Abstract
Results reported in this work suggest a potential therapeutic value of polyunsaturated fatty acids for cerebral pathologies as previously proposed by others for cardiac diseases. We show that the polyunsaturated fatty acid linolenic acid prevents neuronal death in an animal model of transient global ischemia even when administered after the insult. Linolenic acid also protects animals treated with kainate against seizures and hippocampal lesions. The same effects have been observed in an in vitro model of seizure-like activity using glutamatergic neurons and they have been shown to be associated with blockade of glutamatergic transmission by low concentrations of distinct polyunsaturated fatty acids. Our data suggest that the opening of background K+ channels, like TREK-1 and TRAAK, which are activated by arachidonic acid and other polyunsaturated fatty acids such as docosahexaenoic acid and linolenic acid, is a significant factor in this neuroprotective effect. These channels are abundant in the brain where they are located both pre- and post-synaptically, and are insensitive to saturated fatty acids, which offer no neuroprotection.
Keywords: background K+ channels/epilepsy/excitotoxicity/ischemia/polyunsaturated fatty acids
Introduction
There is now a considerable literature describing the beneficial effects of polyunsaturated fatty acids (PUFAs) in the prevention of coronary heart disease (Nordoy, 1999), in decreasing the risk of sudden cardiac death (Leaf and Kang, 1996; Albert et al., 1998) and particularly in preventing fatal ventricular arrhythmias (Nair et al., 1997; Leaf et al., 1999). There are also some indications that PUFAs might have a beneficial effect on various brain functions such as epileptic seizures (Vreugdenhil et al., 1996), depression (Hibbeln, 1998) or bipolar and other behavioral diseases (Stoll et al., 1999). PUFAs seem to exert their beneficial effect by decreasing cardiac and neuronal excitability. Obvious candidates for this effect are the neurotransmitter receptors and ion channels that underly this excitability (Leaf et al., 1999).
The purpose of this work is two-fold. First, it is important to demonstrate that PUFAs are indeed neuroprotectors as well as anti-epileptic molecules in vivo using animal models of transient global ischemia or kainate (KA)-induced seizures. This demonstration having been made, we analyze using an in vitro system how PUFAs exert their protective action. Hypoxic–ischemic and epileptic neuronal injuries have been associated with an excessive activation of post-synaptic glutamate receptors, and glutamate toxicity itself has been linked to lethal influx of Ca2+ mainly through cell membrane channels of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors (Siesjö and Wieloch, 1985; Choi, 1994; Barnard, 1997). We show that PUFAs block neuronal death by inhibiting glutamatergic transmission. How do they exert this effect? We worked out that among the different neurotransmitter receptors and transporters and ionic channels that are modulated by PUFAs, a new class of K+ channels with two pore-forming domains (2-P domains) having the properties of background K+ channels could be particularly interesting candidates. Some of these channels, which have been cloned recently (Lesage and Lazdunski, 1999), are activated by arachidonic acid (AA) and other PUFAs (Kim et al., 1995; Fink et al., 1996, 1998; Patel et al., 1998). These channels are expressed in the heart (Kim and Clapham, 1989) and are abundant in the brain (Kim et al., 1995). Their activation in the neurons would be expected to hyperpolarize synaptic terminals, decreasing glutamate release and/or producing a post-synaptic hyperpolarization, which would favor the blockade of the NMDA receptor-associated channel by Mg2+ and also counterbalance depolarization produced by the glutamate action on other types of ionotropic glutamate receptors. We indeed observed that K+ channels activated by PUFAs are present both pre- and post-synaptically and, consequently, they might be important players in the blockade of glutamatergic transmission by PUFAs, which results in a potent neuroprotective effect.
Results
Linolenic acid, but not palmitic acid, protects against ischemia-induced neuronal death
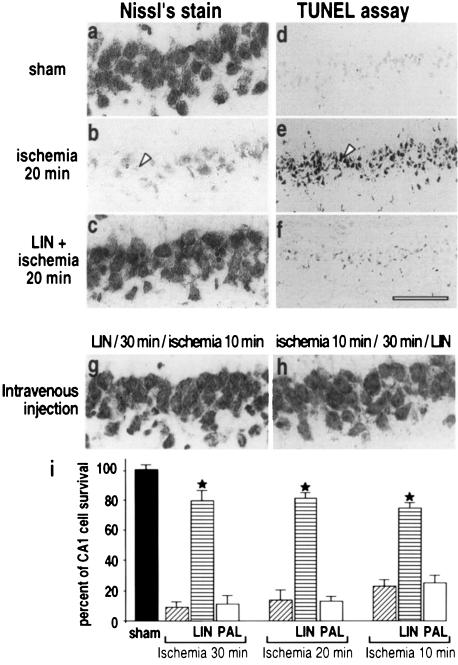
Neuroprotection by the PUFA linolenic acid (LIN) was evaluated in an in vivo model of cerebral global ischemia (Pulsinelli and Brierley, 1979; Schmidt-Kastner and Freund, 1991). Complete forebrain ischemia for 10–30 min induced neuronal cell death of CA1 pyramidal cells in the hippocampus (Figure 1i). Seven days after a 20 min global ischemia, the number of surviving CA1 neurons, as measured by Nissl’s staining, was decreased to 14 ± 5% (Figure 1b and i) as compared with sham control (Figure 1a and i). Injection of LIN [10 µM (5 µl), intracerebroventricularly (i.c.v.)], a PUFA that is found in high quantities in vegetable oils, 30 min before induction of ischemia almost completely inhibited neuronal loss (81 ± 6% of cell survival; Figure 1c and i). These results were confirmed by the lack of TUNEL staining of cells in the CA1 pyramidal cell layer (Figure 1f). The potent neuroprotective effect of LIN was observed for the three different times of ischemia (10, 20 and 30 min) (Figure 1i). In contrast to LIN, administration of the saturated fatty acid palmitic acid (PAL) failed to protect the brain and considerable neuronal loss of CA1 pyramidal neurons was observed after this treatment (Figure 1i). LIN is also protective when administered intravenously (i.v.). LIN (100 nmol/kg, i.v.), injected 30 min before (Figure 1g) or 30 min after (Figure 1h) the toxic insult, fully prevented neuronal loss of CA1 neurons. We also analyzed the effects of another PUFA: AA. Although this fatty acid also had beneficial effects, they were less pronounced and less reproducible than those for LIN; we therefore decided to use LIN throughout all the in vivo experiments.
