Abstract
The TEL–TRKC fusion is expressed as a consequence of t(12;15)(p13;q25), and is associated with two human cancers: congenital fibrosarcoma and acute myelogenous leukemia (AML). We report that the T/T(F) and T/T(L) fusion variants associated with congenital fibrosarcoma and AML, respectively, are constitutively tyrosine phosphorylated, and confer factor-independent growth to the murine hematopoietic cell line Ba/F3. Retroviral transduction of T/T(L) causes a rapidly fatal myeloproliferative disease in a murine bone marrow transplant (BMT) model, whereas T/T(F) causes a long-latency, pre-B-cell lymphoblastic lymphoma. TEL–TRKC variants are potent activators of the MAP kinase pathway, but neither variant activates Stat5 or other Stat family members. T/T(L), but not T/T(F), induces tyrosine phosphorylation of phospholipase Cγ (PLCγ), phosphoinositol-3 kinase and SHC. However, mutation analysis demonstrates that PLCγ tyrosine phos phorylation by T/T(L) is dispensable for induction of the myeloproliferative phenotype by T/T(L). Collectively, these data demonstrate that the TEL–TRKC fusion variants are oncoproteins that activate the MAP kinase pathway, and do not require activation of either PLCγ or Stat5 for efficient induction of a myeloproliferative phenotype in the murine BMT model.
Keywords: fibrosarcoma/leukemia/MAP kinase/Stat5/TEL–TRKC
Introduction
The consequence of t(12;15)(p13;q25) is expression of the TEL–TRKC fusion protein. TEL–TRKC is unusual in that it has been associated with both hematological malignancies [acute myelogenous leukemia (AML); Eguchi et al., 1999] and the non-hematological malignancies infantile fibrosarcoma (Knezevich et al., 1998b) and congenital mesoblastic nephroma (Knezevich et al., 1998a; Rubin et al., 1998). The TEL–TRKC fusion in AML [T/T(L)] includes exons 1–4 of the TEL gene fused in-frame to the tyrosine kinase domain of TRKC. T/T(L) also lacks a 42 bp exon near the C-terminus of the TRKC moiety. In the context of the native TRKC, this splice variation is known to generate a more potent TRKC tyrosine kinase (Lamballe, 1993). In contrast, the TEL–TRKC variant associated with infantile fibrosarcoma [T/T(F)] includes exons 1–5 of the TEL gene, and incorporates the 42 bp exon in the TRKC moiety, which is known to decrease tyrosine kinase activity and affect signal transduction pathways (Lamballe et al., 1993; Valenzuela et al., 1993; Guiton et al., 1995; Tsoulfas et al., 1996).
TEL is a member of the ETS family of transcription factors, and was first identified by its involvement in the t(5;12)(q33;p13) associated with chronic myelomonocytic leukemia (CMML) (Golub et al., 1994). The consequence of the t(5;12) translocation in CMML is fusion of TEL to the platelet-derived growth factor β receptor (PDGFβR). TEL has subsequently been shown to be rearranged in >40 different chromosomal translocations (Golub et al., 1997). TEL contains a C-terminal DNA binding or ETS domain, and is one of a subset of ETS family members that contains an evolutionarily conserved N-terminal pointed (PNT) domain, a self-association motif (Carroll et al., 1996; Jousset et al., 1997).
Other TEL–tyrosine kinase fusions associated with hematological malignancy include the TEL–ABL (Papadopoulos et al., 1995; Golub et al., 1996; Okuda et al., 1996), TEL–JAK2 (Lacronique et al., 1997; Peeters et al., 1997; Schwaller et al., 1998). In each case, the TEL PNT domain is fused to the tyrosine kinase domain of the partner, and serves to oligomerize and constitutively activate the respective tyrosine kinase. Mutations of the ATP binding site in the catalytic domain of the tyrosine kinase partner abrogate transformation. TEL–tyrosine kinase fusion proteins constitutively activate a spectrum of signaling pathways in transformed cells, but one feature shared by each of these is the ability to activate members of the STAT family of latent cytoplasmic transcription factors. It has therefore been suggested that STAT activation is a critical event in transformation mediated by these fusion proteins.
