). Next, bacteria were subcultured into a 24-well plate with F-12-BSA medium in the presence or absence of cholesterol along with different concentrations of bile salts. Bacteria were plated for viability after 16 to 18 h. The data demonstrate that H. pylori bacteria grown in the presence of cholesterol are more resistant to most bile salts than their cholesterol-free counterparts (Fig. 1 B and D; also data not shown). There were no differences in H. pylori bacteria grown under the two culture conditions with two bile salts, sodium taurocholate (Fig. 1A) and sodium glycocholate (Fig. 1D and data not shown), and with sodium cholate (Fig. 1D and data not shown) only a modest difference was present. Cholate-containing bile salts have a hydroxyl residue that is absent from deoxycholate-containing bile salts (Fig. 1C). Interestingly, H. pylori cultured in the absence of cholesterol was much more susceptible to the deoxycholate bile salt derivatives than to cholate-containing counterparts (Fig. 1D, compare C versus DC, GC versus GDC, and TC versus TDC).
FIG. 1.
H. pylori displays cholesterol-dependent resistance to numerous bile salts. H. pylori strain 26695 was grown in F-12-BSA in the absence (no chol) or presence (chol) of cholesterol (50 μg/ml) for 16 to 18 h at the concentration of bile salts noted. Bacteria were harvested, washed, and then serially diluted and plated for viable counts (see Materials and Methods). (A) Taurocholate. Data are representative of three experiments with duplicate measurements. (B) Ursodeoxycholate. Data are representative of three experiments with duplicate measurements. (C) Structural comparison between the bile salts cholate and deoxycholate. The arrow indicates the structural difference. The diagram was drawn with ChemSketch, version 11.0 freeware (Advanced Chemistry Development). (D) Summary of effects of bile salts on H. pylori cultured in the presence or absence of cholesterol. The data for 10 bile salts are summarized. Shown is the log change in viability between H. pylori cultured with cholesterol versus H. pylori cultured without cholesterol in the presence of the bile salt at the concentration listed. The concentration listed is the one that showed the largest difference between the cholesterol-grown versus cholesterol-free cultures. Dose-response curves for all of these bile salts have been performed but are not shown due to space limitations. Data are representative of two to four experiments with duplicate measurements. CDC, chenodeoxycholate; C, cholate; DC, deoxycholate; GCDC, glycochenodeoxycholate; GDC, glycodeoxycholate; GC, glycocholate; TC, taurocholate; TCDC, taurochenodeoxycholate; TDC, taurodeoxycholate; and UDC, ursodeoxycholate.
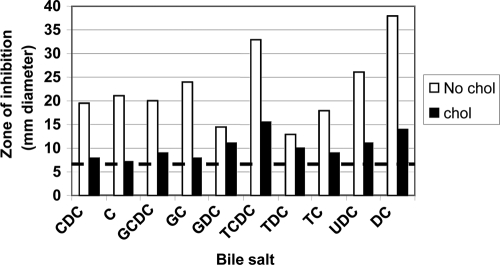
To demonstrate that these observations hold true by using an alternative method and strain of H. pylori, we used disc diffusion assays and strain G27. G27 was inoculated onto F-12-BSA-BCD agar containing or lacking cholesterol along with various bile salts. Cultures grown on F-12-BSA-BCD plates without cholesterol demonstrated 2- to 3-fold larger zones of inhibition from nearly all of the bile salts than cultures grown on F-12-BSA plus cholesterol (Fig. 2). H. pylori G27 cultured with cholesterol was more resistant to most bile salts than G27 cultured without cholesterol. Thus, the two methods and strains were largely in agreement.
FIG. 2.
Disc diffusion assay of bile salts on H. pylori strain G27 grown in the presence or absence of cholesterol. H. pylori strain G27 was plated onto F-12-BSA plates containing beta cyclodextrin in the absence (no chol) or presence (chol) of cholesterol (50 μg/ml). All bile salts (sodium salts) were added to 5 μl of 100 mg/ml stocks, except for chenodeoxycholate and glycochenodeoxycholate, which were 20 mg/ml stocks. Following 5 days of incubation, zones of inhibition were measured in millimeters of diameter. Data are representative of three experiments. Abbreviations are the same as in the legend to Fig. 1. Dotted horizontal line, diameter (in mm) of the filter discs (no zone of inhibition).
The H. pylori cholesteryl glycosyltransferase gene, cgt, is not required for cholesterol-dependent resistance to bile salts.
