Abstract
Cell surface polysaccharides are key determinants of host responses to fungal infection. We determined the effects of alterations in Candida albicans cell surface polysaccharide composition and gross changes in the host immune response in groups of mice challenged intravenously with five C. albicans strains at doses adjusted to give equal disease progression 3 days later. The five strains used were the parental strain NGY152, two mutants with defective cell wall mannosylation, pmr1Δ mutant and mnt1/2Δ mutant, and the same two strains with a copy of PMR1 and MNT1 reintegrated, respectively. Renal and spleen levels of chemokines and cytokines previously shown to be key components of early host response to C. albicans were determined at intervals up to 3 days after challenge. By 12 h after C. albicans challenge, the levels of granulocyte colony-stimulating factor (G-CSF), keratinocyte-derived chemokine (KC), interleukin 6 (IL-6), monocyte chemotactic peptide 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and MIP-2 were higher in the kidneys of mice challenged with the pmr1Δ mutant than in animals challenged with the other strains and were lower by day 3, suggesting an earlier host response to the pmr1Δ mutant. The production of these chemokines also diminished earlier than controls in mice infected with the mnt1/2Δ strain. Although these differences were statistically significant, their magnitude was seldom great, and no unambiguous evidence was obtained for individual responses specific to any cell surface glycosylation change. We conclude that complex, multifactorial local responses offset and obscure any differences resulting from differences in surface mannosylation of C. albicans strains when infection results from pathology-standardized challenges.
Study of the host-fungus interaction in experimental disseminated Candida albicans infections provides information of value in determining significant molecular factors for diagnostics and therapeutic interventions. This is of great importance because systemic human disease caused by Candida species is associated with a high mortality rate (11, 18). In the mouse model of disseminated C. albicans infection, disease progresses particularly in the kidneys, and critical events determining progressive kidney infection occur within the first hours and days following intravenous (i.v.) challenge with C. albicans (8, 17).
We previously showed that massive chemokine and cytokine responses arose within 48 h after i.v. C. albicans challenge (4, 5). Kidney levels of some mouse immune modulators, notably keratinocyte-derived chemokine (KC), interleukin 6 (IL-6), granulocyte colony-stimulating factor (G-CSF), and monokine induced by gamma interferon (MIG), showed strong correlations with parameters assessing the degree of kidney disease by 24 h after challenge, with clinical isolates with low virulence in mice and a mannosylation mutant strain stimulating lower chemokine and cytokine responses than isolates with high virulence in mice (5). Strains that activate a low level of host response might be expected to multiply and spread more readily in vivo than those that stimulate a robust and potentially annihilative response. The opposite finding suggests that kidney disease and microabscess formation in the mouse i.v. challenge model are of immunopathological origin, consequent on the extent to which polymorphonuclear leukocytes (PMNs) are attracted to the site of infection (5). The present study is a continuation of our efforts to investigate mechanisms underlying the generation of a host response to C. albicans that is deleterious to host tissues, particularly the kidneys.
Previous data showed that isolates with different degrees of virulence generated different strain-specific pathological effects and fungal morphology details in the kidney even when the initial challenge inoculum was adjusted to give similar overall clinical responses in experimental infections (15). Since low-virulence mutants of C. albicans deficient in cell wall glycosylation stimulate only a weak immune response in vitro (9, 10, 14) and in the kidney (5), we hypothesized that the principle of using challenge doses adjusted to give equivalent disease pathology might allow us to determine differences in kidney chemokine and cytokine responses that related specifically to the glycosylation defect, and thus identify key, strain-independent host responses leading to immunopathological kidney damage. We utilized infection outcome data on day 3 after challenge (6, 7) and microscopic changes in the kidneys (5) as the basis for pathology-standardized inocula.
We studied two mannosylation mutants, the pmr1Δ mutant and the mnt1Δ mnt2Δ double mutant, which have previously been shown to have low virulence in mice (1, 12). PMR1 encodes an ATPase that is located in the Golgi apparatus and that provides Mn2+ and Ca2+ ions essential for O- and N-mannosyltransferases that accomplish protein glycosylation of most cell wall proteins in C. albicans (1). PMR1 disruption leads to truncation of most O- and N-mannans in the cell wall (1). MNT1 and MNT2 encode partially redundant mannosyltransferases involved in O mannosylation of cell wall proteins: their disruption leads to marked truncation of cell wall O-linked mannans (12).