TRKC is one of a family of neurotrophic growth factor receptors that regulate proliferation, survival, commitment and differentiation of neurons in response to neurotrophin ligands (Kaplan et al., 1991; Lamballe et al., 1991, 1993; Jing et al., 1992; Valenzuela et al., 1993; Guiton et al., 1995; Tsoulfas et al., 1996; Kim et al., 1999). There are four known neurotrophins, nerve growth factor (NGF), brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5 (Lewin and Barde, 1996), that differentially activate members of the neurotrophic growth factor receptor family. For example, brain-derived neurotrophic factor and neurotrophin 4/5 preferentially stimulate TRKB (Berkemeier et al., 1991; Klein et al., 1991), TRKA is the receptor for NGF (Kaplan et al., 1991; Jing et al., 1992) and TRKC is the preferred high-affinity receptor for neurotrophin 3 (Lamballe et al., 1991). Native TRK receptors are activated upon ligand-induced oligomerization, resulting in autophosphorylation of specified tyrosine residues on the cytoplasmic tail of the receptor, which in turn transmit signal through SH2-containing intermediates including SHC and phospholipase Cγ (PLCγ) (Obermeier et al., 1994). For example, in TRKA, Y490 in the juxtamembrane domain is a recognition site for the adapter protein SHC and is also thought to be a phosphoinositide-3-kinase (PI3K) binding site, whereas Y785 is required for the binding and activation of PLCγ (Obermeier et al., 1993, 1994; Stephens et al., 1994). The activated receptor is linked by SHC to the SOS–GRB2 complex, which in turn activates the RAS–MAP kinase pathway (reviewed in Segal and Greenberg, 1996). Both the SHC and PLCγ sites are required for normal neuronal differentiation (Obermeier et al., 1994). TRKC is critical for normal neural development, in that TrkC null animals lack Ia muscle afferent projections to spinal motor neurons, and have fewer large myelinated axons in the dorsal root and posterior columns of the spinal cord, with resultant abnormal movements and postures (Klein et al., 1994).
In this report, we have characterized the signal transduction and transforming properties of the two TEL–TRKC variants associated with human malignancy, as well as related mutants. Our results demonstrate that the TEL–TRKC variants have distinct transforming properties, and have provided new insights into the signal transduction pathways that are required for efficient induction of a myeloproliferative disease (MPD) in the murine bone marrow transplant (BMT) model. Our data indicate that MAP kinase activation is not sufficient for induction of MPD, but that activation of PLCγ is not required. Furthermore, in contrast with all other tyrosine kinase fusion genes characterized thus far, including BCR–ABL (Ilaria and Van Etten, 1996; Ilaria et al., 1999), TEL–ABL (Okuda et al., 1996), TEL–JAK2 (Lacronique et al., 1997; Schwaller et al., 1998), TEL–PDGFβR (Sternberg et al., 1999) and HIP1–PDGFβR (Ross and Gilliland, 1999), TEL–TRKC is able to transform Ba/F3 cells efficiently and induce MPD without activation of Stat5.
