Abstract
Hypoxia has been identified as a contributing factor in the pathophysiology of several diseases and oxygen regulation is important during stem cell development, particularly in early embryogenesis. One aspect that has emerged is the role of hypoxia-inducible factors, or HIFs in regulating the effect of hypoxia. Studies in our laboratory sought to examine the hypoxic regulation of HIF activity in placental trophoblast cells, through the use of dual-reporter luciferase assays. Our study demonstrates that hypoxic conditions cause a significant increase in the level of constitutive luciferase reporter activity. We also show that this induction is not a cell-type or species-specific phenomenon and provides an alternative method for normalizing transfection efficiency in luciferase assays under hypoxic conditions. Our results suggest that in studies dealing with hypoxic conditions, caution should be used when interpreting measurements of transcriptional activity by traditional dual-reporter assays.
Keywords: Hypoxia, Hypoxia-Inducible Factors, Luciferase reporters
INTRODUCTION
Hypoxia (low oxygen) has been identified as a contributing factor in the pathophysiology of a host of diseases [2, 6, 14, 20–22, 41, 42, 56, 57]. Hypoxia is also important during early embryonic development. Embryonic implantation and placental formation occur under extremely low oxygen conditions of 2–3% oxygen and proper placental development is critical for normal fetal growth and embryonic survival [12, 19, 25, 46, 48, 49, 57].
The major mediators of hypoxia are proteins that belong to the family of basic helix-loop-helix transcription factors known as hypoxia inducible factors (HIFs). HIFs are heterodimeric proteins consisting of an alpha (HIF-α) and beta (HIF-β) subunit. Both subunits are highly conserved across species and must dimerize with each other in order to be functionally active. The HIF-β subunit is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT) and is constitutively expressed at all oxygen levels in all cell types [4 10, 25, 34, 54, 57, 60, 63].
Two predominant isoforms for the HIF-α subunits have been identified, HIF-1α and HIF2α / EPAS-1 [28, 50, 61]. HIF-1α mRNA is present in almost all cell types, however, HIF-2α expression is cell-type specific, appearing predominantly in epithelial, neuronal, and fibroblast tissues [4, 12, 28, 50, 51, 61]. Under normal oxygen conditions, key proline and asparagine residues within the oxygen dependent degradation domain (ODD) and C-terminal transactivation domain (CTAD) of the HIF-α protein subunit are hydroxylated by prolyl and asparaginyl hydroxylases. Hydroxylated HIF-α subunits are ubiquitinated through interactions with the von Hippel-Lindau (VHL) tumor suppressor protein and are degraded through the proteosomal degradation pathway [9, 24, 25, 31, 34]. Under low oxygen conditions, hydroxylation of the HIF-α subunits does not occur, allowing for its stabilization. Stabilized HIF-α protein dimerizes with the HIF-β subunit to become transcriptionally active [4, 10, 28, 39, 60, 64]. The functional HIF protein, in the presence of other cofactors such as CBP/p300, binds to consensus sequences (5'- T/G ACGTGCGG-3'), known as Hypoxia Response Elements (HREs), located within the promoter regions of hypoxia-regulated genes, such as erythropoietin (EPO), phosphoglycerate kinase-1 (PGK-1), and vascular endothelial growth factor (VEGF) [17, 39, 42, 45, 58, 60 64, 65].
When delineating the role of HIFs and other transcriptional factors, assaying for transcriptional activity is important to show functionality. One of the easiest ways that this can be accomplished is via reporter assays. Reporter assays are used in numerous studies to test for the transcriptional activity of a variety of proteins. Reporter genes consist of a gene encoding a protein attached to an upstream promoter or enhancer sequence of interest. The encoded proteins are easily quantifiable when transfected in a eukaryotic system. Genes commonly used as reporters are beta galactosidase LacZ), Green fluorescent protein (GFP), Chloramphenicol acetyl transferase (CAT), and Luciferase (Luc/Lux) assays [8, 37, 59].
Luciferase dual-reporter assays are widely used in a variety of scientific fields to study transcriptional activation [1, 11, 29, 33]. The two different types of luciferases commonly used have distinct substrate requirements, producing different wavelengths of fluorescence, thus allowing them to be quantified sequentially from a single sample. In a conventional luciferase dual-reporter assay, cells are transfected with a firefly (Photinus pyralis) luciferase gene under the control of a promoter region from a gene of interest and a Renilla (Renilla remiformis) luciferase gene under the control of the promoter region from a gene that is constitutively expressed. Three commercially available luciferase reporters are commonly used to normalize for transfection efficiency, due to their constitutive activity within most cells types. The pRL-SV40 luciferase reporter (GenBank accession number AF025845) is composed of the Renilla luciferase gene under the control of the constitutively active simian-virus 40 (SV40) early enhancer/promoter. Similarly, the pRL-CMV and pRL-TK (GenBank accession numbers AF025843 and AF025846, respectively) are composed of the Renilla luciferase gene under the control of the constitutively active immediate/early promoter/enhancer of cytomegalovirus (CMV) and herpes simplex virus thymidine kinase (HSV-TK), respectively [7, 22, 45, 52, 59].
Studies in our laboratory sought to examine the transcriptional activity of HIF-1α in response to hypoxia in the Rcho-1 trophoblast cell line, using a conventional luciferase dual-reporter assay [19, 49]. However, during the course of these studies, the level of activation of the constitutive reporters was significantly higher in hypoxic samples than in normoxic controls, creating variability in the results obtained. The current study examines the effects of hypoxia on constitutive luciferase reporters and provides a reliable, alternative for the analysis of transcriptional activation under hypoxic conditions.
MATERIALS AND METHODS
Materials
The Rcho-1 trophoblast cell line was a kind gift from Dr. Michael Soares (Kansas University Medical Center, Kansas City, Kansas). The Cos 7 and NIH-3T3 cell lines were obtained from ATCC. The EPO-Hypoxia Response Element (HRE)-luciferase reporter plasmid, with four copies of the HRE consensus sequence from the promoter of the erythropoietin gene in the pGL3 vector, was a kind gift from Dr. Florent Soubrier (INSERM, Paris, France). The PGK-1-HRE luciferase reporter plasmid, which contains six copies of a 24 base pair sequence including the HRE sequence from the phosphoglycerate kinase-1 (PGK-1) promoter in the pGL3 vector, was a kind gift from Dr. Peter Ratcliffe (University of Oxford, England). The pc3DNAHIF-1α3xSDM construct was a kind gift from Dr. Christine Warnecke (University Erlangen-Nuremberg, Erlangen, Germany). pEGFPN-1 was purchased from Clonetech. RPMI 1640 with L-glutamine (RPMI), Dulbecco's Modified Eagle Medium (DMEM), 1× Dulbecco's Phosphate Buffered Saline (PBS), were purchased from Cellgro. Fetal Bovine Serum (FBS) was purchased from BioWest. HEPES buffer, Trypsin-EDTA, and antibiotic-antimycotic were purchased from Invitrogen. The NXTRACT CelLytic™ NuCLEAR™ Extraction Kit was purchased from Sigma-Aldrich. Rabbit polyclonal HIF-1α antibody (NB100–449) was purchased from Novus Biologicals. Anti-rabbit and anti-mouse horseradish peroxidaseconjugated secondary antibodies were purchased from Promega. Supersignal West Pico Chemiluminescent substrate was purchased from Pierce. Deferoxamine mesylate salt (DFO) and cobalt chloride (CoCl2) were purchased from Sigma. The Dual-Luciferase Assay (DLR) kit, pRL-CMV luciferase constitutive reporter plasmid, pRL-SV40 luciferase constitutive reporter plasmid, and pRL-TK luciferase constitutive reporter plasmid are available from Promega. METAFECTENE™ transfection reagent was purchased from Biontex (Martinsried/Planegg, Germany).