Fig. 1. Neuroprotection by PUFAs against global ischemia. Photomicrographs of hippocampal CA1 regions of rats 7 days (Nissl’s stain) or 3 days (TUNEL labeling) after: sham operation (a and d), 20 min global ischemia (b and e) or injection of LIN [10 µM (5 µl), i.c.v.] 30 min before 20 min ischemia (c and f). (g and h) Nissl’s stained CA1 regions 7 days following an i.v. injection of LIN (100 nmol/kg) 30 min before (g) or 30 min after (h) 10 min ischemia. In each case, the photomicrographs illustrate one representative example of six rats per experimental group. The scale bar in (f) corresponds to 50 µm in (a–c) and (g–h) and 150 µm in (d–f). (i) Histograms representing the percentage of cell survival assessed in Nissl’s stained sections per 1 mm linear length of hippocampal CA1 pyramidal layer in the different experimental groups. Values are plotted as a percentage (± SEM) of control neuronal density (sham-operated rats). Data represent the means (± SEM) of counting 10 brain sections per rat (n = 6 per group). Statistical analysis was performed on normalized percentage neuronal densities by analysis of variance followed by Tukey’s w test for multiple comparisons. Differences compared with the respective ischemic controls that were significant at p <0.001 are indicated by a star.
Linolenic acid, but not palmitic acid, prevents kainate-induced seizures and neuronal death
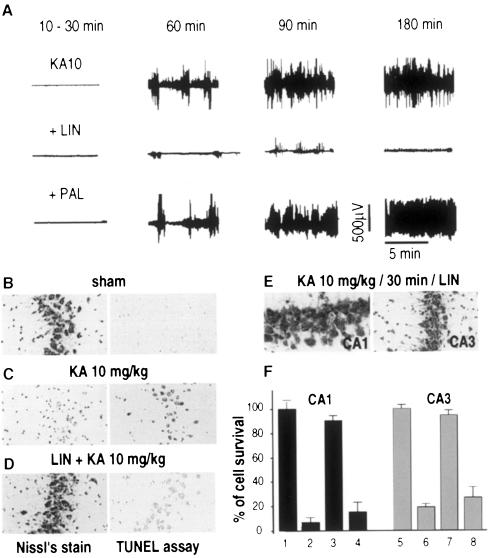
The glutamate analog KA causes a well characterized seizure syndrome resembling human temporal lobe epilepsy and is associated with excitotoxic neurodegeneration in CA1 and CA3 subfields (Nadler et al., 1978; Lothman and Collins, 1981). Administration of KA [10 mg/kg, intraperitoneally (i.p.)] into rats induced behavioral stage 4–5 seizures and electroencephalogram (EEG) seizure activity (Figure 2A). Injection of LIN [10 µM (5 µl), i.c.v.] before KA treatment blocked the epileptiform activity (Figure 2A). In contrast, PAL failed to prevent the epileptiform activity (Figure 2A). Nissl’s staining and TUNEL labeling confirmed the neuroprotection produced by PUFAs against KA-induced excitotoxicity in the hippocampus (Figure 2B–D). LIN [10 µM (5 µl), i.c.v.] administered to rats before KA produced a considerable inhibition of neuronal loss (Figure 2C and F). In this case, 90 ± 5% of CA1 neurons and 95 ± 3% of CA3 neurons survived the KA treatment (Figure 2F). Palmitic acid did not prevent the neuronal damage induced by KA in the hippocampus (Figure 2F). Again, LIN was also found to be neuroprotective when it was administered i.v. either 30 min before (100 nmol/kg) (not shown) or 30 min after (500 nmol/kg) (Figure 2E) the injection of KA.
Fig. 2. Neuroprotection by LIN against kainic acid-induced excitotoxicity. (A) EEG recordings illustrating the effects of LIN and PAL on seizure responses to systemic injection of KA (10 mg/kg) at distinct time points following the injection of KA. LIN and PAL were administered [10 µM (5 µl), i.c.v.] 30 min before KA treatment (n = 6). (B–D) Photographs of CA3 substructures highlighting the effects of LIN and PAL [10 µM (5 µl), i.c.v.] on neuronal damage following KA treatment. Rats were collected 7 or 3 days after KA treatment for Nissl’s staining (left panels) or TUNEL labeling (right panels), respectively. (E) Photographs of CA1 (left) and CA3 (right) substructures of Nissl’s stained sections after i.v. injection of LIN (500 nmol/kg) 30 min after the KA injection. (F) Histograms represent the percentage of cell survival assessed in Nissl’s stained sections per 1 mm linear length of CA1 and CA3 pyramidal layers 7 days following KA injection. Values are plotted as a percentage (± SEM) of control neuronal density (saline-injected rats). 1 and 5, sham; 2 and 6, KA 10 mg/kg; 3 and 7, LIN [10 µM (5 µl), i.c.v.] + KA 10 mg/kg; 4 and 8, PAL [10 µM (5 µl), i.c.v.] + KA 10 mg/kg. Data represent the counting of 10 brain sections per rat (n = 6 per group). Statistical analysis was performed as described in Figure 1.