Results
Both TEL–TRKC fusion variants T/T(L) and T/T(F) are constitutively tyrosine phosphorylated, and confer factor-independent growth to Ba/F3 cells
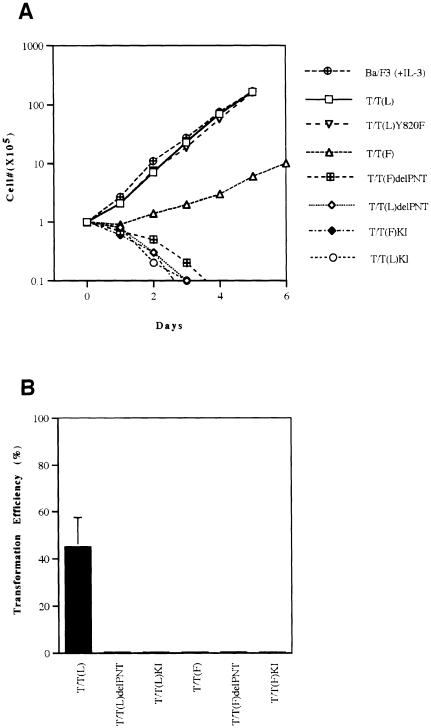
As a measure of transformation in hematopoietic cells, we first tested the ability of T/T(L) and T/T(F) (Figure 1) to transform the interleukin-3 (IL-3)-dependent hematopoietic cell line Ba/F3 to factor-independent growth. Both T/T variants conferred factor-independent growth to Ba/F3 cells, although there was a consistent decrement in repeat experiments in the growth rate of Ba/F3 cells transformed with T/T(F) compared with T/T(L) (Figure 2A). Only the T/T(L) variant was capable of transforming NIH 3T3 cells (Figure 2B), indicating that the T/T(F) variant was a less potent oncogene both in Ba/F3 cells and in NIH 3T3 cells. We hypothesized that transformation to factor-independent growth required both the TRKC tyrosine kinase activity as well as the PNT oligomerization motif. To test this hypothesis, T/T(L) and T/T/(F) mutants were prepared with PNT deletions, and with a K572N substitution that abrogates TRKC tyrosine kinase activity in the context of the native receptor (Figure 1). Neither the PNT deletion of kinase inactive mutants of T/T(L) nor of T/T(F) conferred IL-3-independent growth to Ba/F3 cells. Western blot analysis confirmed that the native and mutant T/T proteins were expressed (Figure 3A). As expected, the T/T(L) and T/T(F) proteins were tyrosine phosphorylated in vivo, while the PNT deletion and kinase inactive mutants of T/T(L) and T/T(F) were not (Figure 3B).
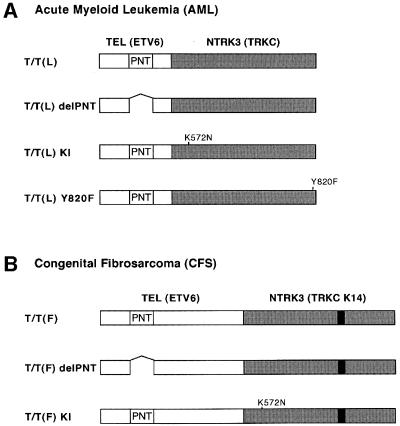
Fig. 1. TEL–TRKC fusion constructs. (A) T/T(L) is encoded by a TEL–TRKC fusion gene isolated from an adult AML patient (Eguchi, 1999). It contains TEL (nucleotides 1–487) and TRKC (nucleotides 1741–2750). (B) T/T(F) is generated by a TEL–TRKC K14 fusion gene containing TEL (nucleotides 1–1033) and TRKC K14 (nucleotides 1601–2715), which is found in congenital fibrosarcoma (CFS) patients (Knezevich, 1998b). T/T(L) delPNT and T/T(F) delPNT are the fusion proteins with deletion of the TEL pointed (PNT) oligomerization domain (amino acids 58–108). T/T(L)KI and T/T(F)KI contain a single mutation (K572N in TRKC), which inactivates the TRKC kinase activity. (K572 in TRKC corresponds to K538 in TRKA, and is an ATP binding site.) T/T(L)Y820F is a mutant that abolishes the putative PLCγ binding site in the context of the native TRKC. (Y820 in TRKC corresponds to Y785 in TRKA.) The nucleotide numbers for TEL, TRKC and TRKC K14 are according to DDBJ/EMBL/GenBank accession Nos U11732, U05012 and S76475, respectively.