MATERIALS AND METHODS
C. albicans mutants.
Previously described strains (1) NGY355, a mutant with both PMR1 alleles specifically disrupted, and NGY356, with reintegration of a single copy of PMR1, were used to examine the effects of O- and N-glycosylation defects on host responses. For ease of comprehension, these two strains, strains NGY355 and NGY356, are referred to as pmr1Δ and pmr1Δ/PMR1 mutants, respectively, throughout this paper. Strains NGY337 and NGY335 were the double allele MNT1 and MNT2 disruptant and MNT1 reintegrant (12), respectively, used to study the effects of O-mannosylation defects on host responses. This single gene reintegrant recovers most of the phenotype of the parental strain. These two strains, NGY337 and NGY335, are referred to as mnt1/2Δ and mnt1/2Δ/MNT1 mutants, respectively, throughout this paper. Control strain NGY152, was derived from CAI-4 (3), the parental strain of the mutants, by reintegration of a copy of URA3 gene in the strongly expressed RPS1 locus. Similar URA3 reintegrations were done with all mutant strains to avoid artifacts resulting from Ura3 deficiency (2).
Conditions for maintenance of the strains and preparation of challenge inocula have been previously described (5).
Animal model.
The animal model of C. albicans infection has been described previously (5-8). Briefly, female BALB/c mice (6 to 8 weeks old; Harlan, United Kingdom) were challenged by i.v. injection of a suspension of C. albicans yeast cells, and subsets of infected animals were humanely terminated 12 h, 24 h, 48 h, and 72 h after challenge. The spleen and pair of kidneys were removed from each animal and homogenized, and the homogenates were utilized for determination of kidney fungal burdens by counting the number of viable cells and measuring the levels of chemokines and cytokines in the spleen and kidney. For a randomly selected subset of 6 mice per strain, sampled at 24 h and 48 h, a section of intact kidney was removed with a scalpel before homogenization for microscopic determination of lesion numbers mm−2 and infiltrate pixels mm−2, as previously described (5). Blood samples were collected for serum preparation at all sample times, except 12 h after challenge. All experimentation conformed to the requirements of the United Kingdom Home Office and our institutional Ethics Committee.
To assess the consequences of infecting mice with different C. albicans strains, we used an outcome score that involves addition of kidney fungal burdens on day 3 and percent weight loss over 3 days after challenge (5, 7). We have previously shown that the change in the body weight of mice from days 0 to 3 correlates strongly with the survival time on a strain by strain basis (6). Expressed as a percentage of day 0 weight, body weight typically changes by approximately −15% (maximally −20%) by day 3 in animals infected with a virulent strain; this datum provides an estimate of the clinical consequence of infection. Kidney fungal burdens provide an estimate of the microbial impact of mouse infections: by 3 days after i.v. challenge, the fungal burden data typically range from 3 to 4 log10 CFU g−1 for infections with a low-virulence strain and 5 to 7 log10 CFU g−1 for infections with a high-virulence strain. In general, we have found that 3-day kidney burdens and weight loss parameters tend to correlate. We have therefore expressed the 3-day status of the C. albicans challenges as a combination outcome score, which adds the kidney burden result to half of the weight loss (dividing the weight loss by 2 ensures a roughly equal contribution of burden and weight loss data to the final score). We have previously shown that the outcome score assessment can be used to assess the effects of antifungal treatments in short-term experiments in vivo (7).
Chemokine and cytokine measurements.
Serum samples and supernatants from kidney and spleen homogenates were assayed for levels of granulocyte-macrophage colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 1β (IL-1β), IL-6, IL-10, IL-17, IL-21, keratinocyte-derived chemokine (KC), monocyte chemotactic peptide 1 (MCP-1), monokine induced by gamma interferon (MIG), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, MIP-2, RANTES, and tumor necrosis factor (TNF). Six other cytokines, gamma interferon (IFN-γ), IL-2, IL-3, IL-4, IL-5, and IL-9, had previously been shown not to be stimulated in kidney or spleen samples by 48 h in the mouse experimental model (5). All the immune modulators were measured by means of BD cytometric bead array (BD, Oxford, United Kingdom) with the exception of MIP-2, which was determined by Quantikine mouse immunoassay (R&D Systems, Abingdon, United Kingdom). As before (5), the concentration of each substance was determined in units of pg/ml, then recalculated as pg g−1 tissue for kidney and spleen samples. Measurements below 25 pg ml−1 were regarded as below the limit of detection and were recorded as 0. The mean pg ml−1 (serum) or pg g−1 (kidney and spleen) determined for 9 mice injected i.v. with sterile saline instead of C. albicans suspensions was subtracted from each measurement from infected tissue samples: in some instances, this led to demonstration of downregulation of production of a chemokine or cytokine.