Cell culture
The Rcho-1 placental trophoblast cell line was cultured as previously described [19, 44]. Briefly, cells were maintained in RPMI 1640 supplemented with 20% FBS, 1% antibiotic-antimycotic, 50μl beta-mercaptoethanol, 1mM sodium pyruvate solution and 20mM HEPES. Cells were not cultured higher than passage 25. Cell number and viability were determined by Trypan Blue exclusion. Cos 7 and NIH-3T3 cells were cultured in DMEM, 10% FBS, and 1% antibiotic-antimycotic. All cells were maintained at a density of 2−3×105/ml and cultured at 37° C in 95% O2 and 5% CO2.
Luciferase Reporter Assay
Reporter activation was determined using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. Briefly, cells were lysed for 15 minutes at room temperature using 1× passive lysis buffer. Lysed cells were collected and centrifuged at 14,000rpm for 15 minutes to eliminate cell debris. The supernatant was used for determination of luciferase activity. Unused supernatant was stored at −80°C until further use. Luciferase activity was measured using a Dynex Revelation 4.06 luminometer (Dynex Technologies, Chantilly, Virginia) [47]. For analysis, the experimental reporter was normalized to the constitutive reporter to control for differences in transfection efficiency.
Conventional and Split Transfection Luciferase Assay
Conventional luciferase assays were performed. Individual plates of Rcho-1 cells (2×105 cells/ml) were transfected with 5 μl Metafectene, 1 μg EPO-HRE plasmid or 1 μg PGK-1-HRE plasmid and 0.2 μg of constitutive reporter plasmid, for 18 hours. The DNA plasmid and Metafectene were allowed to complex at room temperature for 20 minutes in serum-free, antibiotic-free media before application to cells [47]. Twenty-four hours post-transfection, one set of samples was moved to a Coy hypoxia chamber (Coy Laboratories, Grass Lake, Michigan) set at 3%, or 5%, or 8% oxygen balanced with 5% CO2/ N2, while a parallel set was maintained at 21% O2/ 5% CO2 (normoxia). Cells were collected and analyzed for Luciferase activity using Promega Dual luciferase assay kit as described earlier.
Split-transfections, where transfection efficiency is the same in all samples, were also performed; where the induction of the reporter of interest was directly compared to the induction of the same reporter in the normoxic control. For hypoxic experiments, one plate of Rcho-1, Cos 7, or NIH-3T3 cells (2×105 cells/ml) was transfected with 5 μl Metafectene, 1 μg PGK-1-HRE plasmid, and 0.2 μg of either pRL-CMV, pRL-SV40, or pRL-TK luciferase constitutive reporter plasmid, as indicated in the Figure Legends. DNA and Metafectene were mixed and incubated at room temperature for 20 minutes in serum-free, antibiotic-free media then applied to cells in antibiotic-free media for 18 hours at 21% O2.
Twenty-four hours after transfection, cells were trypsinized and replated at equal cell number to ensure equal and comparable transfection efficiency. Twenty-four hours post replating, one plate from each constitutive reporter transfection was moved to the hypoxia chamber, set at 5% O2, while the parallel set was maintained at 21% O2 for 18 hours. Cells were collected and analyzed as described above.
Hypoxia mimetics and constitutive reporter activation
To examine the effect of hypoxia mimetics, one plate of Rcho-1 cells (2×105 cells/ml) per constitutive reporter was transfected as described above. Twenty-four hours post-transfection, the cells were trypsinized and plated at equal cell number into three parallel sets. Cells transfected with each luciferase constitutive reporter were treated with either 100 μM DFO, 100 μM CoCl2, or vehicle for 18 hours at 21% oxygen, to chemically induce HIF-1α protein. Cells were processed and analyzed for luciferase activity as described above.
HIF-1α and constitutive reporter activation
To test the effect of HIF-1α overexpression on constitutive reporter activation, Rcho-1 cells were transfected with PGK-1-HRE reporter and pRL-SV40, or pRL-TK, or pRL-CMV. Twenty -four hours post transfection cells were plated at equal cell number. Each plate was then further transfected with either pc3DNA, or pEGFP-N1, or pc3 HIF-1α3XSDM, or mock transfected with reagent alone. Transfected plates were then maintained at 21% oxygen for 18hrs. Cells were processed and analyzed for luciferase activity as described above.
Western Blot Analysis
Nuclear extracts were collected using the NXTRACT CelLytic™ NuCLEAR™ Extraction Kit per the manufacturer's instructions. Hypotonic buffer was used for Rcho-1 nuclear protein extraction. Protein concentration was determined according to the Bradford method [5]. Nuclear extracts (50 μg protein) were heated in 1× Lammeli Buffer (15.62 mM Tris, 0.5% SDS, 3.125 % glycerol, 0.625% β-mercaptethanol, and 0.025% bromophenol blue) at 95°C for 10 minutes and separated by SDS-PAGE electrophoresis. Proteins were transferred to polyvinylidene fluoride (PVDF) membrane in transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol) overnight at 100 V constant current and 30 mA constant Voltage. The PVDF membrane was stained with Ponceau S Red stain to confirm transfer and then incubated with blocking buffer (5% Non- fat dry milk, 60mM Tris base, 204mM NaCl, 0.025% Tween 20, pH 7.4) for 2 hours at room temperature [19, 49]. Blots were then probed for HIF-1α with a polyclonal rabbit antibody (1ug/ml overnight at 4°C) followed by an HRP-conjugated anti-rabbit secondary (0.02ug/ml; 45 minutes at room temperature). To confirm equal loading, the blot was reprobed with a pan-actin mouse primary antibody (0.25 μg/ml; overnight at 4°C) followed by an HRP-conjugated anti-mouse secondary (0.02μg/ml; 45 minutes at RT). Proteins were visualized using chemiluminescence and Kodak X-ray film (XAR-5).
Sequence Analysis
Transcription factor binding site analysis was performed using PATCH promoter analysis Public Version 1.0 with boundary set at 87.5 (Patch Promoter Analysis Software).
Statistics
Error bars represent standard deviations. Statistical significance was calculated using One Way Anova followed by a Tukeys post hoc test. Significance was set at p≤ 0.05. All experiments were performed independently a minimum of three times.