Fig. 2. Transformation of Ba/F3 cells and NIH 3T3 cells by TEL–TRKC fusion protein and its variants. (A) Ba/F3 cells expressing the various fusion proteins were washed free of IL-3, and 1 × 105 cells were plated in duplicate in bulk in IL-3-free medium, and counted daily as described previously (Carroll et al., 1996; Schwaller et al., 1998). Only cells expressing T/T(L) or T/T(F) were able to grow in the absence of IL-3. (B) MSCVneo retroviruses containing the fusion genes were used to infect NIH 3T3 cells. The infected cells were split and plated in G418-containing medium for up to 2 weeks. After 10–14 days, transformed colonies of cells were apparent under low-magnification microscopy. The transformation efficiencies were calculated as described in Materials and methods. Only the NIH 3T3 cells expressing T/T(L) formed colonies on the plates. The figure is representative of three independent experiments. The standard deviation for each point for number of Ba/F3 cells was always <15% and ranged from 3 to 15%.
Fig. 3. Expression and phosphorylation of TEL–TRKC fusion protein and its variants in Ba/F3 cells. Ba/F3 cells were infected with MSCVneo retroviruses containing T/T(L), T/T(L) delPNT, T/T(L)KI, T/T(F), T/T(F) delPNT or T/T(F)KI as described in Materials and methods. Cells maintained in media containing IL-3 and G418(1 mg/ml) were washed and lysed in lysis buffer. (A) Whole-cell lysates (100 µg) were subjected to 10% SDS–PAGE, followed by transfer to nitrocellulose and Western blotting using a rabbit anti-TRKC antibody (a kind gift from Dr Rosalind A.Segal, Harvard Medical School, Boston, MA). (B) Cell lysates (1 mg) were immunoprecipitated with the anti-TRKC antibody, separated on SDS–PAGE [7.5% gel for T/T(L) and its variants, 10% gel for T/T(F) and its variants], then blotted and probed with an anti-phosphotyrosine antibody (4G10). Blots were then stripped and reprobed with the anti-TRKC antibody. The T/T(L) protein, which lacks amino acids encoded by TEL exon 5, has a predicted mol. wt of ∼52 kDa, whereas the T/T(F) variant, which contains TEL exon 5 as well as the 14 amino acid insertion in the TRKC moiety, has a predicted mol. wt of ∼74 kDa.
Collectively, these data indicated that: (i) T/T(L) and T/T/(F) were transforming oncoproteins that required TRKC kinase activity for transformation; (ii) like other TEL–tyrosine kinase fusions associated with human leukemias, the TEL PNT domain was required for TRKC kinase activation; and (iii) the T/T(F) variant was a less potent oncogene than the T/T(L) variant in both Ba/F3 and NIH 3T3 cells.
PLCγ association and tyrosine phosphorylation by the TEL–TRKC fusion proteins
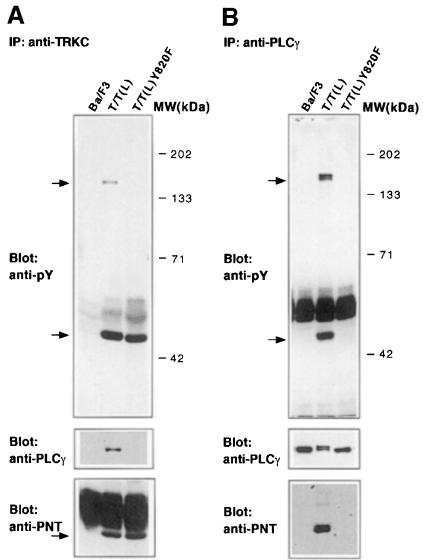
PLCγ is activated by the native TRKC receptor. PLCγ was also strongly tyrosine phosphorylated by T/T(L), but not by T/T(F) (Figure 4A). PLCγ associates with native TRKC through an SH2 interaction with TRKC residue Y820. A tyrosine to phenylalanine substitution at the analogous amino acid in the context of the T/T(L) fusion protein abrogated tyrosine phosphorylation of PLCγ (Figure 4A).