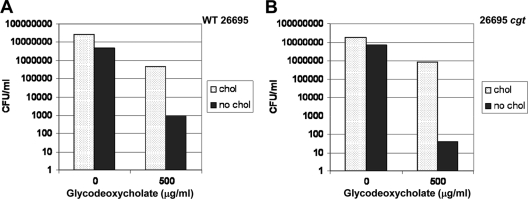
The cgt gene is essential to glycosylation of host cholesterol by H. pylori (46). To date, this is the only gene that has been identified in the H. pylori cholesterol uptake and modification pathway though other genes are thought to exist. The purpose of glycosylation is not entirely understood, so we hypothesized that cgt might be involved in cholesterol-dependent resistance to bile salts, particularly since a cgt mutant is attenuated for virulence in the mouse model (46). Surprisingly, the cgt mutant still retained cholesterol-dependent resistance to glycodeoxycholate (Fig. 3) and deoxycholate (data not shown).
FIG. 3.
The cholesteryl glycosyltransferase, encoded by cgt, is not required for cholesterol-dependent resistance of H. pylori to bile salts. Wild-type H. pylori 26695 (A) and the H. pylori 26695 cgt mutant (B) were grown in the presence or absence of cholesterol as described in the legend to Fig. 1 in the presence of various concentrations of bile salts.
H. pylori displays cholesterol-dependent resistance to several ceragenins.
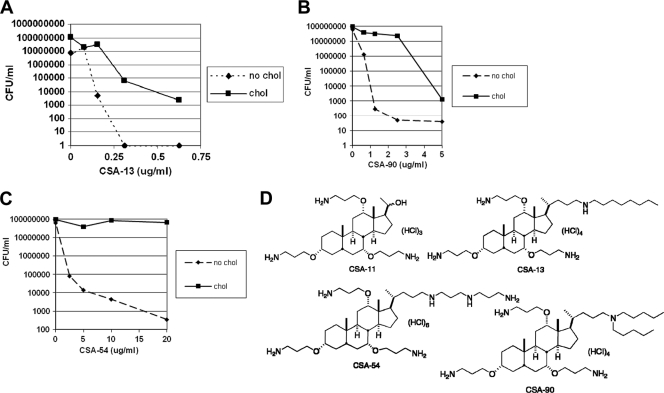
Ceragenins, bile salt derivatives that have cationic charge, were incubated with H. pylori grown in the presence or absence of cholesterol. The data demonstrate that H. pylori 26695 has dramatically more resistance to CSA-13, CSA-54, and CSA-90 when grown in the presence of cholesterol (Fig. 4 A, B, and C). The most potent ceragenin was CSA-13, which killed cholesterol-free H. pylori at a concentration below 0.25 μg/ml. In contrast, there was no killing whatsoever of H. pylori strain 26695 grown in the presence or absence of cholesterol by CSA-11 at concentrations up to 20 μg/ml (data not shown). CSA-11 has also been shown not to have bactericidal activity in other bacterial pathogens. Several of the ceragenin structures are shown in Fig. 4D. To our knowledge, cholesterol is the first identified factor that leads to ceragenin resistance in bacteria.
FIG. 4.
H. pylori displays cholesterol-dependent resistance to ceragenins. H. pylori strain 26695 was grown in F-12-BSA in the presence or absence of cholesterol (50 μg/ml) for 16 to 18 h at the concentrations of CSA-13, CSA-90, or CSA-54 noted. Bacteria were harvested, washed, and then serially diluted and plated for viable counts (see Materials and Methods). (D) Structures of ceragenins used in this study.
The H. pylori cholesteryl glycosyltransferase gene, cgt, is not required for cholesterol-dependent resistance to ceragenins.
We also tested whether cgt was involved in cholesterol-dependent resistance to ceragenins. As found for bile salts, the 26695 cgt mutant surprisingly still retains cholesterol-dependent resistance to CSA-13 and CSA-90, similar to the wild-type strain (data not shown).
H. pylori hefC is required for resistance to bile salts.
Since cgt was eliminated as a candidate for the cholesterol-dependent resistance of H. pylori to bile salts and ceragenins, we began searching for other possibilities. A recent review article investigated possible homologs of bile salt resistance genes in Campylobacter jejuni and H. pylori and related bacteria (35). E. coli AcrB, an established bile salt efflux pump, was one protein highlighted (32). AcrB is a member of the resistance nodulation division (RND) efflux pumps involved in multidrug resistance in Gram-negative bacteria (36) and are comprised of three components: an inner membrane efflux pump, a periplasmic protein, and an outer membrane channel protein.