PUFAs, but not saturated fatty acids, inhibit abnormal synaptic transmission and associated neuronal death in neuronal cultures
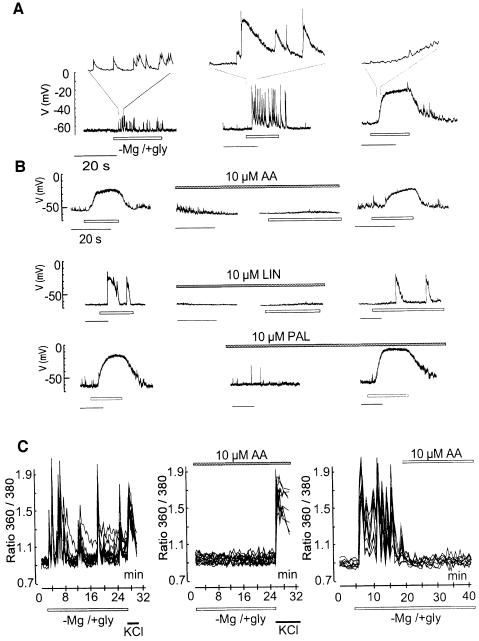
The effects of AA, LIN and other PUFAs were studied in an in vitro model of excitotoxicity (Abele and Miller, 1990; Abele et al., 1990; Rose et al., 1990) in which mouse cerebellar granular neurons were exposed to a Mg2+-depleted glycine-supplemented medium (–Mg/+gly). The removal of Mg2+ acts by enhancing the spontaneous release of the neurotransmitter glutamate and by relieving the Mg2+ block of NMDA receptors, whereas glycine acts as a positive allosteric regulator of the NMDA receptor (Abele et al., 1990). When granule cells are in culture, the –Mg/+gly medium induces a large increase in synaptic activity, observed as EPSPs (excitatory post-synaptic potentials) (Figure 3A and B) producing large and immediate fluctuations in intracellular Ca2+ levels (Figure 3C). These events are due to enhanced glutamate release at presynaptic terminals. They can be completely blocked by both tetrodotoxin (TTX, 1 µM), which eliminates synaptic transmitter release, and by d(–)-2-amino-5-phosphonopentanoic acid (D-AP5; 20 µM), an antagonist of the NMDA receptor at the post-synaptic level (not shown). Exaggerated [Ca2+]i levels in granule neurons exposed to –Mg/+gly for 30–45 min were followed by neuronal death over the next 6–10 h (Figure 4).
Fig. 3. PUFAs abolish the –Mg/+gly-induced excitatory discharges and calcium fluctuations in granule cells. Whole-cell recordings were performed from individual cells in distinct culture dishes. (A) Lower traces: typical patterns of excitatory synaptic activities in response to –Mg/+gly. Upper traces: expanded portions (2 s) from each pattern. Left: increase in the frequency of EPSP. Middle: the synaptic potentials were larger and lasted longer. Right: numerous EPSP of small amplitude led to a sustained depolarization to about –20 mV. In all cases, TTX (1 µM) completely prevented the EPSPs and the effects of –Mg/+gly were reversed upon wash-out. (B) Left: synaptic activities elicited in response to –Mg/+gly. Middle: after a complete recovery upon wash-out, the granule cells were pretreated for 1 min with the different fatty acids (10 µM) prior to the application of –Mg/+gly in the presence of fatty acids. The unsaturated fatty acids (AA, LIN) but not the saturated fatty acid (PAL) prevent the –Mg/+gly-induced intense synaptic activities. Right: removal of the PUFAs led to the restoration of the –Mg/+gly-induced synaptic responses. (C) Internal calcium fluctuations measured by Fura-2-AM. Basal synaptic activity and [Ca2+]i levels were in each case determined by perfusing the cells with a standard salt solution at the beginning of the experiment, and all washes were performed with a similar solution. Data represent [Ca2+]i in eight individual neurons out of a total of ∼30 neurons from the same culture dishes. The Ca2+ peaks in the eight neurons, chosen for this typical illustration, coincide, indicating that these neurons are all interconnected. Experiments were repeated at least three times. AA (10 µM) was present during the whole exposure time (middle panel) or added 10 min after the induction of calcium fluctuations (right panel). In the left and middle panels, cells were exposed to 25 mM KCl upon 25 min –Mg/+gly treatment.
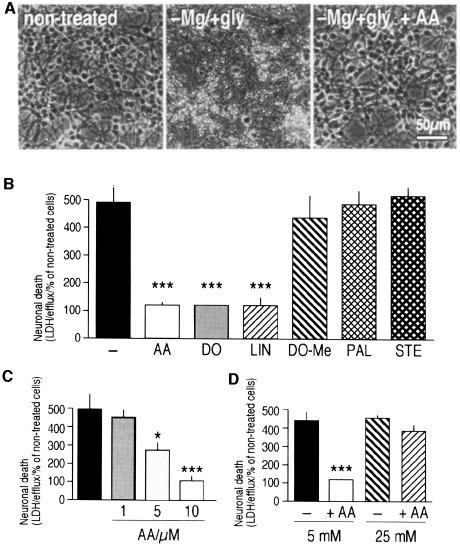
Fig. 4. PUFAs, but not saturated fatty acids, protect against –Mg/+gly-induced excitotoxicity in cultured cerebellar granular neurons. Cultures were treated with –Mg/+gly during 45 min and cell viability estimated at 8 h post-treatment. (A) Phase-contrast photomicrographs. (B–D) Estimation of viability by the LDH-efflux assay. In (B–D), columns represent the sum of the LDH activities released into the culture media during the 8 h post-treatment. Data are in each case shown as averages ± SEM obtained from nine dishes in three independent experiments. *p <0.05 and ***p <0.001 as compared with –Mg/+gly. In (D), cells were exposed to –Mg/+gly in the presence of AA (10 µM) and then shifted to culture medium containing either 5 mM KCl or 25 mM KCl. Viability in control cells, exposed to 5 mM or 25 mM KCl, was identical.
AA (10 µM) completely inhibited both electrical discharges (Figure 3B), [Ca2+]i fluctuations(Figure 3C) and neuronal death (Figure 4, p <0.001). Even when applied 10 min after the induction of [Ca2+]i fluctuations by the –Mg/+gly medium, AA effectively reduced the [Ca2+]i fluctuations (Figure 3C, right panel). Not only AA, but other PUFAs such as LIN (10 µM) (Figure 3B) and docosahexaenoic acid (DO) (10 µM) (not shown), also completely abolished the induction of EPSPs discharges, [Ca2+]i fluctuations (not shown) and neuronal death determined by the lactate dehydrogenase (LDH)-efflux assay (Figure 4B) or TUNEL labeling (not shown). No protection was obtained by the methylester of DO or the saturated fatty acids PAL and stearic acid at similar concentrations (Figure 4B). The effect of AA is concentration dependent (Figure 4C); full protection was observed at 10 µM AA.