Fig. 4. Tyrosine phosphorylation of PLCγ, PI3 kinase and SHC by TEL–TRKC fusion protein and its variants. Ba/F3 cells expressing the indicated fusion proteins were grown in medium without IL-3. Cell lysates were immunoprecipitated with (A) anti-PLCγ monoclonal IgG (Upstate Biotechnology, Lake Placid, NY), (B) anti-PI3 kinase rabbit antiserum (Upstate Biotechnology, Lake Placid, NY) or (C) anti-SHC rabbit polyclonal antibody (Transduction Laboratories, Lexington, KY). Immunoprecipitates were separated by SDS–PAGE, transferred to nitrocellulose and Western blotting was performed using the 4G10 anti-phosphotyrosine antibody. Blots were then stripped and reprobed with the anti-PLCγ monoclonal IgG, the anti-PI3 kinase rabbit antiserum or anti-SHC mouse monoclonal antibody (Transduction Laboratories, Lexington, KY). As a control, parental Ba/F3 cells were washed free of IL-3 and grown in the absence of IL-3 for 2 h. Stimulation of these IL-3-starved Ba/F3 cells with 1.0 ng/ml IL-3 for 5 min restored phosphorylation of SHC.
Immunoprecipitation of extracts from stable cell lines expressing T/T(L) or T/T(L)Y820F with anti-TRKC antibody demonstrated association of PLCγ with T/T(L), but not with the T/T(L)Y820F mutant (Figure 5A). The association of PLCγ with T/T(L), but not T/T(L)Y820F, was confirmed in immunoprecipitation experiments using anti-PLCγ antibody (Figure 5B). These data indicated that, like the native TRKC, the Y820F mutation abrogated PLCγ association with T/T(L).
Fig. 5. PLCγ is the only tyrosine-phosphorylated protein associated with TEL–TRKC with high affinity. Ba/F3 cells expressing the indicated fusion proteins were maintained in the absence of IL-3. Cells were lysed and immunoprecipitated with either a rabbit anti-TRKC antibody (A) or anti-PLCγ monoclonal IgG (B) as described in previous figures. The immunoprecipitates were subjected to 7.5% SDS–PAGE and probed with 4G10, and then stripped and sequentially reprobed with the anti-PLCγ antibody and a rabbit anti-TEL PNT domain antibody (a kind gift from Peter Marynen, Leuven, Belgium). Two tyrosine-phosphorylated bands were observed of ∼52 and 140 kDa, respectively. Stripping and reprobing the blot with anti-PLCγ antibody identified the 140 kDa band as PLCγ, and blotting with antibody directed against the TEL PNT domain confirmed the identity of the 52 kDa band as T/T(L).
PLCγ was strongly tyrosine phosphorylated by T/T(L), but not by T/T(F). To determine whether differential activation of PLCγ could account for the difference in transformation by T/T(L) and T/T(F), stable Ba/F3 cell lines expressing the T/T(L)Y820F mutant were prepared by retroviral transduction. There was no difference in the growth rate of hematopoietic cells transformed by T/T(L) and T/T(L)Y820F (Figure 2A), although there were equivalent levels of expression of the fusion proteins by Western blot analysis (data not shown). This result was confirmed in the murine BMT assay, as described below. Taken together, these data indicate that although PLCγ is the major tyrosine-phosphorylated protein associated with T/T(L) in transformed Ba/F3 cells, tyrosine phosphorylation of PLCγ is dispensable for transformation.