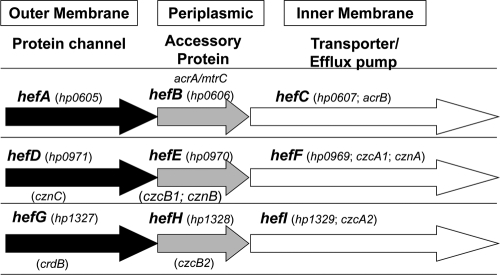
By BlastP analysis, we found that H. pylori has three homologs of AcrB: HefC, HefF, and HefI; these proteins were also highlighted in a previous review (35) but had not been thoroughly investigated for bile salt resistance. We therefore hypothesized that one or more of these putative efflux pump proteins would be involved in the ability of H. pylori to resist bile salts. Of these, HefC shows the highest amino acid identity to both E. coli AcrB and C. jejuni CmeF (Table 2). To address the above hypothesis, we investigated the involvement of hefC, hefF, and hefI in the cholesterol-dependent resistance of H. pylori to bile salts. These three genes encode putative inner membrane efflux pumps (Fig. 5). Bactericidal experiments were conducted as described in Materials and Methods. While the wild-type H. pylori strain AH244 grown in the absence of cholesterol is susceptible to killing by deoxycholate, AH244 grown in the presence of cholesterol is resistant (6 orders of magnitude difference) (Fig. 6 A). Similar phenotypes were observed for the hefF and hefI mutants. In contrast, the hefC mutant was susceptible to deoxycholate even when the strain was grown in the presence of cholesterol. To determine whether the hefC mutant was susceptible to other bile salts, we also investigated glycodeoxycholate (Fig. 6B) and cholate (Fig. 6C). The hefC mutant grown in the presence of cholesterol displayed increased susceptibility to these other bile salts as well, compared with wild-type H. pylori. There are clear differences in susceptibility of H. pylori to individual bile salts.
TABLE 2.
Homology of H. pylori HefC, HefF, and HefI to E. coli AcrB and C.jejuni CmeF
| Protein | Homology to the indicated proteina |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcrB |
HefC |
HefF |
HefI |
|||||||||
| Identity (%) | Similarity (%) | P | Identity (%) | Similarity (%) | P | Identity (%) | Similarity (%) | P | Identity (%) | Similarity (%) | P | |
| CmeF | 25 | 48 | 10−84 | 37 | 58 | 10−177 | 22 | 45 | 10−55 | 22 | 44 | 10−48 |
| AcrB | 27 | 50 | 10−106 | 24 | 46 | 10−64 | 20 | 41 | 10−42 | |||
| HefC | 25 | 45 | 10−66 | 22 | 45 | 10−53 | ||||||
| HefF | 28 | 52 | 10−127 | |||||||||
Of the three Hefs, HefC shows the strongest homology to AcrB and CmeF.
FIG. 5.
Schematic of the putative efflux pump systems in H. pylori. The many alternative names for the genes are shown. For simplicity, all the genes are referred to in the text as hef, after the authors that first named these genes (see reference 4). The hef mutants (not shown in the figure) are all internal deletions with an inserted kanamycin resistance cassette.
FIG. 6.
The H. pylori hefC mutant displays increased susceptibility to bile salts over that of the wild type or the hefF or hefI mutant. Strains were grown overnight in F-12-BSA broth in the presence (chol) or absence (no chol) of cholesterol (50 μg/ml) in various concentrations of bile salt. Cultures were diluted and plated on CBA for viable counts.
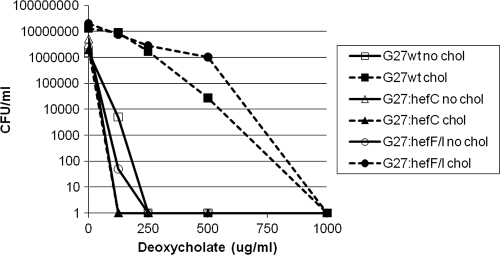
To determine whether this observation held for other H. pylori strains, the hefC mutation was introduced into three additional strains: 26695, G27, and SS1. All three hefC mutants showed increased susceptibility to deoxycholate in a manner similar to that of the hefC mutant of AH244 (Fig. 7; also data not shown).
FIG. 7.
The hefF hefI double mutant still displays cholesterol-dependent resistance to the bile salt deoxycholate. Strains (G27 background) were grown overnight in F-12-BSA broth in the presence (chol) or absence (no chol) of cholesterol (50 μg/ml) in various concentrations of deoxycholate. Cultures were diluted and plated on CBA for viable counts.
Neither the hefF nor the hefI mutations in H. pylori resulted in bile salt susceptibility.
To determine whether HefF and HefI might have overlapping functions that compensate for each other, we constructed an hefF hefI double mutant in strain G27 (see Materials and Methods). This double mutant strain still displayed cholesterol-dependent resistance to the bile salt deoxycholate that was indistinguishable from that of the wild-type strain (Fig. 7), suggesting that neither HefF nor HefI is involved in bile salt resistance.