The neuroprotective effects of PUFAs may be linked to K+ channel opening
Several lines of evidence suggest that the potent neuroprotective effects of PUFAs may be linked at least in part to K+ channel opening. First, the protective effects of PUFAs are strongly dependent on external K+ concentrations. Protection was observed only as cerebellar granular neurons were exposed to physiological [K+]e (5 mM), whereas it was not seen in a medium containing 25 mM external K+ (Figure 4D). Viability of cells exposed to –Mg/+gly alone for 45 min and estimated 8 h later was the same at low and high K+ concentrations. Moreover, AA was unable to prevent a rise in [Ca2+]i when cells were exposed to high external K+ following the –Mg/+gly exposure (Figure 3C). The calcium rise provoked by high external K+ was higher and more long-lasting in the presence of PUFAs. If driving the membrane potential close to the K+ equilibrium potential (EK) by activating background K+ channels is the mechanism by which PUFAs exert neuroprotection, then protection can only occur in low [K+]e, as observed in this work.
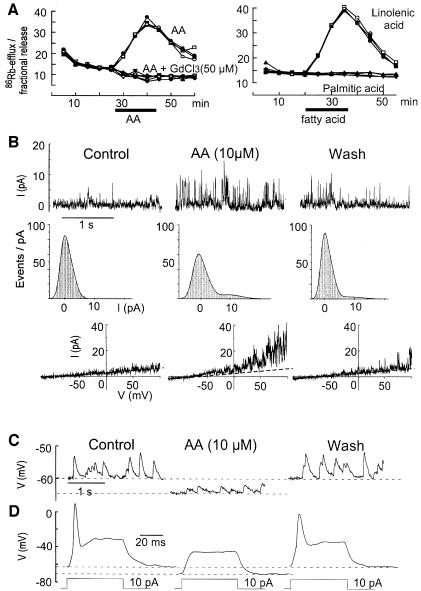
86Rb+ efflux analysis is an effective way to analyze the properties of cloned PUFA-activated K+ channels (Maingret et al., 1999). It has been used here to provide information about both synaptic and non-synaptic channels activated by fatty acids in the cerebellar granule cell cultures. AA, LIN (Figure 5A) and DO (not shown) all stimulate a large 86Rb+ efflux in these cultures with properties that are the same as those previously observed with COS cells transfected with the TRAAK channel, a 2-P domain K+ channel with specific expression in neurons (Maingret et al., 1999). Neither the methyl derivative of DO, nor saturated fatty acids, were capable of inducing any 86Rb+ efflux. Gadolinium completely blocked the 86Rb+ efflux (Figure 5A, left panel). The classical blockers of K+ channels, TEA, 4-AP or charybdotoxin, had no effects, whereas Ba2+ at high concentrations (up to 2 mM) partially reduced the 86Rb+ efflux (not shown). Outside-out patch analysis also independently revealed the presence of non-inactivating K+ channels, which are reversibly activated by 10 µM AA. These channels have electrophysiological properties close to those of the recently cloned AA- and PUFA-activated TRAAK and TREK-1 (another 2-P domain K+ channel) channels (Fink et al., 1996, 1998) and to the native AA-activated channels previously recorded in cultured mesencephalic and hypothalamic neurons (Kim et al., 1995) (Figure 5B). Whole-cell recordings showed an inhibitory effect of PUFAs on neuronal excitability (Figure 5C and D). In most neurons, addition of AA resulted in a decrease of the excitability due to a hyperpolarization of the cell membrane by 5–10 mV (Figure 5C and D). Other authors have similarly described a potent inhibitory effect of PUFAs on the frequency of action potentials in CA1 and CA3 neurons together with a hyperpolarization of the resting membrane potential (Xiao and Li, 1999).
Fig. 5. AA contributes to synaptic inhibition in granule cells by activating background K+ channels. (A) Kinetics of 86Rb+ efflux evoked by 50 µM AA (left) or 50 µM LIN (right) in cerebellar granular cultures. Four independent kinetics are shown per experimental condition. Fatty acids were added as indicated by a horizontal bar. (B) Activation of background K+ channels by AA. Upper traces: single channel K+ currents recorded in an outside-out patch held at +60 mV. Left, control; middle, after 2 min exposure to 10 µM AA; right, after a 5 min wash. Middle traces: same outside-out patch. Density histograms corresponding to 10 s recordings. Lower traces: I–V curves from another outside-out patch constructed with a voltage ramp of 500 ms duration from –100 to +100 mV. (C and D) Whole-cell recordings of the effects of AA (10 µM) on neuronal excitability. (C) Left, spontaneous EPSP. Middle, 10 µM AA induced a membrane hyperpolarization and a reduction of both the mean EPSP amplitude and frequency. Right, after a 10 min wash period. (D) Left, control action potential evoked by a current step of 10 pA. Middle, AA caused membrane hyperpolarization associated with a decreased input resistance and 10 pA was unable to trigger an action potential. Right, after a 10 min wash.
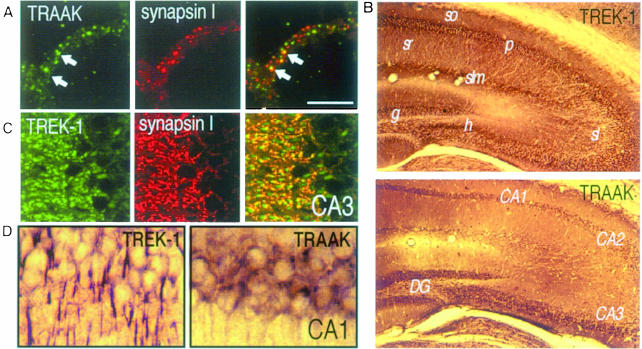
The RT–PCR analysis (not shown) and immunocytochemistry, using polyclonal antibodies directed against the channel proteins (Figure 6A), confirmed the presence of both TREK-1 or TRAAK channels in cultured granule neurons. Both channel proteins are clustered throughout cell body membranes and fiber bundles. Both TRAAK (Figure 6A) and TREK-1 (not shown) are present at both non-synaptic and synaptic sites identified by the synaptic marker synapsin I. TREK-1 and TRAAK proteins are highly expressed in the hippocampus (Figure 6B–D), a particularly vulnerable structure in global ischemia and KA-induced seizures. In this region, TRAAK is located to cell bodies (Figure 6D), whereas TREK-1 is absent from cell bodies but abundant in neuropile, notably in dendrites (Figure 6C and D). AA activation of sustained dendritic K+ channels has recently been identified in CA1 pyramidal neurons (Colbert and Pan, 1999).