RESULTS
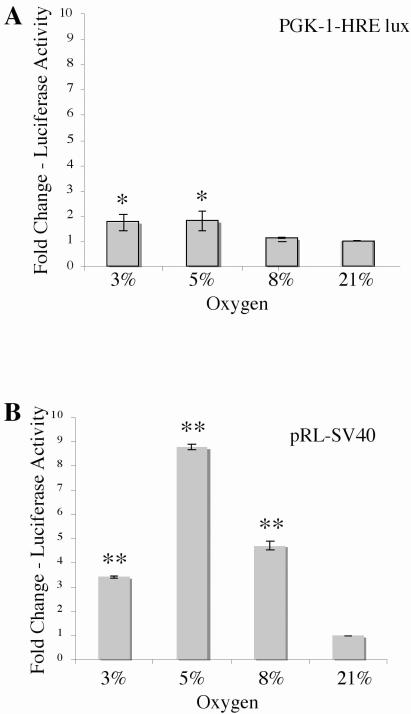
Studies in our laboratory examined the transcriptional activity of HIF-1 in Rcho-1 trophoblast cells utilizing the conventional luciferase dual-reporter assay. To determine the effects of hypoxia on HIF-1 activity, Rcho-1 trophoblast cells were transfected with the PhosphoGlycerate Kinase-1 (PGK-1)-HRE experimental luciferase reporter plasmid, which contains the firefly luciferase gene under the control of six copies of the consensus HRE sequence from Phospho Glycerate Kinase -1 and the pRL-SV40 constitutive luciferase reporter. PGK-1 has been shown to be a direct target of HIF-1 [17, 39, 45, 58, 60, 64]. The transfected cells were then exposed to varying levels of hypoxia (3–8% O2) or normoxia (21% O2) and the amount of luciferase was quantified to determine HIF-1 activity (Figure 1A). We observed that the PGK-1-HRE reporter was induced 1.7 fold in 3% oxygen, a 1.8 fold induction of the reporter was observed at 5% oxygen whereas, 1.1 fold induction of the reporter was observed at 8% oxygen, when compared with the 21% control.
Figure 1. Induction of PGK-1-HRE and pRL-SV40 luciferase reporters by hypoxia.
In the conventional luciferase assay, two or more plates are plated with equal numbers of cells and individually transfected with an experimental and a constitutive luciferase reporter construct. During analysis, differences in initial transfection efficiency are accounted for by normalizing the luminescence obtained for the experimental reporter to the luminescence obtained for the constitutive reporter. Normalized reporter luminescence under experimental conditions is then compared with that of the control condition to obtain results as fold change over control. Rcho-1 trophoblast cells were plated at equal cell number of 1.0 × 105 cells and transfected with 1 μg PGK-1-HRE and 0.2μg pRL-SV40 and incubated at the O2 concentration indicated for 18hrs and analyzed for (A) PGK-1-HRE reporter activation and (B) pRL-SV40 constitutive reporter activation. Results are the average of three independent experiments. Luciferase activity at each concentration was analyzed and is shown relative to activity at 21% oxygen. Error bars represent standard deviation. Significance was denoted as *p≤0.01 or **p≤0.001 and was determined using a one way Anova with Tukeys post hoc test.
We further analyzed for the induction of the constitutive reporter pRL-SV40 in the hypoxia samples. We observed a significant induction of the constitutive reporter, pRL-SV40, in hypoxia with a 3.4 fold induction at 3% oxygen, 8.7 fold induction at 5% oxygen and a 4 fold induction of the constitutive reporter at 8% oxygen, when compared with normoxic (21%) controls (Figure 1B).
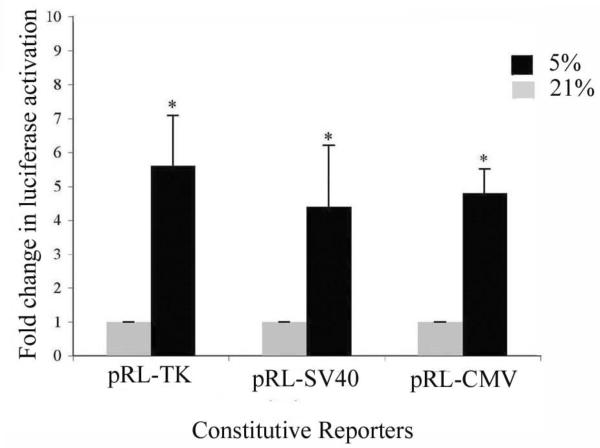
To test if the hypoxic induction of the constitutive reporter was restricted to pRL-SV40, we analyzed for the induction of two other commonly used constitutive reporters, pRL-CMV and pRL-TK. Since maximum induction of pRL-SV40 was observed at 5% oxygen, this level of oxygen was used for treatment (Figure 2). Rcho-1 cells, transfected with constitutive reporters (0.2μg), were placed in hypoxia of 5% or at 21% for 18hrs and analyzed for induction of luciferase constitutive reporters. Significant induction of all three reporters was observed at the 5% oxygen, with a 5.6 fold induction of pRL-TK, 4.4 fold induction of pRL-SV40 and a 4.79 fold induction of the pRL-CMV reporter, suggesting that hypoxia is inducing the constitutive luciferase reporters.
Figure 2. Hypoxia induces activation of pRL-SV40, pRL-CMV, and pRL-TK constitutive reporters.
Rcho-1 trophoblast cells were plated at equal cell number of 1.0 × 105 cells and transfected with 1 μg of PGK-1-HRE and 0.2μg of pRL-SV40, or pRL-CMV or pRL-TK as indicated using the conventional transfection method. Samples were incubated at 5% or 21% oxygen for 18hrs and analyzed for activation of the constitutive reporter. Results are the average of three independent experiments. Luciferase activity at each concentration was analyzed and is shown relative to activity at 21% oxygen. Error bars represent standard deviation. Significance was denoted as *p≤0.01 or **p≤0.001 and was determined using a one way Anova with Tukeys post hoc test.
In order to minimize the potential variability induced by the hypoxic activation of the constitutive reporters, we developed an alternative transfection protocol. A split-transfection protocol normalizes the transfection efficiency among samples prior to treatment, thus allowing for experimental (firefly) reporter induction to be directly compared between samples without the need for a constitutive (Renilla) reporter to normalize for transfection efficiency.
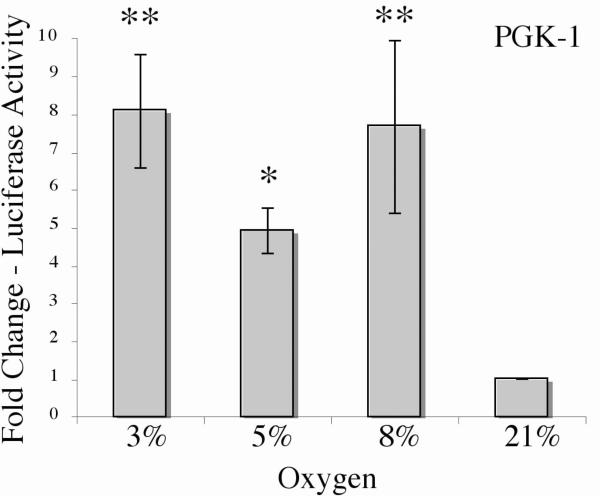
Using the split-transfection method, Rcho-1 cells were transfected with the PGK-1-HRE construct and treated at 3%, or 5%, or 8% oxygen and control at 21%, oxygen for 18hrs, and samples were analyzed for reporter activation. In contrast to conventional dual-luciferase assays, significantly higher activation of the PGK-1-HRE reporter was observed at all levels of hypoxia, with 8.1 fold induction at 3%, 4.9 fold induction at 5% and 7.6 fold induction at 8% oxygen (Figure 3A).