These results were confirmed by using disc diffusion assays similar to those presented in Fig. 2 except that 10 μl of 100 mg/ml bile salt was used, and four experiments were conducted on CBA plates. The hefC AH244 mutant displayed greater susceptibility than the wild-type, hefF, or hefI mutant strains to five different bile salts, as indicated by larger zones of inhibition with the hefC mutant. The zones of inhibition (in mm of diameter) for the hefC mutant were 20 to 25 mm for deoxycholate, cholate, glycodeoxycholate, and taurodeoxycholate and 11 mm for taurocholate; the WT, hefF, and hefI mutant zones of inhibition were 12 to 15 mm for deoxycholate and 7 to 8 mm for glycocholate and taurodeoxycholate, and there were no zones at all for cholate and taurocholate. Additionally, it was likewise determined that the hefC mutant grown with cholesterol was at least 100-fold more susceptible to deoxycholate than wild-type H. pylori (data not shown) using the previously validated ATP assay to measure viability of H. pylori (40).
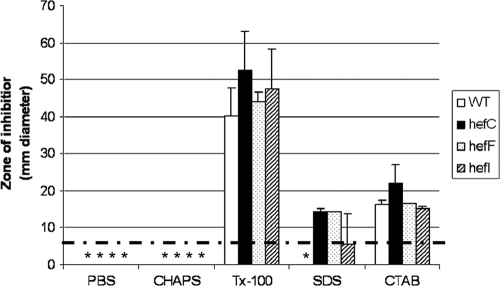
Since bile salts are membrane-disrupting antimicrobial compounds, we wondered whether the hefC mutant would also display increased susceptibility to other membrane-disrupting agents. The wild type and hef mutants were therefore assessed for susceptibility to the structurally diverse detergents sodium dodecyl sulfate (SDS; anionic), cetyl triammonium bromide (CTAB; cationic), Triton X-100 (nonionic), and 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS, zwitterionic) by the disc diffusion method. All four strains had similar zones of inhibition with SDS, Triton X-100, and CTAB treatment but were not killed at all by CHAPS (Fig. 8).
FIG. 8.
The hefC mutant does not display increased susceptibility to detergents over that of the wild type or the hefF or hefI mutant. H. pylori strains (∼109 CFU/ml) were plated onto CBA plates, and susceptibility to detergents was assessed by disc diffusion assay. Ten microliters of 100 mg/ml stocks was used. Shown are averages ± standard deviations of duplicates. The experiments were conducted twice with similar results. Dotted horizontal line, diameter (in mm) of the filter discs; *, no zone of inhibition occurred.
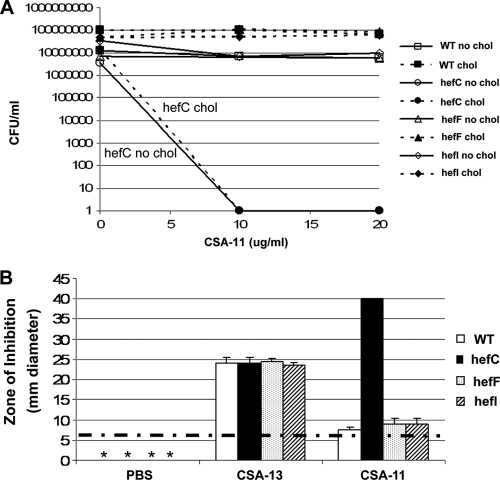
H. pylori hefC is required for resistance to ceragenin 11.
To determine whether the hef genes were involved in resistance to ceragenins, we investigated hefC, hefF, and hefI mutants for susceptibility to ceragenin 11, which was shown above not to kill wild-type H. pylori 26695. Similarly, CSA-11 did not kill wild-type AH244 (Fig. 9 A). The hefF and hefI mutants also resisted killing by CSA-11. In contrast, the hefC mutant is 6 logs more susceptible to CSA-11 than the wild-type strain (Fig. 9A). There was also a trend for the hefC mutant to be more susceptible than the wild-type strain to CSA-54, but this was not as dramatic as for CSA-11 (data not shown). In disc diffusion assays, the hefC mutant displayed a much larger zone of inhibition to CSA-11 than the wild type or the hefF or hefI mutant (Fig. 9B). As a control, the wild type and each hef mutant were incubated with CSA-13, and no differences in susceptibilities among the strains were noted.
FIG. 9.