Fig. 6. (A–D) Immunostaining with α-TREK1 and α-TRAAK antibodies on cerebellar granule cultures (A), or brain sections at the level of the hippocampus (B–D). (A) Clustering of TRAAK proteins (left) and synapsin I (middle) in fiber bundles of granule neurons with some overlap of the two proteins (right). (B) Low-power photomicrographs of the hippocampus. CA1–CA3, Ammon's horn; DG, dentate gyrus; g, granular cells; h, hilus; p, pyramidal neurons; sl, stratum lucidum; slm, stratum lacunosum moleculare; so, stratum oriens; sr, stratum radiatum. (C) Confocal microscopic images of TREK-1 (left) and synapsin I (middle) labeling in stratum lucidum of the CA3. Right, significant although not complete overlap of the two proteins. (D) High-power photomicrograph of the CA1 region following α-TREK-1 or α-TRAAK labeling, respectively. The scale bar corresponds to 10 µm in (A), (C) and (D) and to 50 µm in (B).
Discussion
The present study shows a major neuroprotective effect of the n-3 PUFA LIN in in vivo models of both global ischemia and KA-induced epilepsy. LIN, which is present in vegetable oils, is neuroprotective when administered preventively in one single i.v. dose, 30 min before the ischemic or epileptic insult. It also provides potent neuroprotection when administered as long as 30 min after the neurotoxic insult. Therefore, the potential therapeutic value of this PUFA and other related compounds in neurological diseases associated with hyperexcitability, excessive glutamate release and neuronal death is worth exploring in the same way as is being done for ischemic-induced cardiac sudden death (Leaf et al., 1999).
The neuroprotective effects of LIN and other PUFAs have also been observed in an in vitro model of excitotoxicity with mouse cerebellar granular neurons in culture. PUFAs potently eliminated the build-up of neurotoxic exaggerated [Ca2+]i levels in granule cells and the associated neuronal death. Their protective effect is probably largely due to the fact that PUFAs abolish EPSP discharges by inhibiting glutamatergic synaptic transmission; none of the effects observed with PUFAs in vivo and in vitro are observed with saturated fatty acids.
The most important message in this work is the very potent neuroprotective effect of PUFAs associated with PUFA-induced blockade of glutamatergic transmission. However, it seemed to us that it would also be useful to try to identify the molecular target(s) involved in the beneficial action of these PUFAs. AA and other PUFAs have multiple effects on glutamate receptors, glutamate transporters and ion channels. Some of these effects would be clearly expected to favor hyperexcitability and/or neurotoxicity instead of providing neuroprotection. They include PUFA inhibition of the activity of several voltage-sensitive K+ channels (Honoré et al., 1994; Meves, 1994; Keros and McBain, 1997), the inhibition of glutamate transporters (Gegelashvili and Schousboe, 1997) and the activation of NMDA receptors (Miller et al., 1992; Nishikawa et al., 1994) [other types of glutamate receptors seem to be only modestly changed in their function (Miller et al., 1992; Nishikawa et al., 1994)]. Other previously described effects of PUFAs will certainly contribute to a decrease of synaptic glutamate transmission and an increase of neuroprotection. They include the partial inhibition of voltage-sensitive Na+ channels (Fraser et al., 1993; Vreugdenhil et al., 1996) and of voltage-sensitive Ca2+ channels (Vreugdenhil et al., 1996). Another important class of candidates for the neuroprotective action of PUFAs are the recently cloned TREK-1 and TRAAK channels (Fink et al., 1996, 1998), which seem to correspond closely to a class of background channels identified in the heart (Kim and Clapham, 1989) and in the brain (Kim et al., 1995) and now in the cerebellum (this work), and which are potently activated by AA and other PUFAs but are unaltered in their function by saturated fatty acids.
Although K+ efflux has been proposed to contribute to neuronal apoptosis (via the NMDA receptor) in non-physiological concentrations of external Na+ and Ca2+ (Yu et al., 1999), we and others have shown that K+ channel openers such as those that activate ATP-sensitive K+ channels (KATP) (Quast, 1993) are potent neuroprotectors (Abele and Miller, 1990; Heurteaux et al., 1993; Lauritzen et al., 1997) and potent inhibitors of hyperexcitability (Abele and Miller, 1990). However, these compounds, such as cromakalim or pinacidil, protect only when administered before the ischemic insult (Heurteaux et al., 1993) and they have side-effects, since they also produce hypotension by activating vascular KATP channels (Quast, 1992).
Activators of background K+ channels will in general be more potent than inhibitors of other types of ionic channels. Indeed, an activation of only a small fraction of these channels, and particularly of TREK-1 and TRAAK channels, which are not very active in the absence of PUFA stimulation, might produce substantial changes in the resting potential as well as in the firing properties of neurons. The dependence on external K+ of the protection by AA and other PUFAs would be consistent with a dominant effect of the activation of background K+ channels. However, the final demonstration would require the use of specific blockers of TREK-1 and TRAAK channels, as has been performed previously for KATP channels using the blocker glibenclamide (Heurteaux et al., 1993). Specific TREK-1 and TRAAK blockers have not yet been discovered, and unfortunately we cannot use Gd3+, which has multiple effects and inhibits many different types of ion transport systems. Therefore, at the present time, it is probably safe to conclude that if PUFA-activated K+ channels have a significant role in neuroprotection, the beneficial effects of PUFAs might well be due to a combination of actions on TREK-1 and TRAAK-like channels and on voltage-dependent Na+ and Ca2+ channels. Interestingly, other activators of PUFA-activated 2-P K+ channels, such as riluzole (Duprat et al., 2000) or volatile anesthetics (Patel et al., 1999), also inhibit neuronal excitability and are also neuroprotective (Mizoule et al., 1985; Ettaiche et al., 1999; Lang-Lazdunski et al., 1999; Walker et al., 1999).