Figure 3. Induction of PGK-1-HRE luciferase reporter by hypoxia using the split transfection protocol.
In the split transfection luciferase assay, cells are transfected with an experimental luciferase reporter alone. Post-transfection, the plate is split equally into multiple plates as required. Differences in transfection efficiency are accounted for by splitting out the original plate at equal cell number, thus eliminating the need for normalization using a constitutive reporter. Experimental reporter luminescence under test conditions can then be directly compared with that of the control condition to obtain results as fold change over control. Rcho-1 trophoblast cells were plated at equal cell number of 1.0 × 105 cells and transfected with PGK-1 and then treated with the indicated level of oxygen for 18hrs. Cells were collected and PGK-1-HRE reporter activation was analyzed. Results are the average of three independent experiments. Luciferase activity at each concentration was analyzed and is shown relative to activity at 21% oxygen. Error bars represent standard deviation. Significance was denoted as *p≤0.01 or **p≤0.001 and was determined using a one way Anova with Tukeys post hoc test.
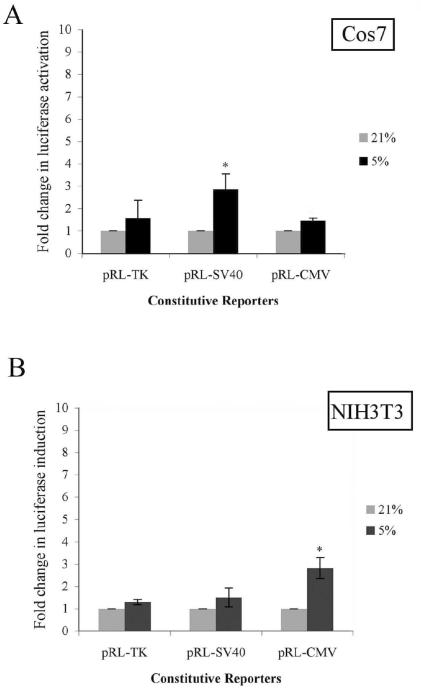
Rcho-1 trophoblasts are a rodent, choriocarcinoma-derived cell line that serves as a model of the placental giant cell lineage [19, 44]. To determine if the induction of constitutive luciferase reporters by hypoxia was restricted to the placental cells, the split transfection assays were repeated in Cos7 cells, an immortalized SV40-transformed African green monkey kidney cell line [5], and NIH-3T3 cells, a mouse embryonic fibroblast cell line (Figure 4) [26]. In Cos 7 cells, pRL-SV40 was significantly induced (3.25-fold) by exposure to hypoxia of 5% oxygen; pRL-TK and pRL-CMV were induced by exposure to hypoxia, but did not reach statistical significance. In NIH-3T3 cells, pRL-CMV showed a significant, 3-fold induction upon exposure to hypoxia of 5%, while pRL-SV40 and pRL-TK had only a slight induction that was not statistically significant.
Figure 4. Hypoxia induces constitutive luciferase activation in Cos7 and NIH3T3 cells.
(A) Cos 7 cells and (B) NIH 3T3 cells were transfected with pRL-CMV, pRL-SV40, or pRL-TK constitutive luciferase reporter using the split transfection protocol as described. Samples were treated at the oxygen concentration indicated and analyzed as described in Materials and Methods. Results are average of three independent experiments. Luciferase activity at each concentration was analyzed and is shown relative to activity at 21% oxygen. Error bars represent standard deviation. Significance was denoted as *p≤0.01 or **p≤0.001 and was determined using a one way Anova with Tukeys post hoc test.
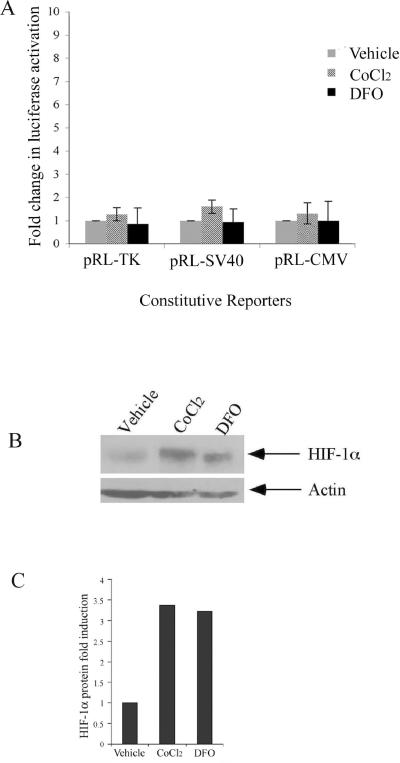
Hypoxia inducible factor 1 (HIF-1) is known to be a major effector of hypoxic responses. To test whether the hypoxic activation of constitutive luciferase reporters was a HIF-1α dependent event, we evaluated the effect of hypoxia mimetics cobalt chloride (CoCl2) and deferoxamine (DFO) on constitutive luciferase reporters (Figure 5). DFO and CoCl2 act to stabilize HIF-1α protein thus allowing transcriptional activation to occur. Both compounds act by blocking the action of the prolyl hydroxylases, which target HIF-1α for degradation by hydroxylating conserved proline residues. Surprisingly, no significant luciferase induction of pRL-TK, pRL-SV40, or pRL-CMV was observed in Rcho-1 cells treated with 100μM CoCl2 or DFO for 18hrs at 21% oxygen (Figure 5A). To confirm HIF-1α protein stabilization, Western blot analysis of nuclear extracts obtained after treating Rcho-1 cells for 18hrs with CoCl2 and DFO was performed. The results indicate a significant stabilization of HIF-1α protein (110 kDa) in both the CoCl2 as well as DFO treated Rcho-1 cells when compared with the vehicle only treated cells (Figure 5B). Densitometric analysis, when normalized to actin (45 kDa), revealed a greater than 3-fold induction in HIF-1α protein in CoCl2 as well as DFO treated sample when compared with vehicle treated controls alone (Figure 5C). HIF-1α is stabilized under low oxygen conditions and previous studies have shown that HIF-1α protein levels increase when trophoblasts are exposed to hypoxia. The mechanism of stabilization of HIF-1α in hypoxia differs from the stabilization with hypoxia mimetics such as DFO and CoCl2. Therefore, we examined the induction of constitutive luciferase reporters after transfecting Rcho-1 cells with a constitutively-active, site-directed mutant HIF-1α construct (pc3HIF-1α 3xSDM) that is protein stable and present under normoxic conditions. The pc3HIF-1α 3xSDM construct is stably expressed and active in normoxia due to two site directed point mutations within the oxygen dependent degradation domain and one site directed point mutation within the transactivation domain, that prevent the hydroxylation-dependent degradation of the HIF-1α protein [60].