Analysis of STAT, PI3K, SHC and MAP kinase signaling in TEL–TRKC-transformed Ba/F3 cells
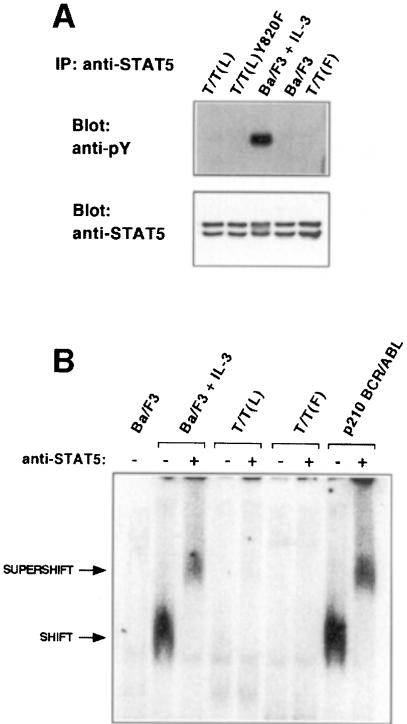
Although STAT activation by native TRKC has not been tested, STAT5 is activated by a broad spectrum of tyrosine kinase fusions associated with hematological malignancy, including BCR–ABL, TEL–ABL, TEL–JAK2 and TEL–PDGFβR. In striking contrast to other TK fusions, neither the T/T(L) nor the T/T(F) variants activated Stat5, as assessed by tyrosine phosphorylation (Figure 6A) or electrophoretic mobility shift assays (EMSAs) (Figure 6B). Similar experiments showed no evidence for activation of any other Stat family member, including Stat1, Stat2, Stat3, Stat4 and Stat6, although all Stat family members were expressed in Ba/F3 cells (data not shown). Control experiments demonstrated activation of Stat5 in Ba/F3 cells stimulated with IL-3 (Figure 6A and B) and in Ba/F3 cells transformed by BCR–ABL (Figure 6B).
Fig. 6. Stat5 is not activated in TEL–TRKC transformed Ba/F3 cells. (A) Ba/F3 cells expressing the indicated fusion proteins were grown in the absence of IL-3. Cell lysates were immunoprecipitated with a rabbit anti-Stat5 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), separated in 7.5% gel and probed with 4G10, then stripped and reprobed with the anti-Stat5 antibody. As a control, parental Ba/F3 cells were washed and grown in the absence of IL-3 for 2 h. Stimulation of the starved Ba/F3 cells with 1.0 ng/ml IL-3 for 5 min restored phosphorylation of Stat5. (B) Nuclear extracts from the indicated Ba/F3 cells were analyzed by EMSA using 32P-labeled FcγRI-derived GAS probe as described in Materials and methods. As a negative control, parental Ba/F3 cells were washed and grown in the absence of IL-3 for 4 h. Stimulation of the starved Ba/F3 cells with 1.0 ng/ml IL-3 for 15 min completely reactivated Stat5.
PI3K and SHC, known targets of the native TRKC, were assayed for tyrosine phosphorylation in transformed Ba/F3 cells. PI3K was tyrosine phosphorylated in stable cell lines expressing T/T(L), but not T/T(F) (Figure 4B). We were not able to demonstrate association of PI3K with either T/T(L) or T/T(F) (data not shown). SHC was strongly tyrosine phosphorylated in T/T(L) cell lines, or in control experiments in which Ba/F3 cells were stimulated with IL-3, but was only weakly phosphorylated by T/T(F) (Figure 4C).