Data analysis.
The database for statistical analysis was built up by incremental addition of experimental data. To determine the level of challenge required for equivalent disease pathology, initial experiments were done with groups of three mice infected with different challenge doses. The outcome score on day 3 after challenge was used to determine appropriate levels of challenge for experiments involving chemokine and cytokine assays. The main experiments involved groups of 9 mice for each time point analyzed. The experiments were undertaken piecemeal, with at least four of the strains under investigation included in each experimental session to avoid introduction of temporal bias in the accumulation of results. Eight experimental sessions were required to complete the database. All cytokine and chemokine concentrations are expressed to two significant figures.
Statistical tests were done with the PASW statistical package, version 18.0. To avoid type 1 errors with large numbers of test comparisons, results were considered statistically significant only when the probability P that they arose by chance was <0.01.
RESULTS
Pathology-standardized challenge inocula.
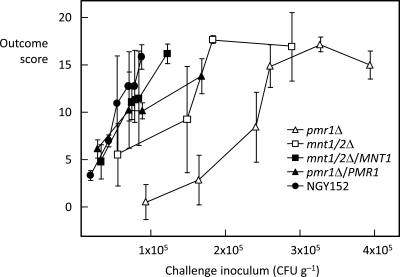
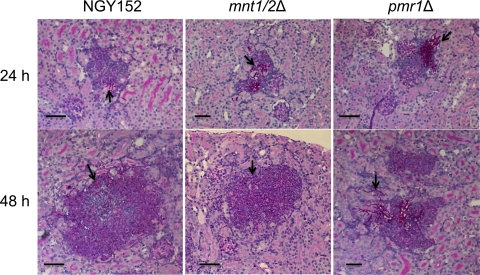
To determine the challenge levels that would give similar disease outcomes for each C. albicans strain, groups of 3 mice were infected with different inoculum concentrations. Dose-response curves for 3-day outcome scores versus a series of challenge inocula are shown in Fig. 1. The lower mouse virulence of the pmr1Δ and mnt1/2Δ mutants is self-evident from these results. The curves for the two reintegrants almost overlapped the curve for the NGY152 control. It was decided to use challenge inocula that generated 3-day scores in the outcome score range of 12 to 16 for the main experimental series. At lower challenges, a number of mice show little evidence of infection, and at higher challenges, acute, fatal disease may result within 24 to 48 h. All five C. albicans strains generated comparable pathology at all sample times. Figure 2 shows histopathological sections of lesions from mice infected with control strain NGY152 and mnt1/2Δ and pmr1Δ mutants after 24 h and 48 h. All three strains generated lesions containing hyphal and pseudohyphal elements surrounded by host leukocyte infiltrates. Table 1 shows the pathology-related parameters that were measured for each strain among the groups of experimental sessions involved. Tissue burdens—the only parameter measured at all five sample times—showed a steady increase from 12 h (median burden of 4.5 log CFU g−1) through 72 h (median burden of 6.4 log CFU g−1). The data used to determine the means and standard deviations shown in Table 1 were analyzed statistically for interstrain variation at each sample time by the nonparametric Kruskal-Wallis analysis of variance (ANOVA) test. All parameters showed no significant intergroup distribution variation in this test, with the exception of tissue burdens at 12 h, which did show intergroup variation (P = 0.001).
FIG. 1.
Day 3 outcome score versus challenge dose for 5 C. albicans strains injected i.v. in BALB/c mice. Data show means ± standard errors of the means (SEMs) (error bars) for groups of mice (3 mice in each group).
FIG. 2.
Periodic acid-Schiff stain (PAS)-hematoxylin-stained sections of mouse kidneys. Samples from animals infected with the indicated C. albicans strain at 24 and 48 h. Hyphal elements (indicated by black arrow and red color) are surrounded by a purple-colored infiltrate of polymorphonuclear leukocytes. Bars, 50 μm.