Figure 5. Hypoxia mimetics do not induce constitutive luciferase reporter activation.
Equal numbers of Rcho-1 trophoblasts were seeded and incubated for 24 hours. Rcho-1 trophoblasts were then transfected with 0.2 μg pRL-CMV, pRL-SV40, or pRL-TK constitutive luciferase reporter using the split transfection protocol as described earlier. Cells were treated with 100μM Deferoxamine (DFO) or 100μM Cobalt chloride (CoCl2) or vehicle for 18 hours. (A) Samples were analyzed for constitutive reporter activation. Results are the average of three independent experiments. Luciferase activity at each concentration was analyzed and is shown relative to activity at 21% oxygen. Error bars represent standard deviation. Significance was determined using a one way Anova with Tukeys post hoc test. (B) Nuclear extracts were collected, separated by SDS-PAGE, and Western blotting was performed using a polyclonal HIF-1α antibody and the appropriate secondary antibody as indicated in the Materials and Methods. The same blot was reprobed with actin monoclonal antibody for equal protein loading. Arrows identify HIF-1α actin (45kDa). The figure is a representative of three independent experiments. (C) Densitometric analysis of HIF-1α protein Rcho-1 cells treated with vehicle, CoCl2 or DFO. Protein levels were normalized to levels of actin.
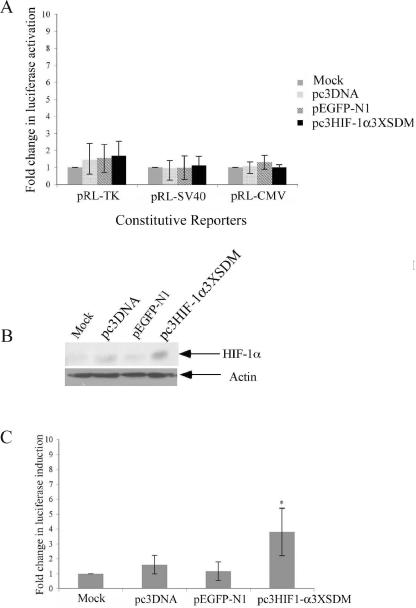
Rcho-1 trophoblasts were transfected with the PGK-1-HRE luciferase reporter along with one of the three constitutive luciferase reporters using the split transfection method. One plate was mock-transfected while the other three plates were transfected with either pc3DNA, pEGFP-N1, or pc3HIF-1α3xSDM. The results indicate that transfection with the stable HIF-1α3xSDM construct did not significantly induce any of the three constitutive luciferase reporters above levels seen in control transfections (Figure 6A), despite the presence of HIF-1α protein in the Rcho-1 cells transfected with the constitutively active HIF-1α construct (Figure 6B). Additionally, the pc3HIF-1α 3xSDM construct significantly induced PGK-1-HRE experimental reporter activity above that of mock transfected, vector transfected, or enhanced GFP expressing (pEGFPN-1) transfected cells (Figure 6C), indicating that the constitutively active HIF-1construct can transcriptionally activate direct targets but does not affect the constitutive luciferase reporters.
Figure 6. HIF-1α does not induce constitutive luciferase reporter activation.
Rcho-1 cells were transfected with PGK-1-HRE luciferase reporter and pRL-CMV, pRL-SV40, or pRL-TK constitutive luciferase reporter and either pc3DNA, pEGFP-N1 (as an internal control), or pc3HIF-1α 3xSDM, or mock transfected, as indicated. Samples were maintained in normoxia (21% oxygen) for 24hrs and analyzed for, (A) constitutive reporter activation and (C) PGK-1-HRE reporter. Results are average of three independent experiments. Luciferase activity at each concentration was analyzed and is shown relative to activity at 21% oxygen. Error bars represent standard deviation. Significance was denoted as *p≤0.01 was determined using a one way Anova with Tukeys post hoc test. (B) Nuclear extracts were collected, separated by SDS-PAGE, and Western blotting was performed using a polyclonal HIF-1α antibody and the appropriate secondary antibody as indicated in the Materials and Methods. The same blot was reprobed with actin monoclonal antibody for equal protein loading. Arrows identify HIF-1α and actin. The figure is a representative of three independent experiments.
Because all three reporters share a common backbone but have different promoter regions, we hypothesized that the promoter regions present in constitutive reporters would contain sequences that were activated by hypoxia. HIFs are the transcription factors most commonly associated with the up-regulation of genes in response to hypoxia. Using PATCH promoter analysis software, we searched for the consensus HRE sequence, 5'-T/G ACGTGCGG-3', within pRL-CMV, pRL-SV40 and pRL-TK constitutive luciferase reporters, however, no full HRE consensus sequences was found [data not shown, 13, 32, 40]. In addition, sequence analysis of the promoterless backbone luciferase construct, pRL-null, indicated that no HRE consensus sequences were present.
Using the results of the PATCH promoter analysis (data not shown), we looked for other sequences within the constitutive reporter promoter regions of each construct that could be potential targets of the proteins that may be activated during hypoxic stress. It has been previously shown that the CMV promoter contains at least one cAMP response element (CRE) and that the SV40 promoter contains at least one Activator Protein-2 (AP-2) binding site [15, 27]. The CRE is the binding site for the cAMP-Response Element Binding (CREB) protein, a transcription factor activated under a variety of physiological conditions, including hypoxia [13, 35, 38]. The consensus sequence for CRE has been defined as 5'-TGACGTCA-3' [3, 36]. Our search revealed that the CMV promoter contained eleven CREs and the TK promoter contained two. The SV40 and TK promoters each contained three AP-2 binding sites, while the CMV promoter contained four. Other consensus sequences found in varying levels within the luciferase constitutive reporters include AhR/ARNT binding sites, SP-1 binding sites, and NF-κB binding sites (Patch software, 40). AhR/ARNT is constitutively present in most cells types and is a binding partner for HIF-1α while the SP-1 and NF-κB have been shown to be upregulated under hypoxic conditions [4, 12, 30, 43, 60]. We also found high numbers of steroid hormone response elements in all three promoter regions. Taken together, our results suggest that the transcriptional activation of constitutive luciferase reporters in response to hypoxia is independent of HIF-1 activity.
DISCUSSION
Effect of hypoxia has been studied in various systems. Hypoxia is known to affect various developmental processes, including early embryonic development. The effect of hypoxia is mediated by transcription factors known as hypoxia inducible factors or HIFs. Luciferase reporter assays are frequently used to study the roles of HIFs in the activation of several hypoxia-regulated genes. In this study, we describe the induction of constitutive luciferase reporters under hypoxic conditions.
The luciferase dual-reporter assay is an easy to use, effective, and extremely sensitive means of measuring transcriptional activity and is widely used under a variety of experimental conditions. The two reporters used for the assay include the experimental reporter, ie. promoter derived from the gene under study tagged with firefly luciferase gene and a constitutive reporter, which consists of a promoter derived from a constitutively active gene tagged with a renilla luciferase gene. The constitutive luciferase reporter serves as a measure of control for the transfection efficiency of the reporters in this transfection-sensitive assay.