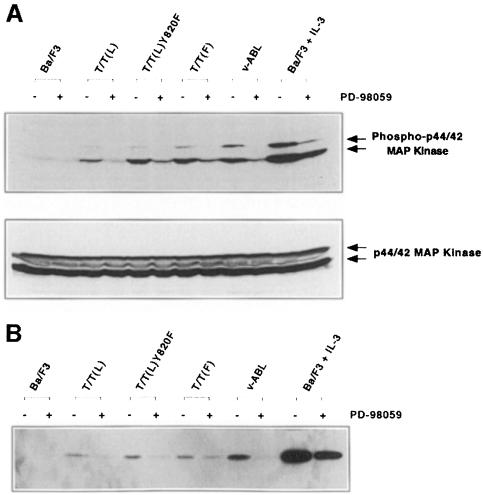
The MAP kinase pathway is also activated by native TRKC. T/T(L) and T/T(F) activation of the MAP kinase pathway was tested by assaying for phosphorylation of p44/42 MAP kinase (Erk1 and Erk2) in whole-cell lysates. T/T(L), T/T(F) and T/T(L)Y820F all activated the MAP kinase pathway in Ba/F3 cells (Figure 7A), and activation was inhibitable by the MAP kinase kinase (MEK) specific inhibitor PD98059 (Figure 7A). Similar data were obtained using Elk-1 as a substrate (Figure 7B). Of note, T/T(F) appeared to be a more potent activator of the MAP kinase pathway than the T/T(L) variant.
Fig. 7. MAP kinase activation by TEL–TRKC fusion protein and its variants. Ba/F3 cells expressing the indicated fusion proteins were grown in medium without IL-3. (A) Approximately 300 µg of whole-cell lysate were loaded in each lane, and phosphorylation of p44 and p42 MAP kinases (Erk1 and Erk2) was visualized with an antibody to the phosphorylated MAP kinase p44/42 proteins (New England Biolabs, Beverly, MA). The membrane was then stripped and reprobed with p44/42 MAP kinase antibody (New England Biolabs, Beverly, MA). (B) Cell lysates (400 µg) were analyzed for p44/42 MAP kinase activity using the p44/42 MAP Kinase Assay Kit (New England Biolabs, Beverly, MA). Two micrograms of purified Elk-1 fusion protein were used as the MAP kinase substrate as described in Materials and methods. Phosphorylation of Elk-1 was detected using Phospho-Elk-1(Ser383) antibody. As a control, parental Ba/F3 cells were washed and grown in the absence of IL-3 for 4 h. Stimulation of the starved Ba/F3 cells with 1.0 ng/ml IL-3 for 5 min restored MAP kinase activity. Control experiments also demonstrated that stable cell lines expressing v-ABL activated the MAP kinase pathway, and as expected, IL-3 stimulation was a potent inducer of MAP kinase activity (A and B).
Transforming properties of TEL–TRKC fusion proteins in primary hematopoietic progenitors in a murine BMT assay
T/T(L) and T/T(L)Y820F cause a rapidly fatal MPD. We next assessed the ability of the TEL–TRKC fusion proteins to transform primary hematopoietic cells in a murine BMT assay. Bone marrow harvested from 5-fluorouracil-primed whole bone marrow was transduced with retrovirus containing the TEL–TRKC fusion genes and related mutants. Each retroviral vector contained a cassette with an internal ribosomal entry site (IRES) and EGFP. TEL–TRKC-transduced bone marrow cells were introduced by tail vein injection into lethally irradiated syngeneic recipient mice. In control experiments, animals transplanted with bone marrow cells retrovirally transduced with the kinase inactive mutants of T/T, T/T(L)KI and T/T(F)KI did not develop hematological disease. However, the T/T(L) variant caused a rapidly fatal hematopoietic malignancy with a median latency of ∼20 days (Figure 8), and the disease was readily transplantable into secondary recipients (data not shown). The T/T(L)Y820F mutant was indistinguishable in disease latency from the T/T(L) fusion (Figure 8; Table I).
Fig. 8. Comparative survival analysis of TEL–TRKC BMT (Kaplan–Meier). Cumulative survival was plotted against days post-transplantation. Mice transplanted with primary murine bone marrow infected with T/T(L) or T/T(L)Y820F developed a rapidly fatal MPD with a median latency of ∼20 days. In contrast, mice transplanted with bone marrow retrovirally transduced with the T/T(F) variant developed B-cell lymphomas, and had a prolonged latency with a median survival of ∼50 days. Control experiments in which the kinase inactive mutants T/T(L)KI and T/T(F)KI were transduced into murine bone marrow caused no disease in lethally irradiated syngeneic recipients.