TABLE 1.
Data indicating progression of infection and kidney pathological changes in mice infected i.v. with five C. albicans strainsa
| Time (h) | Strain | Lesion area (mm2) | No. of infiltrate pixels (mm−2)b | Log CFU g of kidney−1 | Weight change (%) (day 3 to day 0) | Outcome score |
|---|---|---|---|---|---|---|
| 12 | NGY152 | 4.4 ± 0.4 | ||||
| pmr1Δ mutant | 4.9 ± 0.2 | |||||
| pmr1Δ/PMR1 mutant | 4.5 ± 0.2 | |||||
| mnt1/2Δ mutant | 4.5 ± 0.2 | |||||
| mnt1/2Δ/MNT1mutant | 4.3 ± 0.2 | |||||
| 24 | NGY152 | 2.0 ± 0.4 | 36 ± 17 | 5.4 ± 0.2 | ||
| pmr1Δ mutant | 2.1 ± 0.8 | 35 ± 31 | 5.3 ± 0.3 | |||
| pmr1Δ/PMR1 mutant | 1.9 ± 1.0 | 31 ± 18 | 5.0 ± 0.3 | |||
| mnt1/2Δ mutant | 1.8 ± 0.8 | 43 ± 16 | 5.4 ± 0.4 | |||
| mnt1/2Δ/MNT1mutant | 1.8 ± 0.5 | 32 ± 11 | 5.2 ± 0.2 | |||
| 48 | NGY152 | 2.4 ± 0.7 | 80 ± 26 | 5.9 ± 0.5 | ||
| pmr1Δ mutant | 3.2 ± 0.4 | 120 ± 100 | 5.9 ± 0.5 | |||
| pmr1Δ/PMR1mutant | 2.5 ± 0.7 | 95 ± 35 | 6.0 ± 0.3 | |||
| mnt1/2Δ mutant | 2.6 ± 1.8 | 110 ± 71 | 5.9 ± 0.5 | |||
| mnt1/2Δ/MNT1mutant | 3.6 ± 1.7 | 140 ± 53 | 6.1 ± 0.5 | |||
| 72 | NGY152 | 6.5 ± 0.5 | −15 ± 4.5 | 14 ± 2.7 | ||
| pmr1Δ mutant | 6.5 ± 0.8 | −15 ± 6.7 | 14 ± 3.9 | |||
| pmr1Δ/PMR1mutant | 6.3 ± 0.5 | −15 ± 4.2 | 14 ± 2.5 | |||
| mnt1/2Δ mutant | 6.4 ± 0.4 | −15 ± 2.3 | 14 ± 1.2 | |||
| mnt1/2Δ/MNT1mutant | 6.1 ± 0.5 | −18 ± 1.8 | 16 ± 1.0 |
All data expressed to 2 significant figures and shown as means ± standard deviations. Microscopy data (lesion area and number of infiltrate pixels mm−2) were determined with groups of six mice sampled at random from among the mice tested. Fungal burdens in kidneys, 3-day weight changes, and outcome scores were determined for the same groups of 9 mice that were used for chemokine and cytokine assays.
The number of pixels is shown in thousands (e.g., 36 ± 17 in the table means 36,000 ± 17,000).
Cytokine responses to C. albicans challenge.
Kidney, spleen, and serum concentrations of a range of cytokines were measured for groups of 9 mice infected with pathology-standardized inocula of the five C. albicans strains. IL-17 was not detected in all samples. The maximum levels measured for GM-CSF and IL-10 from any sample were 36 and 113 pg g−1, respectively, with most measurements below 50 pg g−1 or pg ml−1. No further analysis of these two substances was attempted. Serum concentrations of the immune effectors were lower than those in the kidney and spleen in almost all instances; the patterns of concentration changed with time, when they were consistent, they usually mirrored those measured for spleen samples. Only three of the serum data sets showed statistically significant interstrain variation in the Kruskal-Wallis test: MIP-1β at 24 h and IL-21 at 24 h and 48 h. In all three cases, similar differences were noted in the corresponding spleen samples. Serum data were, therefore, not analyzed further. Full data on cytokine responses are given in the table in the supplemental material available online.