In this study, we investigated the role of hypoxia in activating constitutive luciferase reporters. We first detected this phenomena in the Rcho-1 cell line, which is an established placental trophoblast cell line. We observed a significant induction of the constitutive reporters, pRL-SV40, pRL-CMV and pRL-TK, at varying levels of hypoxia (3%, 5% and 8% oxygen). The induction of constitutive reporters has also been previously reported in response to steroid treatment, stimulation with LPS (lipopolysaccharide), GATA transcription factors, and stress activated MAPK protein kinases [7, 22, 45, 55].
Our study is one of the first to identify the induction of constitutive reporters by hypoxia. Using various cotransfection plasmids, we have confirmed that the constitutive activation of luciferase reporters is not an effect of the cotransfection plasmid alone. We were also able to demonstrate that the hypoxic induction of constitutive luciferase reporters pRL-SV40, pRL-CMV, and pRL-TK was not a limited phenomenon, but occurred in other cell types such as Cos7 and NIH3T3, also confirming that the phenomena was not species specific. The levels of hypoxic induction varied between the three different constitutive reporters and also between cell types. Higher induction of the constitutive reporters was observed in Rcho-1 cells when compared with Cos7 and NIH3T3. This may be due to stem cell-like nature of the Rcho-1 cells. We developed an alternative transfection protocol for luciferase assays performed in hypoxia. This approach ensures equal transfection efficiency among treatment sets, thus eliminating the need for a constitutive luciferase reporter. With this approach we show significant differences in the result obtained with the HIF-1α specific PGK-1 HRE reporter. (Figure 1 and Figure 3).
To determine if the hypoxic induction of the constitutive reporters was a HIF-1α - mediated effect of hypoxia, we treated the luciferase-transfected cells with two purported chemical mimetics of hypoxia, DFO and CoCl2, that have been shown to stabilize HIF-1α, even at 21% O2. Surprisingly DFO and CoCl2 failed to significantly induce the luciferase constitutive reporters over the vehicle treatment, even though increased levels of HIF-1α protein were observed. This suggests that stabilization of HIF-1α, through the use of DFO or CoCl2, is not an exact replication of hypoxia and that other, more complex factors may be involved during hypoxia, which may play a role in the hypoxic induction of the constitutive reporters. We further analyzed the effect of HIF-1α constitutive luciferase reporter-induction, by transfecting a mutant HIF-1α, known to be stable in normoxia [60]. However, this also failed to significantly induce the constitutive luciferase reporter activation above the levels seen in mock-transfected cells, or cells transfected with other vectors; even though HIF-1α transfected with the pc3HIF-1α3xSDM construct. This data suggested that constitutive luciferase reporter induction by hypoxia may occur in a HIF-α independent manner and was further supported by the lack of consensus HREs in any of the constitutive luciferase reporters.
It is possible that the hypoxic induction of the constitutive luciferase reporters is the result of some hypoxically-induced phenomenon such as the generation of reactive oxygen species, which have been shown to up-regulate a number of factors, including CREB, NF-κB, and AP-2, with binding sites within the luciferase constitutive reporters [18, 38, 53, 62]. This may explain the variance seen not only between the different reporters, but also between the different cell types, as factors may not be present or induced in equal amounts across species or cell types. Future studies are needed to clarify the exact mechanisms of the observed hypoxic induction of constitutive luciferase reporters, particularly analysis of the shared plasmid backbone in the three reporters. Future studies using the pRL-null vector (Invitrogen), which contains the plasmid backbone but no promoter, should indicate if hypoxic responsiveness is within individual promoters or in the shared pRL backbone sequence via a nonconsensus HRE.
In conclusion, we have defined a phenomenon that has major implications for the interpretation of data obtained by luciferase dual-reporter assays in the study of hypoxia by showing that hypoxia induces commonly used, constitutive luciferase reporters. The induction of constitutive luciferase reporters that are used to control for transfection efficiency may alter data interpretation and lead to erroneous conclusions. Hypoxic regulation of gene transcription has often been attributed primarily to HIF-1α [66], however, our study suggests that hypoxia evokes a more complex set of responses than previously known. Finally, this study proposes a highly reliable method of controlling for transfection efficiency that eliminates the need to use a constitutive reporter to normalize for transfection efficiency, overcoming the problem of hypoxic induction of constitutive luciferase reporters.
ACKNOWLEDMENTS
We would like to thank Dr. I. Michael Leffak, Dr. Robert Putnam, and Dr. Amy Gultice for helpful discussions and contributions and Ms. Christina Wicker for critical analysis of the manuscript. We would also like to thank Dr. James Lessard (Cincinnati Children's Hospital Research Foundation) for providing the pan-actin antibody. We would like to thank Chanel Keoni, Amanda Vince and Charles Dutton for their excellent technical support. This work was supported in part by NIH grant HD045750 (TLB).
This work was supported in part by NIH grant HD045750 (TLB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Abboud ER, Coffelt SB, Figueroa YG, Zwezdaryk KJ, Nelson AB, Sullivan DE, et al. 2007 Integrin-linked kinase: A hypoxia-induced anti-apoptotic factor exploited by cancer cells. Int J Oncol. 2007;30:113–122. [PubMed] [Google Scholar]
- [2].Al-Shukaili AK, Al-Jabri AA. Rheumatoid arthritis, cytokines, and hypoxia. What is the link? Saudi Med J. 2006;27:1642–1649. [PubMed] [Google Scholar]
- [3].Berkowitz LA, Gilman MZ. Two distinct forms of active transcription factor CREB (cAMP response element binding protein) PNAS. 1990;87:5258–5262. doi: 10.1073/pnas.87.14.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bracken CP, Whitelaw ML, Peet DJ. The hypoxia-inducible factors: key transcriptional regulators of hypoxic responses. Cell Mol Life Sci. 2003;60:1376–1393. doi: 10.1007/s00018-003-2370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:243–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- [6].Brahimi-Horn MC, Pouyssegur J. Harnessing the hypoxia-inducible factors in cancer and ischemic disease. Biochem Pharmacol. 2007;73:450–457. doi: 10.1016/j.bcp.2006.10.013. [DOI] [PubMed] [Google Scholar]
- [7].Bruening W, Glasson B, Mushynski W, Durham HD. Activation of stress-activated MAP protein kinases up-regulates expression of transgenes driven by the cytomegalovirus immediate/early promoter. Nucleic Acids Res. 1998;26:486–489. doi: 10.1093/nar/26.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Choi KH, Basma H, Singh J, Cheng P. Activation of CMV promoter-controlled glycosyltransferase and β-galactosidase glycogens by butyrate, tricostatin A, and 5-Aza-2'-deoxycytidine. Glycoconjugate Journal. 2005;22:63–69. doi: 10.1007/s10719-005-0326-1. [DOI] [PubMed] [Google Scholar]