There is no efficacious treatment of irreversible brain damage following cerebral ischemia and, whatever the molecular mechanism, the observation that PUFAs such as LIN that are present in our diets are neuroprotective, even after the neurotoxic insult has taken place, seems promising and worth further clinical exploration. Our work shows that neuroprotection is not restricted to n-3 PUFAs, such as LIN and DO (a major component of fish oil), since n-6 PUFAs such as AA also have comparable effects on excitability. However, the beneficial effects of AA in the in vivo models were less pronounced. These findings are likely to be explained by the fact that AA can be transformed into various other compounds by the cyclooxygenase and lipoxygenase pathways and has many toxic oxidized metabolites (Farooqui and Horrocks, 1991; Lafon-Cazal et al., 1993). Therefore, further therapeutic assays with PUFAs should probably be restricted to n-3 PUFAs.
Materials and methods
Cell cultures and toxicity experiments
Primary cultures of cerebellar granule cells were prepared from 5- to 6-day-old Balb-C mice (Charles River, France) as described (Schousboe et al., 1989). Cells were plated at a density of 3 × 106 cells/35 mm culture dish. For toxicity experiments, neurons were washed once and incubated for 45 min in physiological salt solution of the following composition (in mM): 118.2 NaCl, 4.8 KCl, 1.2 NaH2PO4, 2.5 CaCl2, 10 HEPES and 33 glucose, with or without 3 MgCl2. The Mg2+-free buffer contained 100 nM glycine. Following treatments, the cells were returned to culture medium containing either 5 or 25 mM KCl as indicated. All drugs were added at the beginning of the toxic exposure and removed at the end.
Cell survival was evaluated 8 h after the treatment using the LDH assay (Koh and Choi, 1987). At this time point, no cell death had occurred in control cells, as verified by both LDH efflux and TUNEL labeling, performed as described in Lauritzen et al. (1997). LDH activities, measured as OD340, were expressed as activity present in the total medium volume/total LDH activity (media + cells), and presented in the figures as release of LDH as a percentage of that in control cells. The percentage LDH activities were used for statistical analysis by ANOVA followed by a Fischer PLSD for multiple comparisons (*p <0.05, **p <0.01 and ***p <0.001).
[Ca2+]i measurements
Intracellular calcium levels were measured in single neurons using Fura-2 microspectrofluorimetry, as described (Grynkiewicz et al., 1985; Lauritzen et al., 1997).
86Rb+ efflux studies
All the 86Rb+ efflux studies were conducted at room temperature (RT) on 5- to 6-day-old cultures (grown in Falcon 12 well dishes at 2 × 106 cells/well) as described in Maingret et al. (1999). 86Rb+ release was expressed as fractional release using a computer program adapted for the calculations. In each experiment, background efflux was determined by incubating sister cultures in vehicle without drugs.
Electrophysiology
The whole-cell and outside-out configurations of the patch–clamp technique were used (Patel et al., 1998). The external solution at pH 7.4 contained (in mM): 140 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES–NaOH. Pipette solutions contained either the external medium (cell attached) or an internal solution at pH 7.3 with (in mM): 140 KCl, 2 MgCl2, 10 HEPES–KOH, 5 EGTA. Cells were continuously superfused with a microperfusion system during the time course of the experiments (0.1 ml/min) at RT.
Global ischemia and kainate administration
Transient complete forebrain ischemia (Pulsinelli and Brierley, 1979) was produced in male Wistar rats (250 g) as described previously (Heurteaux et al., 1993).
Kainate was administered i.p. at a dose of 10 mg/kg body weight. The EEG was recorded using a twisted stainless steel bipolar electrode implanted under stereotaxic guidance in CA1 field (A3.8, L2, H2.7) and a ground electrode screwed in front of the olfactory bulb in the middle plane. EEG recordings were monitored for four consecutive hours.
LIN and PAL were injected either i.c.v. at a dose of 10 µM (5 µl) 30 min before the induction of ischemia or KA or i.v. 30 min (100 nmol/kg body weight) before or 30 min (100 or 500 nmol/kg body weight) after the neurotoxic insult. Sham-operated animals and rats injected with vehicle were used as controls.
Analysis of ischemia- or kainic acid-induced hippocampal damage
Three or 7 days (TUNEL assay or Nissl’s stain, respectively) following ischemia or KA treatment, the animals were killed and their brains were removed. Frozen coronal sections (12 µm) were taken of the hippocampal area, stained with cresyl violet (Nissl’s stain) and assessed for neuronal death. The neuronal density of CA1 and CA3 sectors, i.e. the number of intact pyramidal cells per 1 mm linear length of the CA1/CA3 stratum pyramidale observed in each section, was quantified according to the method of Kirino et al. (1986). The TUNEL assay (Gavrieli et al., 1992) was performed using the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling kit from Boehringer Mannheim. TUNEL labeling was visualized with 3′-diaminobenzidine and nickel chloride.
Immunostaining
α-TREK-1 and α-TRAAK antibodies were raised against a glutathione S-transferase fusion protein containing the C-terminus (Pro296–Val398) of TRAAK and the N-terminus (Met1–Trp44) of TREK-1, as described (Lesage et al., 1996). Neuronal cultures were fixed, blocked with goat serum and incubated with α-TREK-1 (1:600) or α-TRAAK (1:600) and mouse monoclonal antibody to synapsin I (1:1000; Bioproducts) for 2 h at RT. Appropriate secondary antibodies (goat anti-rabbit tetramethylrhodamine isothiocyanate and donkey anti-mouse–fluorescein isothiocyanate; Jackson Laboratories) were used to visualize the proteins. For immunohistochemistry on mice brain sections, adult BalbC mice were killed after transcardial perfusion with phosphate-buffered 4% paraformaldehyde. Floating brain sections (50 µm) were cut on a vibratome (Leica). Immunostaining with α-TREK-1 and α-TRAAK antibodies was performed using the Vector Elite ABC kit. Sections were incubated overnight at 4°C with TREK-1 or TRAAK antibodies, respectively (1:800 dilution). The antigen–antibody complexes were visualized by reaction with 3′-diaminobenzidine and H2O2.