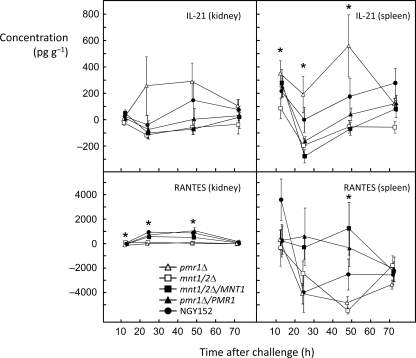
For all chemokines and cytokines tested, the levels in the kidney samples tended to be higher than levels measured in spleen samples. The exceptions were IL-21 and RANTES (Fig. 3). IL-21 was not measured in high concentrations in the kidney, but for all strains, a similar pattern was noted in the spleen samples, with a decrease in levels between 12 and 24 h, followed by a recovery at 48 h and 72 h. Throughout the time course, in both tissues, IL-21 levels were highest in samples from mice infected with the pmr1Δ mutant, while those in mice infected with the mnt1Δ mutant were among the lowest. Interstrain variation was statistically significant in the spleen samples, but not in the kidney samples, with significant differences at 12 h, 24 h, and 48 h (Fig. 3). Kidney levels of RANTES were highest at 24 h and 48 h and then fell close to zero at 72 h. At 24 h and 72 h, the kidney RANTES levels were significantly lower for mice infected with the two mannosylation-defective mutants than for the control and reintegrant strains (Fig. 3). RANTES levels in the spleen showed high mouse-to-mouse variation and a tendency to suppression of levels relative to saline-injected controls after 12 h (Fig. 3). At 48 h, the mean spleen RANTES levels showed significant interstrain variation, with the lowest mean levels in mice infected with the two mannosylation-defective mutants.
FIG. 3.
Concentrations of IL-21 and RANTES in homogenates of kidney and spleen over 72 h after i.v. infection of BALB/c mice with five strains of C. albicans. An asterisk indicates statistically significant interstrain variation in the data (Kruskal-Wallis test; P < 0.01). The concentrations are shown as means ± SEMs (error bars) (n = 9). A small amount of jitter has been applied to the x-axis data to facilitate visualization of the points. The symbol key is included in the bottom left panel.
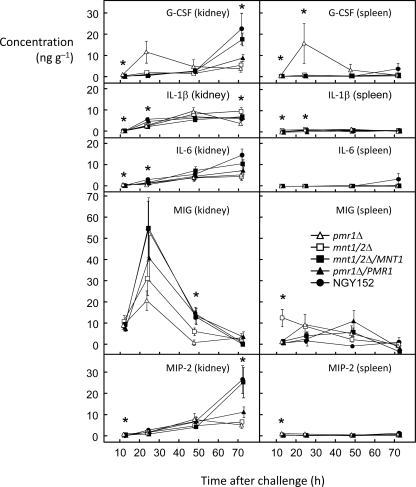
Five cytokines (G-CSF, IL-1β, IL-6, MIG, and MIP-2) were measured in kidneys with mean concentrations in excess of 5,000 pg g−1 in one or more samples (Fig. 4). For G-CSF, IL-1β, IL-6, and MIP-2, the levels in kidney samples rose progressively throughout the 72-h period. At 12 h postchallenge, the kidney data showed statistically significant interstrain variation for all four of these cytokines. The levels of G-CSF, IL-6, and MIP-2 in the kidney at 12 h were notably higher for the pmr1Δ mutant than for mice infected with the other four strains. The mean ± SD levels of G-CSF, IL-6, and MIP-2 in the pmr1Δ strain were 940 ± 600, 600 ± 470, and 1,100 ± 520 pg g−1, respectively, compared with mean ranges, respectively, for the other strains of 4 to 270, 10 to 68, and 170 to 780 pg g−1. For the same three effectors, G-CSF, IL-6, and MIP-2, the renal levels by 72 h were lower for the two mannosylation-defective mutants than for the controls (Fig. 4), suggesting that these cytokines may have been induced more rapidly in response to the mannosylation-defective mutants but that the response to these mutants fell off as infection progressed. MIG gave the highest measured concentrations of any of the immune effectors in kidneys with a large peak 24 h after challenge followed by decreased levels at 48 h and 72 h (Fig. 4). At 24 h and 48 h, the MIG concentrations were lower in kidneys from mice infected with the two mannosylation-defective mutants than for the control strains: the interstrain differences were statistically significant only at 48 h. The levels of the five most abundant immune effectors in the kidney in the spleen were mostly substantially lower than the levels in the kidney (Fig. 4), with IL-6 not detected in almost all samples. The mean levels of G-CSF, MIG, and MIP-2 in the spleen were notably higher at 24 h for the two mannosylation-defective mutants than for the control strains. For MIP-2, this difference was striking: the mean levels of MIP-2 ± SDs (pg g−1) at 12 h were 410 ± 120 for the mnt1/2Δ mutant and 990 ± 800 for the pmr1Δ mutant compared with 77 ± 150 for strain NGY152, 100 ± 140 for the mnt1/2Δ/MNT1 mutant and 300 ± 44 for the pmr1Δ/PMR1 mutant. Only the spleens from mice infected with the pmr1Δ mutant showed detectable levels of G-CSF at 12 h (240 ± 260 pg g−1).