- [9].Cockman ME, Lancaster DE, Stolze IP, Hewitson KS, McDonough MA, Coleman ML, et al. Posttranslational hydroxylation of ankyrin repeats in IκB proteins by the hypoxia-inducible factor (HIF) asparaginyl hydroxylases, factor inhibiting HIF (FIH) PNAS. 2006;103:14767–14772. doi: 10.1073/pnas.0606877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cockman ME, Masson N, Mole DR, Jaakkola P, Chang G, Clifford SC, et al. Hypoxia Inducible Factor-α Binding and Ubiquitylation by the von Hippel-Lindau Tumor Suppressor Protein. J Biol Chem. 2000;275:25733–25741. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- [11].Cosgrave N, Hill AD, Young LS. Growth factor-dependent regulation of survivin by c-myc in human breast cancer. J Mol Endocrinol. 2006;37:377–390. doi: 10.1677/jme.1.02118. [DOI] [PubMed] [Google Scholar]
- [12].Dahl K.D. Cowden, Fryer BH, Mack FA, Compernolle V, Maltepe E, Adelman DM. Hypoxia-Inducible Factors 1α and 2α Regulate Trophoblast Differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic Regulation of Lactate Dehydrogenase A. J Biol Chem. 1995;270:21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- [14].Foster GE, Poulin MJ, Hanley PJ. Intermittent hypoxia and vascular function: implications for obstructive sleep apnea. Exp Physiol. 2007;92:51–65. doi: 10.1113/expphysiol.2006.035204. [DOI] [PubMed] [Google Scholar]
- [15].Geist LJ, Hopkins HA, Dai LY, He B, Monick MM, Hunninghake GW. Cytomegalovirus Modulates Transcription Factors Necessary for the Activation of the Tumor Necrosis Factor-α Promoter. Am J Respir Cell Mol Biol. 1997;16:31–37. doi: 10.1165/ajrcmb.16.1.8998076. [DOI] [PubMed] [Google Scholar]
- [16].Gluzman Y. SV40-Transformed Simian Cells Support the Replication of Early SV40 Mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- [17].Hamrick SE, McQuillen PS, Jiang X, Mu D, Madan A, Ferriero DM. A role for hypoxia-inducible factor-1α in deferoxamine neuroprotection. Neuroscience Letters. 2005;379:96–100. doi: 10.1016/j.neulet.2004.12.080. [DOI] [PubMed] [Google Scholar]
- [18].Grether-Beck S, Olaizola-Horn S, Schmitt H, Grewe M, Jahnke A, Johnson JP, et al. Activation of transcription factor AP-2 mediates UVA radiation- and singlet oxygen-induced expression of the human intercellular adhesion molecule 1 gene. PNAS. 1996;93:14586–14591. doi: 10.1073/pnas.93.25.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gultice AD, Selesniemi KL, Brown TL. Hypoxia Inhibits Differentiation of Lineage Specific Rcho-1 Trophoblast Giant Cells. Biol Reprod. 2006;74:1041–1050. doi: 10.1095/biolreprod.105.047845. [DOI] [PubMed] [Google Scholar]
- [20].Hara T, Bansal A, Degrado TR. Effect of hypoxia on the uptake of (methyl(3)H)choline, (1-(14)C) acetate and ((18)F)FDG in prostate cancer cells. Nucl Med Biol. 2006;33:977–984. doi: 10.1016/j.nucmedbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [21].Hengstler JG, Bockamp EO, Hermes M, Brulport M, Bauer A, Schormann W, et al. Oncogene-blocking therapies: new insights from conditional mouse tumor models. Curr Cancer Drug Targets. 2006;6:603–612. doi: 10.2174/156800906778742488. [DOI] [PubMed] [Google Scholar]
- [22].Ho CKM, Strauss JF., III Activation of the control reporter plasmids pRL-TK and pRL-SV40 by multiple GATA transcription factors can lead to aberrant normalization of transfection efficiency. BMC Biotechnology. 2004;4:10–11. doi: 10.1186/1472-6750-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ibrahim NM, Marinovic AC, Price SR, Young LG, Fröhlich O. Pitfall of an Internal Control Plasmid: Response of Renilla Luciferase (pRL-TK) Plasmid to Dihydrotestosterone and Dexamethasone. BioTechniques. 2000;29:782–784. doi: 10.2144/00294st04. [DOI] [PubMed] [Google Scholar]
- [24].Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIF-α Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- [25].Jaakkola P, Mole DR, Tian Y, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- [26].Jainchill JL, Aaronson SA, Todaro GJ. Murine Sarcoma and Leukemia Viruses: Assay Using Clonal Lines of Contact-Inhibited Mouse Cells. J Virol. 1969;4:549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Johnson AC. Activation of Epidermal Growth factor Receptor Gene Transcription by Phrbol 12-Myristate 13-Acetate Is Mediated by Activator Protein 2. J Biol Chem. 1996;271:3033–3038. [PubMed] [Google Scholar]
- [28].Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- [29].Kim SH, Hwang CI, Juhnn YS, Lee JH, Park WY, Song YS. GADD153 mediates celecoxib-induced apoptosis in cervical cancer cells. Carcinogenesis. 2007;28:223–231. doi: 10.1093/carcin/bgl227. [DOI] [PubMed] [Google Scholar]
- [30].Kunz M, Bloss G, Gillizer R, Gross G, Goebeler M, Rapp UR, et al. Hypoxia/reoxygenation induction of monocyte chemoattractant protein-1 in melanoma cells: involvement of nuclear factor-κB, stimulatory protein-1 transcription factors and mitogen-activated protein kinase pathways. Biochem J. 2002;366:299–306. doi: 10.1042/BJ20011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Landazuri MO, Vara-Vega A, Viton M, Cuevas Y, del Peso L. Analysis of HIF-prolyl hydroxylases binding to substrate. Biochem Biophys Res Comm. 2006;351:313–320. doi: 10.1016/j.bbrc.2006.09.170. [DOI] [PubMed] [Google Scholar]
- [32].Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, Finley J, Thompson HW, et al. Coordinate Activation of HIF-1 and NF-κB DNA Binding and COX-2 and VEGF Expression in Retinal Cells by Hypoxia. Invest Opthalmol Vis Sci. 2003;44:4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- [33].Maor S, Mayer D, Yarden RI, Lee AV, Sarfstein R, Werner H, et al. Estrogen receptor regulates insulin-like growth factor-I receptor gene expression in breast tumor cells: involvement of transcription factor Sp1. J Endocrinol. 2006;191:605–612. doi: 10.1677/joe.1.07016. [DOI] [PubMed] [Google Scholar]
- [34].Maxwell PH, Wiesener MS, Chang G, Clifford SC, Vaux EC, Cockman ME, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- [35].Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. Phosphorylation of cAMP Response Element Binding Protein During Hypoxia in Cerebral Cortex of Newborn Piglets and the Effect of Nitric Oxide Synthase Inhibition. Neurosci. 2002;115:985–991. doi: 10.1016/s0306-4522(02)00275-0. [DOI] [PubMed] [Google Scholar]
- [36].Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc. Nat'l Acad. Sci. 1986;83:6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nabirochkina EN, Ivanov AV. Treatment by Methyl Methanesulfonate Induces Up-Regulation of Cytomegalovirus Immediate/Early Promoter. BioTechniques. 2000;29:732–736. doi: 10.2144/00294bm11. [DOI] [PubMed] [Google Scholar]
- [38].O'Reilly S, Leonard MO, Kieran N, Comerford KM, Cummins E, Pouliot M, et al. Hypoxia induces epithelial amphiregulin gene expression in a CREB-dependent manner. Am J Physiol Cell Physiol. 2006;290:C592–C600. doi: 10.1152/ajpcell.00278.2005. [DOI] [PubMed] [Google Scholar]
- [39].O'Rourke JF, Tian Y, Ratcliffe PJ, Pugh CW. Oxygen-regulated and Transactivating Domains in Endothelial PAS Protein 1: Comparison with Hypoxia-inducible Factor 1α. J Biol Chem. 1999;274:2060–2071. doi: 10.1074/jbc.274.4.2060. [DOI] [PubMed] [Google Scholar]
- [40].Patch Promoter Analysis Software. www.gene-regulation.com/pub/programs.html#patch.