Acknowledgments
Acknowledgements
We are grateful to Drs F.Lesage and R.Reyes for the preparation of antibodies and to Drs W.Ferlin and A.Patel for careful reading of the manuscript. Thanks are due to F.Aguila, D.Doume and V.Lopez for technical assistance. This work was supported by the Centre National de la Recherche Scientifique (CNRS), the Association pour la Recherche sur le Cancer (ARC), the Conseil Regional (PACA) and the Association Française contre les Myopathies (AFM).
References
- Abele A.E. and Miller,R.J. (1990) Potassium channel activators abolish excitotoxicity in cultured hippocampal pyramidal neurons. Neurosci. Lett., 115, 195–200. [DOI] [PubMed] [Google Scholar]
- Abele A.E., Scholz,K.P., Scholz,W.K. and Miller,R.J. (1990) Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron, 4, 413–419. [DOI] [PubMed] [Google Scholar]
- Albert C.M., Hennekens,C.H., O’Donnell,C.J., Ajani,U.A., Carey,V.J., Willett,W.C., Ruskin,J.N. and Manson,J.E. (1998) Fish consumption and risk of sudden cardiac death. J. Am. Med. Assoc., 279, 23–28. [DOI] [PubMed] [Google Scholar]
- Barnard E.A. (1997) Ionotropic glutamate receptors: new types and new concepts. Trends Pharmacol. Sci., 18, 141–148. [DOI] [PubMed] [Google Scholar]
- Choi D.W. (1994) Glutamate receptors and the induction of excitotoxic neuronal death. Prog. Brain Res., 100, 47–51. [DOI] [PubMed] [Google Scholar]
- Colbert C.M. and Pan,E. (1999) Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K+ channels in hippocampal CA1 pyramidal neurons. J. Neurosci., 19, 8163–8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat F., Lesage,F., Patel,A.J., Fink,M., Romey,G. and Lazdunski,M. (2000) The neuroprotective agent riluzole activates the two P-domain K+ channels TREK-1 and TRAAK. Mol. Pharmacol., in press. [PubMed] [Google Scholar]
- Ettaiche M., Fillacier,K., Widmann,C., Heurteaux,C. and Lazdunski,M. (1999) Riluzole improves functional recovery following ischemia in the rat retina. Invest. Ophthalmol. Vis. Sci., 40, 729–736. [PubMed] [Google Scholar]
- Farooqui A.A. and Horrocks,L.A. (1991) Excitatory amino acid receptors, neural membrane phospholipid metabolism and neurological disorders. Brain Res. Rev., 16, 171–191. [DOI] [PubMed] [Google Scholar]
- Fink M., Duprat,F., Lesage,F., Reyes,R., Romey,G., Heurteaux,C. and Lazdunski,M. (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J., 15, 6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Fink M., Lesage,F., Duprat,F., Heurteaux,C., Reyes,R., Fosset,M. and Lazdunski,M. (1998) A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acid. EMBO J., 17, 3297–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D.D., Hoehn,K., Weiss,S. and MacViar,B.A. (1993) Arachidonic acid inhibits sodium currents and synaptic transmission in cultured striatal neurons. Neuron, 11, 633–644. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman,Y. and Ben-Sason,S.A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol., 119, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G. and Schousboe,A. (1997) High affinity glutamate transporters: regulation of expression and activity. Mol. Pharmacol., 52, 6–15. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie,M. and Tsien,R.Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem., 260, 3440–3450. [PubMed] [Google Scholar]
- Heurteaux C., Bertaina,V., Widmann,C. and Lazdunski,M. (1993) K+ channel openers prevent global ischemia-induced expression of c-fos, c-jun, heat shock protein and amyloid β-protein precursor genes and neuronal death in rat hippocampus. Proc. Natl Acad. Sci. USA, 90, 9431–9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln J.R. (1998) Fish consumption and major depression. Lancet, 351, 1210–1213. [DOI] [PubMed] [Google Scholar]
- Honoré E., Barhanin,J., Attali,B., Lesage,F. and Lazdunski,M. (1994) External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc. Natl Acad. Sci. USA, 91, 1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keros S. and McBain,C.J. (1997) Arachidonic acid inhibits transient potassium currents and broadens action potentials during electrographic seizures in hippocampal pyramidal and inhibitory interneurons. J. Neurosci., 17, 3476–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. and Clapham,D.E. (1989) Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science, 244, 1174–1179. [DOI] [PubMed] [Google Scholar]
- Kim D., Sladek,C.D., Aguado-Velasco,C. and Mathiasen,J.R. (1995) Arachidonic acid activation of a new family of K+ channels in cultured rat neuronal cells. J. Physiol. (Lond.), 484, 643–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T., Tamura,A. and Sano,K. (1986) A reversible type of neuronal injury following ischemia in the gerbil hippocampus. Stroke, 17, 455–459. [DOI] [PubMed] [Google Scholar]
- Koh J.Y. and Choi,D.W. (1987) Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J. Neurosci. Methods, 20, 83–90. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M., Pietri,S., Culcasi,M. and Bockaert,J. (1993) NMDA-dependent superoxide production and neurotoxicity. Nature, 364, 535–537. [DOI] [PubMed] [Google Scholar]
- Lang-Lazdunski L., Vaillant,N., Widmann,C., Heurteaux,C. and Lazdunski,M. (1999) Riluzole improves neurological function following severe spinal cord ischemia. J. Thorac. Cardiovasc. Surg., 117, 881–889. [DOI] [PubMed] [Google Scholar]