FIG. 4.
Changes in the levels of the five chemokines or cytokines produced in highest concentration in response to C. albicans over time. The symbol key is included in the MIG (spleen) panel.
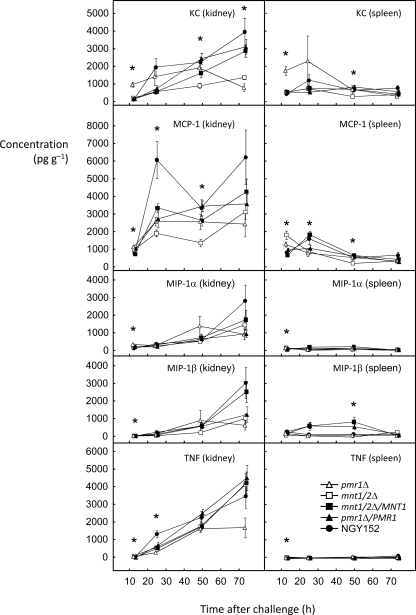
The five immune modulators detailed in Fig. 5 were produced in lower concentrations than those shown in Fig. 4, but the trends for the levels in the kidney to increase with time were similar. The previously described trend toward higher levels in tissues from mice infected with the mannosylation-deficient mutants than controls at 12 h and lower levels by 72 h was also noted for the data for KC, MCP-1, and MIP-1α (Fig. 5). A similar trend was also evident for samples from spleens.
FIG. 5.
Changes in the levels of the final five chemokines or cytokines assayed over time. The symbol key is included in the bottom right panel.
DISCUSSION
We hypothesized that i.v. infection of mice with challenge doses adjusted to give comparable pathological effects for each C. albicans strain tested would reveal differences in tissue chemokine and cytokine responses to two mannosylation-deficient mutants compared with the appropriate controls (two reintegrant strains and the parental strain NGY152). We have provided clear evidence confirming that under these conditions the mice sampled for kidney and spleen cytokine assays had comparable renal pathologies. The levels of immune effectors measured in this study were consistent with those previously determined for infections with clinical isolates of the fungus (5) and confirm KC, MCP-1, MIG, MIP-1α, and MIP-2 as the earliest upregulated chemokines in the kidney.
Our pathology-standardized challenge doses of all five strains of C. albicans induced statistically indistinguishable levels of leukocyte infiltrates in kidneys at 24 h and 48 h, along with statistically indistinguishable kidney fungal burdens and lesion densities (Table 1). Our data therefore confirm our previous conclusion that renal pathology in experimental C. albicans infection involves immunologically induced tissue damage (5), associated with very high expression of genes encoding soluble immune effectors (4). The five earliest induced chemokines all play a role in chemoattraction of leukocytes (21) consistent with the view of C. albicans disease resulting at least partly from collateral damage due to host responses. In the spleen, where C. albicans is steadily eliminated from the tissue without progression of infection (8), the overall level of cytokine response was much lower than in the kidney, where disease progresses, a further observation consistent with the concept of immunopathological impact on renal disease.