The hefC mutant is susceptible to ceragenin-11. (A) Bactericidal assay of the wild type or hef mutants grown in F-12-BSA medium in the presence or absence of cholesterol and different concentrations of ceragenin 11 (CSA-11). Minus, no cholesterol; plus, with cholesterol. Shown are results from a representative of two experiments, each conducted in duplicate (average ± standard deviation) with essentially identical results. (B) Disc diffusion assay on the wild type or hef mutants on CBA. CSA-13, ceragenin 13; CSA-11, ceragenin 11. Zones of inhibition were measured (mm diameter). Shown are results from a representative of two experiments, each conducted in duplicate (average ± standard deviation), with essentially identical results. Dotted horizontal line, diameter in mm of the filter discs; *, no zone of inhibition occurred.
DISCUSSION
In this study we demonstrate that H. pylori resistance to bile salts and ceragenins increases in a cholesterol-dependent fashion. H. pylori has been known for some time to steal host cholesterol, modify it by glycosylation, and incorporate these modified cholesteryl glucosides into its membrane (1, 16-18, 20, 21). However, the significance of this was largely unexplored until recently when it was shown that these cholesteryl glucosides play an important role in colonization of mice and immune evasion (46). These observations, accompanied by those presented here, suggest that the cholesterol allows H. pylori to evade not only the adaptive immune response but also innate immune mechanisms such as bile salts encountered by H. pylori in vivo. Remarkably, the glycosylated cholesterol derivatives generated by the cholesteryl glycosyltransferase, encoded by cgt, are not involved in this cholesterol-dependent resistance of H. pylori to bile salts or ceragenins in vitro. Incorporation of cholesterol could lead to decreased membrane permeability to bile salts or altered expression of membrane-bound proteins that play a role in bile salt resistance, such as HefC. We are currently exploring whether expression of H. pylori hefC is altered in response to cholesterol or bile salts.
For the bile salts in which both a cholate and deoxycholate derivative were available, H. pylori was more resistant to the cholate derivative. Loss of the hydroxyl group to yield the deoxycholate derivatives (Fig. 1C) resulted in dramatic differences between H. pylori cultured with cholesterol versus cultures without cholesterol (Fig. 1). This suggests that H. pylori susceptibility to bile salts could involve the interaction between a specific surface constituent of the bacterium that recognizes the deoxycholate derivatives better than the cholate derivatives. This interaction appears to be more pronounced when H. pylori is cultured in the absence of cholesterol since H. pylori is much more susceptible to the deoxycholate bile salts under these conditions. Other bacteria, such as Vibrio cholerae, likewise display greater susceptibility to the deoxycholate derivative than the cholate derivative (5, 6).
This study is the first report to discover a ceragenin resistance mechanism, namely, that cholesterol confers resistance of H. pylori to ceragenins. Ceragenins are gaining momentum as novel cationic antimicrobial compounds related to bile salts that can kill antibiotic-resistant pathogens (8, 9). Since these are easily synthesized in the laboratory yet are not peptides, studies can be conducted in vitro and in vivo. The first in vivo efficacy experiments with CSA-13 are under way (P. B. Savage, unpublished observations). H. pylori grown in the absence of cholesterol has about 10- to 100-fold increased susceptibility to ceragenins versus other Gram-negative bacteria (29, 37). In the presence of cholesterol H. pylori has susceptibility similar to the susceptibilities of other Gram-negative bacteria (which are typically grown without cholesterol). CSA-13 was shown to have a minimum bactericidal concentration of 0.275 μg/ml to 8.9 μg/ml in different H. pylori strains using Mueller-Hinton agar plus 5% sheep blood (which contains cholesterol), in line with the observations here using F-12-BSA medium containing cholesterol (28).
In the closely related organism C. jejuni, there are two known efflux pump systems, CmeABC and CmeDEF. CmeF bears stronger amino acid identity than CmeC to the H. pylori putative membrane protein HefC and E. coli AcrB. Whether CmeF plays a role in bile salt resistance in C. jejuni has not been reported, but the homologous CmeC does play an important role in bile salt resistance (30). Given the established role of AcrB (11, 32, 36) and HefC (this study) in bile salt resistance in E. coli and H. pylori, respectively, it seems likely that the CmeF protein from C. jejuni will likewise be involved.