- Lauritzen I., De Weille,J.R. and Lazdunski,M. (1997) The potassium channel opener (–)-cromakalim prevents glutamate-induced cell death in hippocampal neurons. J. Neurochem., 69, 1570–1579. [DOI] [PubMed] [Google Scholar]
- Leaf A. and Kang,J.X. (1996) Prevention of cardiac sudden death by n-3 fatty acids: a review of the evidence. J. Intern. Med., 240, 5–12. [DOI] [PubMed] [Google Scholar]
- Leaf A., Kang,J.X., Xiao,Y.-F., Billman,G.E. and Voskuyl,R.A. (1999) The antiarrhythmic and anticonvulsant effects of dietary n-3 fatty acids. J. Membr. Biol., 172, 1–11. [DOI] [PubMed] [Google Scholar]
- Lesage F. and Lazdunski,M. (1999) Potassium channels with two P-domains. In Kurachi,Y., Jan,L.Y. and Lazdunski,M. (eds), Potassium Ion Channels. Molecular Structure, Function and Diseases—Current Topics in Membranes. Academic Press, San Diego, CA, pp. 199–222. [Google Scholar]
- Lesage F., Guillemare,E., Fink,M., Duprat,F., Lazdunski,M., Romey,G. and Barhanin,J. (1996) TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J., 15, 1004–1011. [PMC free article] [PubMed] [Google Scholar]
- Lothman E.W. and Collins,R.C. (1981) Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res., 218, 299–318. [DOI] [PubMed] [Google Scholar]
- Maingret F., Fosset,M., Lesage,F., Lazdunski,M. and Honoré,E. (1999) TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem., 274, 1381–1387. [DOI] [PubMed] [Google Scholar]
- Meves H. (1994) Modulation of ion channels by arachidonic acid. Prog. Neurobiol., 43, 175–186. [DOI] [PubMed] [Google Scholar]
- Miller B., Sarantis,M., Traynelis,S.F. and Attwell,D. (1992) Potentiation of NMDA receptor currents by arachidonic acid. Nature, 355, 722–725. [DOI] [PubMed] [Google Scholar]
- Mizoule J., Meldrum,B., Mazadier,M., Croucher,M., Ollat,C., Uzan,A., Legrand,J.J., Gueremy,C. and Fur,G.L. (1985) 2-amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission. I. Anticonvulsant properties. Neuropharmacology, 24, 767–773. [DOI] [PubMed] [Google Scholar]
- Nadler J.V., Perry,B.W. and Cotman,C.W. (1978) Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature, 271, 676–677. [DOI] [PubMed] [Google Scholar]
- Nair S.S., Leitch,J.W., Falconer,J. and Garg,M.L. (1997) Prevention of cardiac arrhythmia by dietary (n-3) polyunsaturated fatty acids and their mechanism of action. J. Nutr., 127, 383–393. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Kimura,S. and Akaike,N. (1994) Facilitatory effect of docosahexaenoic acid on N-methyl-d-aspartate response in pyramidal neurones of rat cerebral cortex. J. Physiol., 475, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordoy A. (1999) Dietary fatty acids and coronary heart disease. Lipids, 34, S19–S22. [DOI] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Maingret,F., Lesage,F., Fink,M., Duprat,F. and Lazdunski,M. (1998) A mammalian two pore domain mechano-gated S-type K+ channel. EMBO J., 17, 4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.J., Honoré,E., Lesage,F., Fink,M., Romey,G. and Lazdunski,M. (1999) Inhalational anesthetics activate two-pore-domain background K+ channels. Nature Neurosci., 2, 422–426. [DOI] [PubMed] [Google Scholar]
- Pulsinelli W.A. and Brierley,J.B. (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke, 10, 267–272. [DOI] [PubMed] [Google Scholar]
- Quast U. (1992) Potassium channel openers: pharmacological and clinical aspects. Fundam. Clin. Pharmacol., 6, 279–293. [DOI] [PubMed] [Google Scholar]
- Quast U. (1993) Do the K+ channel openers relax smooth muscle by opening K+ channels? Trends Pharmacol. Sci., 14, 332–337. [DOI] [PubMed] [Google Scholar]
- Rose K., Christine,C.W. and Choi,D.W. (1990) Magnesium removal induces paroxysmal neuronal firing and NMDA receptor-mediated neuronal degeneration in cortical cultures. Neurosci. Lett., 115, 313–317. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R. and Freund,T.F. (1991) Selective vulnerability of the hippocampus in brain ischemia. Neuroscience, 40, 599–636. [DOI] [PubMed] [Google Scholar]
- Schousboe A., Meier,E., Drejer,J. and Hertz,L. (1989) Preparation of primary cultures of mouse (rat) cerebellar granule neurons. In Shahar,A., de Vellis,J., Vernadakis,A. and Haber,B. (eds), A Dissection and Tissue Culture Manual of the Nervous System. Alan R.Liss, New York, NY, pp. 203–206. [Google Scholar]
- Siesjö B.K. and Wieloch,T. (1985) Brain injury: neurochemical aspects. In Becker,D.P. and Povlishock,J.T. (eds), System Trauma. William Byrd Press, Richmond, VA, pp. 513–532. [Google Scholar]
- Stoll A.L., Severus,E., Freeman,M.P., Rueter,S., Zboyan,H.A., Diamond,E., Gress,K.K. and Marangell,L.B. (1999) Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch. Gen. Psychiatry, 56, 413–416. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M., Bruehl,C., Voskuyl,R.A., Kang,J.X., Leaf,A. and Wadman,W.J. (1996) Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc. Natl Acad. Sci. USA, 93, 12559–12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.C., Perry,H., Scaravilli,F., Patsalos,P.N., Shorvon,S.D. and Jefferys,J.G. (1999) Halothane as a neuroprotectant during constant stimulation of the perforant path. Epilepsia, 40, 359–364. [DOI] [PubMed] [Google Scholar]
- Xiao Y.-F. and Li,X. (1999) Polyunsaturated fatty acids modify mouse hippocampal neuronal excitability during excitotoxic or convulsant stimulation. Brain Res., 846, 112–121. [DOI] [PubMed] [Google Scholar]
- Yu S.P., Yeh,C.-H., Strasser,U., Tian,M. and Choi,D.W. (1999) NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science, 284, 336–339. [DOI] [PubMed] [Google Scholar]