Our expectation that we would easily discern differences in chemokine or cytokine responses between tissues from mice infected with glycosylation mutants and control tissues was not robustly fulfilled. The considerable mouse-to-mouse variation found in the cytokine assays was a confounding factor, and differences between mean cytokine levels for different strains were seldom large (Fig. 3 to 5). In the mouse infection model, animal-to-animal variation and even lesion-to-lesion variation are always substantial (Table 1 and Fig. 1), a situation that inevitably reduces the precision of measurements in whole-animal experimentation. One possible conclusion from these data is that a high challenge dose, even of a mannosylation-defective strain, places sufficient antigen load in the kidney parenchyma to stimulate the same host response as a control strain, leading to the same attraction of leukocytes to the sites of fungal growth. Notwithstanding this interpretation of the data, several intriguing differences were apparent in the levels of cytokines and chemokines measured, particularly in kidney samples, and particularly 12 h after challenge, when statistically significant interstrain variation was found for most of the immune modulators. For mice infected with the pmr1Δ mutant, which is far more mannan deficient than the mnt1/2Δ mutant, 12-h kidney levels of G-CSF, KC, IL-6, MCP-1, MIP-1α, MIP-1β, and MIP-2 were the highest measured (Fig. 3 to 5), suggesting that the mutants triggered an earlier initial production of these immunoeffectors than the control strains did, but this production represents the core response to C. albicans required to establish immunopathological effects. The subsequent fall of these chemokines and cytokines to levels below those of controls by 72 h suggests that their continued production is not required to sustain disease when other effectors have come into play. Similarly, MIG production at an early stage is a core component of the renal response to C. albicans, but MIG levels need not be sustained high beyond 24 h for disease to progress in the kidney (Fig. 4).
The levels of RANTES in the kidney and spleen in mannosylation-defective infections were the lowest measured (Fig. 3), implying that C. albicans surface mannans are important inducers of this chemokine. In contrast, only in animals infected with the pmr1Δ mutant (in the kidney and spleen) were IL-21 levels raised above those of other mice (Fig. 3), pointing to a role for N-linked mannans more than O-linked mannans in stimulation of IL-21. The putative mechanism for differential response to mutants deficient in cell wall mannan would be exposure of epitopes that are ordinarily concealed by mannans (bringing early upregulation of cytokine production) or absence of stimulation of receptors that require mannan epitopes for activation (giving lower and/or slower cytokine production). Our results therefore suggest that initial production of RANTES and IL-21 depends on local recognition of O- or N-linked mannans, while the elevated early levels of other effectors result from recognition of surface epitopes that are normally concealed by mannans. O- and N-linked mannans and cell wall glucans all contribute to recognition of C. albicans by receptors such as dectin 1, Toll-like receptor 2 (TLR2), and TLR4 (13, 14, 19). Moreover, renal epithelial cells themselves can directly produce cytokines in response to antigenic stimuli, with MCP-1 and RANTES resulting from interactions of stimuli with TLR4 and TLR2, respectively (16, 20). The magnitude of gross differences in cytokine response in the kidney to C. albicans cells with different surface mannans may therefore be offset by the inevitable complexity of secondary immune phenomena consequent on initial activation of innate responses.
Supplementary Material
Acknowledgments
This work was supported by grants 076954/Z/05/Z and 080088/Z/06/Z from the Wellcome Trust.
Editor: G. S. Deepe, Jr.