There appears to be some specificity among the three efflux pump systems of H. pylori since HefF and HefI play no role in bile salt resistance while HefC is critical. Moreover, the pumps can also discriminate between bile salts versus other amphipathic molecules since the wild type and the hef mutants displayed similar susceptibilities to charged and uncharged detergents. Lack of an effect with detergents in the hefC mutant of H. pylori differs from that of the RND multidrug efflux pump in V. cholerae, which does play an important role in resistance to SDS and Triton X-100 (6). HefF (also designated CznA and CzcA1) plays an important role in metal ion homeostasis since an hefF mutant had increased susceptibility to cadmium, zinc, and nickel (39). The other hef efflux pumps were not tested in that study, but these data suggest that the HefF efflux pump has a primary role in pumping out toxic heavy metals yet does not have a role in bile salt resistance (this study). The genes upstream of hefI (czcA2), hefG (crdB), and hefH (czcB2) are involved in copper resistance (44, 45). The hefC gene has been linked to multidrug resistance in H. pylori (25). The hefA gene (upstream of hefC in the same operon) has also been linked to multiple-antibiotic resistance (31), including novobiocin resistance (43), but an earlier study by Bina and coworkers (4) did not reach the same conclusion, perhaps due to the use of different strain backgrounds and a less sensitive method. In a more recent study, an hefA mutant of H. pylori displayed increased susceptibility to the bile salt sodium deoxycholate (31), supporting the work in this study with the gene downstream in the same operon, hefC. HefA, HefD, and HefG are all homologs of TolC, which is an outer membrane protein channel involved in multidrug resistance in E. coli, as well as type I protein secretion (24).
The hefA homolog has also been disrupted from Helicobacter hepaticus, and similar conclusions were reached: hefA is involved in bile salt resistance and multidrug resistance (amoxicillin, gentamicin, ofloxacin, and rifamycin but not cefotaxime, vancomycin, erythromycin, metronidazole, tetracycline, or ciprofloxacin) but not resistance to detergents (SDS) or nickel (3).
Collectively, the data suggest that the H. pylori HefDEF efflux pump is for pumping out toxic heavy metals (cadmium, zinc, and nickel), the HefGHI efflux pump is for pumping out copper, and the HefABC efflux pump is specific for bile salts and some, but not all, antibiotics. How the HefABC pump can distinguish among antibiotics, while still having rather broad substrate specificity, remains a mystery. Future work will center on investigating the other two hef genes upstream of hefC (hefA and hefB) for their role in bile salt resistance in H. pylori. Additionally, transport studies will be conducted to directly test whether the HefABC system constitutes a bile salt efflux pump.
The specific mechanism of how hefC is directly involved in the cholesterol-dependent resistance to bile salts is not yet clear. One intriguing hypothesis is that HefC can import cholesterol directly. Since bile salts are structurally similar to cholesterol, this hypothesis is not unreasonable. Another possibility is that HefC interacts with the yet-to-be-discovered cholesterol transport machinery in the cytoplasmic membrane.
The data presented in this study suggest that two mechanisms are involved in resistance of H. pylori to bile salts and ceragenins. The first mechanism is dependent upon HefC. Since bile salts constitute an innate immune defense mechanism, H. pylori may have evolved HefC to pump out the bile salts it encounters in vivo before the bile salts exert their antibacterial effect on the membrane. If this system fails, the bacteria have a second system that is dependent upon incorporation of cholesterol into its membrane to counteract bile salts, perhaps by decreasing bile salt permeability. Having this two-pronged resistance strategy may help the bacteria persist in the unique gastric niche in case one system becomes inoperable or becomes downregulated. Novel therapeutics that target the ability of H. pylori to utilize cholesterol, as recently reported (23, 27), as well as the HefC efflux pump, may be important alternative strategies to consider as antibiotic resistance in this gastric pathogen continues to rise.
Acknowledgments
This work was supported by Public Health Service grant R01 CA101931 from the National Institutes of Health to D.J.M. and by a grant from Ceragenix Pharmaceuticals to P.B.S. P.B.S. is a paid consultant for Ceragenix.
We are grateful to R. Alm for providing the hef mutants of H. pylori.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 25 October 2010.