- [41].Petrella BL, Brinckerhoff CE. Tumor cell invasion of von Hippel Lindau renal cell carcinoma is mediated by membrane type metalloproteinase. Mol Cancer. 2006;5:66–80. doi: 10.1186/1476-4598-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ralph GS, Parham S, Lee SR, Beard GL, Craigon MH, Ward N, et al. Identification of Potential Stroke Targets by Lentiviral Vector Mediated Overexpression of HIF-1α and HIF-2α in a Primary Neuronal Model of Hypoxia. J Cereb Blood Flow Metab. 2004;24:245–258. doi: 10.1097/01.WCB.0000110532.48786.46. [DOI] [PubMed] [Google Scholar]
- [43].Royds JA, Dower SK, Qwarnstrom EE, Lewis CE. Response of tumour cells to hypoxia: role of p53 and NF-κB. Mol. Pathol. 1998;51:55–61. doi: 10.1136/mp.51.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Saghal N, Canham LN, Canham B, Soares MJ. Rcho-1 Trophoblast Stem Cells: A Model System for Studying Trophoblast Cell Differentiation. Methods Mol Med. 2004;121:159–178. [PubMed] [Google Scholar]
- [45].Salnikow K, Su W, Blagosklonny MV, Costa M. Carcinogenic Metals Induce Hypoxia-inducible Factor-stimulated Transcription by Reactive Oxygen Species-independent Mechanism. Cancer Res. 2000;60:3375–3378. [PubMed] [Google Scholar]
- [46].Schaffer L, Vogel J, Breymann C, Gassmann M, Marti HH. Preserved placental oxygenation and development during severe systemic hypoxia. Am J Physiol Regul Inter Comp Physiol. 2006;290:844–851. doi: 10.1152/ajpregu.00237.2005. [DOI] [PubMed] [Google Scholar]
- [47].Selesniemi KL, Brown TL. Http://www.biontex.com/con_4_6_4/cms/front_content.php?idcat=67.
- [48].Selesniemi KL, Reedy MA, Gultice AD, Brown TL. Identification of committed placental stem cell lines for studies of differentiation. Stem Cells Dev. 2005;14:535–547. doi: 10.1089/scd.2005.14.535. [DOI] [PubMed] [Google Scholar]
- [49].Selesniemi KL, Reedy MA, Gultice AD, Guilbert LJ, Brown TL. Transforming growth factor beta induces differentiation of the labyrinthine trophoblast stem cell line SM10. Stem Cells Dev. 2005;14:697–709. doi: 10.1089/scd.2005.14.697. [DOI] [PubMed] [Google Scholar]
- [50].Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- [51].Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Ann Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- [52].Sherf B, Navarro S, Wood KV. Dual-Luciferase™ Reporter Assay: An Advanced Co-reporter Technology. Promega Notes. 1996;57:2–5. [Google Scholar]
- [53].Simon MC. Mitochondrial reactive oxygen species are required for hypoxic HIFα stabilization. Hypoxia and Exercise. 2006;15:165–170. doi: 10.1007/978-0-387-34817-9_15. [DOI] [PubMed] [Google Scholar]
- [54].Srinivas V, Zhu X, Salceda S, Nakamura R, Caro J. Hypoxia-inducible Factor 1α (HIF-1α) Is a Non-heme Iron Protein. J Biol Chem. 1998;273:18019–18022. doi: 10.1074/jbc.273.29.18019. [DOI] [PubMed] [Google Scholar]
- [55].Sulentic CE, Kang JS, Na YJ, Kaminski NE. Interactions at a dioxin responsive element (DRE) and an overlapping kappaB site within the hs4 domain of the 3' alpha immunoglobin heavy chain enhancer. Toxicology. 2004;200:235–246. doi: 10.1016/j.tox.2004.03.015. [DOI] [PubMed] [Google Scholar]
- [56].Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc. Nat'l. Acad. Sci. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vengellur A, LaPress JJ. The role of Hypoxia Inducible Factor 1α in cobalt chloride induced cell death in mouse embryonic fibroblasts. Tox Sci. 2004;82:638–646. doi: 10.1093/toxsci/kfh278. [DOI] [PubMed] [Google Scholar]
- [58].Wang GL, Semenza GL. 1993 Desferoxamine Induces Erythropoietin Gene Expression and Hypoxia-Inducible Factor 1 DNA-Binding Activity: Implications for Models of Hypoxia Signal Transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- [59].Ward CM, Stern PL. The Human Cytomegalovirus Immediate-Early Promoter is Transcriptionally Active in Undifferentiated Mouse Embryonic Stem Cells. Stem Cells. 2002;20:472–475. doi: 10.1634/stemcells.20-5-472. [DOI] [PubMed] [Google Scholar]
- [60].Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1α and HIF-2 α (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2 α target gene on Hep3B and Kelly cells. FASEB J. 2004;18:1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- [61].Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, et al. Widespread hypoxia-inducible expression of HIF-2α in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- [62].Xu Y, Porntadavity S, St Clair DK. Transcriptional regulation of the human manganese superoxide dismutase gene: the role of specificity protein 1 (Sp1) and activating protein-2 (AP-2) Biochem J. 2002;362:401–412. doi: 10.1042/0264-6021:3620401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, et al. Hypoxia-Inducible Factor-1 Mediates Activation of Cultured Vascular Endothelial Cells by Inducing Multiple Angiogenic Factors. Circ Res. 2003;93:664. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- [64].Zhong H, Simons JW. Activation of Hypoxia-Inducible Factor 1 α by Oxygen Independent Pathways. Exp Onco. 2001;23:88–95. [Google Scholar]
- [65].Zimmer M, Doucette D, Siddiqui N, Ilipopoulos O. Inhibition of Hypoxia-Inducible Factor is Sufficient for Growth Suppression of VHL−/− Tumors. Mol Cancer Res. 2004;2:89–95. [PubMed] [Google Scholar]
- [66].Gultice AD, Kulkarni-Datar K, Brown TL. HIF1alpha Mediates Distinct Steps of Rat Trophoblast Differentiation in Gradient Oxygen. Biology of Reproduction. 2009;80:184–193. doi: 10.1095/biolreprod.107.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]