Footnotes
Published ahead of print on 8 November 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Bates, S., D. M. MacCallum, G. Bertram, C. A. Munro, H. B. Hughes, E. T. Buurman, A. J. P. Brown, F. C. Odds, and N. A. R. Gow. 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+-ATPase, is required for glycosylation and virulence. J. Biol. Chem. 280:23408-23415. [DOI] [PubMed] [Google Scholar]
- 2.Brand, A., D. M. MacCallum, A. J. P. Brown, N. A. R. Gow, and F. C. Odds. 2004. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Mol. Microbiol. 3:900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonzi, W., and M. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacCallum, D. M. 2009. Massive induction of innate immune response to Candida albicans in the kidney in a murine intravenous challenge model. FEMS Yeast Res. 9:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacCallum, D. M., L. Castillo, A. J. P. Brown, N. A. R. Gow, and F. C. Odds. 2009. Early-expressed chemokines predict kidney immunopathology in experimental disseminated Candida albicans infections. PLoS One 4:e6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacCallum, D. M., L. Castillo, K. Nather, C. A. Munro, A. J. P. Brown, N. A. R. Gow, and F. C. Odds. 2009. Property differences among the four major Candida albicans strain clades. Eukaryot. Cell 8:373-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacCallum, D. M., A. Coste, F. Ischer, M. D. Jacobsen, F. C. Odds, and D. Sanglard. 2010. Genetic dissection of azole resistance mechanisms in Candida albicans and their validation in a mouse model of disseminated infection. Antimicrob. Agents Chemother. 54:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacCallum, D. M., and F. C. Odds. 2005. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48:151-161. [DOI] [PubMed] [Google Scholar]
- 9.Mora-Montes, H. M., S. Bates, M. G. Netea, L. Castillo, A. Brand, E. T. Buurman, D. F. Diaz-Jimenez, B. J. Kullberg, A. J. P. Brown, F. C. Odds, and N. A. R. Gow. 2010. A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions. J. Biol. Chem. 285:12087-12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora-Montes, H. M., S. Bates, M. G. Netea, D. F. Diaz-Jimenez, E. Lopez-Romero, S. Zinker, P. Ponce-Norla, B. J. Kullberg, A. J. P. Brown, F. C. Odds, A. Flores-Carreon, and N. A. R. Gow. 2007. Endoplasmic reticulum alpha-glycosidases of Candida albicans are required for N glycosylation, cell wall integrity, and normal host-fungus interaction. Eukaryot. Cell 6:2184-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan, J. 2005. Global trends in candidemia: review of reports from 1995-2005. Curr. Infect. Dis. Rep. 7:429-439. [DOI] [PubMed] [Google Scholar]
- 12.Munro, C. A., S. Bates, E. T. Buurman, H. B. Hughes, D. M. MacCallum, G. Bertram, A. Atrih, M. A. J. Ferguson, J. M. Bain, A. Brand, S. Hamilton, C. Westwater, L. M. Thomson, A. J. P. Brown, F. C. Odds, and N. A. R. Gow. 2005. Mnt1p and Mnt2p of Candida albicans are partially redundant α-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence. J. Biol. Chem. 280:1051-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netea, M. G., G. D. Brown, B. J. Kullberg, and N. A. R. Gow. 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6:67-78. [DOI] [PubMed] [Google Scholar]
- 14.Netea, M. G., N. A. R. Gow, C. A. Munro, S. Bates, C. Collins, G. Ferwerda, R. P. Hobson, G. Bertram, H. B. Hughes, T. Jansen, L. Jacobs, E. T. Buurman, K. Gijzen, D. L. Williams, R. Torensma, A. McKinnon, D. M. MacCallum, F. C. Odds, J. W. M. Van der Meer, A. J. P. Brown, and B. J. Kullberg. 2006. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116:1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odds, F. C., L. Van Nuffel, and N. A. R. Gow. 2000. Survival in experimental Candida albicans infections depends on inoculum growth conditions as well as animal host. Microbiology 146:1881-1889. [DOI] [PubMed] [Google Scholar]
- 16.Segerer, S., P. J. Nelson, and D. Schlöndorff. 2000. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J. Am. Soc. Nephrol. 11:152-176. [DOI] [PubMed] [Google Scholar]
- 17.Spellberg, B., A. S. Ibrahim, J. E. Edwards, and S. G. Filler. 2005. Mice with disseminated candidiasis die of progressive sepsis. J. Infect. Dis. 192:336-343. [DOI] [PubMed] [Google Scholar]
- 18.Spellberg, B. J., S. G. Filler, and J. E. Edwards. 2006. Current treatment strategies for disseminated candidiasis. Clin. Infect. Dis. 42:244-251. [DOI] [PubMed] [Google Scholar]
- 19.Tada, H., E. Nemoto, H. Shimauchi, T. Watanabe, T. Mikami, T. Matsumoto, N. Ohno, H. Tamura, K. Shibata, S. Akashi, K. Miyake, S. Sugawara, and H. Takada. 2002. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14-and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 46:503-512. [DOI] [PubMed] [Google Scholar]
- 20.Tsuboi, N., Y. Yoshikai, S. Matsuo, T. Kikuchi, K.-I. Iwami, Y. Nagai, O. Takeuchi, S. Akira, and T. Matsuguchi. 2002. Roles of Toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J. Immunol. 169:2026-2033. [DOI] [PubMed] [Google Scholar]
- 21.Viola, A. A., and A. D. Luster. 2008. Chemokines and their receptors: drug targets in immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 48:171-197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.