REFERENCES
- 1.Ansorg, R., K. D. Muller, G. von Recklinghausen, and H. P. Nalik. 1992. Cholesterol binding of Helicobacter pylori. Zentralbl. Bakteriol. 276:323-329. [DOI] [PubMed] [Google Scholar]
- 2.Baltrus, D. A., M. R. Amieva, A. Covacci, T. M. Lowe, D. S. Merrell, K. M. Ottemann, M. Stein, N. R. Salama, and K. Guillemin. 2009. The complete genome sequence of Helicobacter pylori strain G27. J. Bacteriol. 191:447-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belzer, C., J. Stoof, S. Breijer, J. G. Kusters, E. J. Kuipers, and A. H. van Vliet. 2009. The Helicobacter hepaticus hefA gene is involved in resistance to amoxicillin. Helicobacter 14:72-79. [DOI] [PubMed] [Google Scholar]
- 4.Bina, J. E., R. A. Alm, M. Uria-Nickelsen, S. R. Thomas, T. J. Trust, and R. E. Hancock. 2000. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob. Agents Chemother. 44:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bina, X. R., J. A. Philippart, and J. E. Bina. 2009. Effect of the efflux inhibitors 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide on antimicrobial susceptibility and virulence factor production in Vibrio cholerae. J. Antimicrob. Chemother. 63:103-108. [DOI] [PubMed] [Google Scholar]
- 6.Bina, X. R., D. Provenzano, N. Nguyen, and J. E. Bina. 2008. Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infect. Immun. 76:3595-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalker, A. F., H. W. Minehart, N. J. Hughes, K. K. Koretke, M. A. Lonetto, K. K. Brinkman, P. V. Warren, A. Lupas, M. J. Stanhope, J. R. Brown, and P. S. Hoffman. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 183:1259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, J. N., R. N. Jones, H. S. Sader, P. B. Savage, and M. J. Rybak. 2008. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J. Antimicrob. Chemother. 61:365-370. [DOI] [PubMed] [Google Scholar]
- 9.Chin, J. N., M. J. Rybak, C. M. Cheung, and P. B. Savage. 2007. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 51:1268-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, B., Q. Guan, J. P. Walsh, J. S. Boswell, T. W. Winter, E. S. Winter, S. S. Boyd, C. Li, and P. B. Savage. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 45:663-669. [DOI] [PubMed] [Google Scholar]
- 11.Drew, D., M. M. Klepsch, S. Newstead, R. Flaig, J. W. De Gier, S. Iwata, and K. Beis. 2008. The structure of the efflux pump AcrB in complex with bile acid. Mol. Membr. Biol. 25:677-682. [DOI] [PubMed] [Google Scholar]
- 12.Epand, R. M., R. F. Epand, and P. B. Savage. 2008. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect. 21:307-311. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin, A., D. Kersulyte, G. Sisson, S. J. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 14.Han, S. W., D. G. Evans, F. A. el-Zaatari, M. F. Go, and D. Y. Graham. 1996. The interaction of pH, bile, and Helicobacter pylori may explain duodenal ulcer. Am. J. Gastroenterol. 91:1135-1137. [PubMed] [Google Scholar]
- 15.Hanninen, M. L. 1991. Sensitivity of Helicobacter pylori to different bile salts. Eur. J. Clin. Microbiol. Infect. Dis. 10:515-518. [DOI] [PubMed] [Google Scholar]
- 16.Haque, M., Y. Hirai, K. Yokota, N. Mori, I. Jahan, H. Ito, H. Hotta, I. Yano, Y. Kanemasa, and K. Oguma. 1996. Lipid profile of Helicobacter spp.: presence of cholesteryl glucoside as a characteristic feature. J. Bacteriol. 178:2065-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haque, M., Y. Hirai, K. Yokota, and K. Oguma. 1995. Lipid profiles of Helicobacter pylori and Helicobacter mustelae grown in serum-supplemented and serum-free media. Acta Medica Okayama 49:205-211. [DOI] [PubMed] [Google Scholar]
- 18.Haque, M., Y. Hirai, K. Yokota, and K. Oguma. 1995. Steryl glycosides: a characteristic feature of the Helicobacter spp.? J. Bacteriol. 177:5334-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrandt, E., and D. J. McGee. 2009. Helicobacter pylori lipopolysaccharide modification, Lewis antigen expression, and gastric colonization are cholesterol-dependent. BMC Microbiol. 9:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirai, Y., M. Haque, T. Yoshida, K. Yokota, T. Yasuda, and K. Oguma. 1995. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J. Bacteriol. 177:5327-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inamoto, Y., S. Hamanaka, Y. Hamanaka, T. Nagate, I. Kondo, T. Takemoto, and K. Okita. 1995. Lipid composition and fatty acid analysis of Helicobacter pylori. J. Gastroenterol. 30:315-318. [DOI] [PubMed] [Google Scholar]
- 22.Itoh, M., K. Wada, S. Tan, Y. Kitano, J. Kai, and I. Makino. 1999. Antibacterial action of bile acids against Helicobacter pylori and changes in its ultrastructural morphology: effect of unconjugated dihydroxy bile acid. J. Gastroenterol. 34:571-576. [DOI] [PubMed] [Google Scholar]
- 23.Kawakubo, M., Y. Ito, Y. Okimura, M. Kobayashi, K. Sakura, S. Kasama, M. N. Fukuda, M. Fukuda, T. Katsuyama, and J. Nakayama. 2004. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003-1006. [DOI] [PubMed] [Google Scholar]
- 24.Koronakis, V., J. Eswaran, and C. Hughes. 2004. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu. Rev. Biochem. 73:467-489. [DOI] [PubMed] [Google Scholar]
- 25.Kutschke, A., and B. L. de Jonge. 2005. Compound efflux in Helicobacter pylori. Antimicrob. Agents Chemother. 49:3009-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langford, M. L., J. Zabaleta, A. C. Ochoa, T. L. Testerman, and D. J. McGee. 2006. In vitro and in vivo complementation of the Helicobacter pylori arginase mutant using an intergenic chromosomal site. Helicobacter 11:477-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, H., P. Wang, H. Hoshino, Y. Ito, M. Kobayashi, J. Nakayama, P. H. Seeberger, and M. Fukuda. 2008. α1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol alpha-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 18:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leszczynska, K., A. Namiot, D. E. Fein, Q. Wen, Z. Namiot, P. B. Savage, S. Diamond, P. A. Janmey, and R. Bucki. 2009. Bactericidal activities of the cationic steroid CSA-13 and the cathelicidin peptide LL-37 against Helicobacter pylori in simulated gastric juice. BMC Microbiol. 9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, C., M. R. Lewis, A. B. Gilbert, M. D. Noel, D. H. Scoville, G. W. Allman, and P. B. Savage. 1999. Antimicrobial activities of amine- and guanidine-functionalized cholic acid derivatives. Antimicrob. Agents Chemother. 43:1347-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, J., C. Cagliero, B. Guo, Y. W. Barton, M. C. Maurel, S. Payot, and Q. Zhang. 2005. Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni. J. Bacteriol. 187:7417-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Z. Q., P. Y. Zheng, and P. C. Yang. 2008. Efflux pump gene hefA of Helicobacter pylori plays an important role in multidrug resistance. World J. Gastroenterol. 14:5217-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 33.Mathai, E., A. Arora, M. Cafferkey, C. T. Keane, and C. O'Morain. 1991. The effect of bile acids on the growth and adherence of Helicobacter pylori. Aliment. Pharmacol. Ther. 5:653-658. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama, F. 1967. Quantitative microanalysis of bile. J. Lab. Clin. Med. 69:594-609. [PubMed] [Google Scholar]
- 35.Okoli, A. S., T. Wadstrom, and G. L. Mendz. 2007. Minireview: bioinformatic study of bile responses in Campylobacterales. FEMS Immunol. Med. Microbiol. 49:101-123. [DOI] [PubMed] [Google Scholar]
- 36.Pos, K. M. 2009. Drug transport mechanism of the AcrB efflux pump. Biochim. Biophys. Acta 1794:782-793. [DOI] [PubMed] [Google Scholar]
- 37.Saha, S., P. B. Savage, and M. Bal. 2008. Enhancement of the efficacy of erythromycin in multiple antibiotic-resistant gram-negative bacterial pathogens. J. Appl. Microbiol. 105:822-828. [DOI] [PubMed] [Google Scholar]
- 38.Schindlbeck, N. E., C. Heinrich, F. Stellaard, G. Paumgartner, and S. A. Muller-Lissner. 1987. Healthy controls have as much bile reflux as gastric ulcer patients. Gut 28:1577-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stahler, F. N., S. Odenbreit, R. Haas, J. Wilrich, A. H. Van Vliet, J. G. Kusters, M. Kist, and S. Bereswill. 2006. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 74:3845-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Testerman, T. L., P. B. Conn, H. L. Mobley, and D. J. McGee. 2006. Nutritional requirements and antibiotic resistance patterns of Helicobacter species in chemically defined media. J. Clin. Microbiol. 44:1650-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Testerman, T. L., D. J. McGee, and H. L. Mobley. 2001. Helicobacter pylori growth and urease detection in the chemically defined medium Ham's F-12 nutrient mixture. J. Clin. Microbiol. 39:3842-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 43.van Amsterdam, K., A. Bart, and A. van der Ende. 2005. A Helicobacter pylori TolC efflux pump confers resistance to metronidazole. Antimicrob. Agents Chemother. 49:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waidner, B., K. Melchers, I. Ivanov, H. Loferer, K. W. Bensch, M. Kist, and S. Bereswill. 2002. Identification by RNA profiling and mutational analysis of the novel copper resistance determinants CrdA (HP1326), CrdB (HP1327), and CzcB (HP1328) in Helicobacter pylori. J. Bacteriol. 184:6700-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waidner, B., K. Melchers, F. N. Stahler, M. Kist, and S. Bereswill. 2005. The Helicobacter pylori CrdRS two-component regulation system (HP1364/HP1365) is required for copper-mediated induction of the copper resistance determinant CrdA. J. Bacteriol. 187:4683-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wunder, C., Y. Churin, F. Winau, D. Warnecke, M. Vieth, B. Lindner, U. Zahringer, H. J. Mollenkopf, E. Heinz, and T. F. Meyer. 2006. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat. Med. 12:1030-1038. [DOI] [PubMed] [Google Scholar]