Table I. Summary of BMT experiments with TEL–TRK C variants and related mutants.
| No. transplanted/No. diseased | Latency (weeks) | WBC (106/ml) | Spleen weight (mg) | Histopathology | |
|---|---|---|---|---|---|
| T/T(L) | 9/9 | 2.2 (2–4) | 317 (135–500) | 400 (350–500) | MPD |
| T/T(L)Y820F | 8/8 | 2.4 (2–3) | 83 (23–120) | 362 (300–400) | MPD |
| T/T(F) | 8/8 | 7.8 (6–9) | 2 (1–3) | 240 (170–350) | LBL |
| T/T(L)KI | 4/0 | n.s. ≥40 | 4.5 (4–5) | 100 | normal |
| T/T(F)KI | 3/0 | n.s. ≥34 | 4 | 120 | normal |
WBC, white blood count; MPD, myeloproliferative disease; LBL, lymphoblastic lymphoma; n.s., no symptoms.
Pathological examination was performed on four T/T(L), three T/T(L)Y820F and two control mice. Seven out of seven mice transplanted with either T/T(L) or T/T(L)Y820F developed a morphologically indistinguishable myeloproliferative disorder. All animals had enlarged spleens (weight = 300–400 g; normal = 100–150 g; Table I) and expansion of the red pulp by maturing myeloid elements (Figure 9B and E). In addition, all animals had elevated white blood cell (WBC) counts (WBC: 23–500 × 106/ml; Table I) with mature and immature myeloid progenitors seen in peripheral blood smears (Figure 9C and F), and prominent extramedullary hematopoiesis primarily involving the liver (Figure 9A and D).
Fig. 9. Histopathological characterization of mice transplanted with T/T(L)-transduced bone marrow (A–C), T/T(L)Y820F-transduced bone marrow (D–F) and T/T(F)-transduced bone marrow (G–L). Peripheral blood smears (C, F, I): Wright–Giemsa stain; paraffin-embedded sections (A, B, D, E, G, H, J–L): hematoxylin and eosin stain. (A and D) Livers from representative T/T(L) and T/T(L)Y820F animals showed extensive perivenular and sinusoidal extramedullary hematopoiesis, comprised predominantly of maturing myeloid forms. (B and E) Spleens from representative T/T(L) and T/T(L)Y820F animals showed expansion of red pulp by sheets of maturing myeloid forms. (C and F) Peripheral blood smears from T/T(L) and T/T(L)Y820F animals showed marked leukocytosis consisting predominantly of mature and immature myeloid cells. Lymph node (G and H) from a representative T/T(F) mouse demonstrated diffuse effacement of the nodal architecture by a homogeneous population of immature cells (G). Higher power (H) views demonstrated a blast-like appearance of cells with irregular nuclear contours, dispersed chromatin and small amounts of cytoplasm. Note the numerous apoptotic cell bodies present. A peripheral blood smear (I) from a T/T(F) mouse demonstrated leukopenia with rare atypical lymphoid cells. Bone marrow from this mouse (J) showed extensive focal infiltrates of these immature blast-like cells. Extensive parasacral soft tissue infiltration was seen in a cross-section through the lower abdomen of a T/T(F) mouse (K). At higher power, immature blast-like lymphoid cells were seen infiltrating through the skeletal muscle fibers of this parasacral region (L).
Flow cytometric analysis of single-cell suspensions from the spleen of animals receiving bone marrow cells retrovirally transduced with either T/T(L) or T/T(L)Y820F demonstrated a large population of cells in the spleen that were Gr-1+ and also positive for EGFP (Figure 10A, top panels). In contrast, animals transplanted with the kinase inactive mutant T/T(KI) had few Gr-1+ cells in the spleen (Figure 10A, lower left panel), similar to the low number of Gr-1+ cells in spleens from normal animals (data not shown).
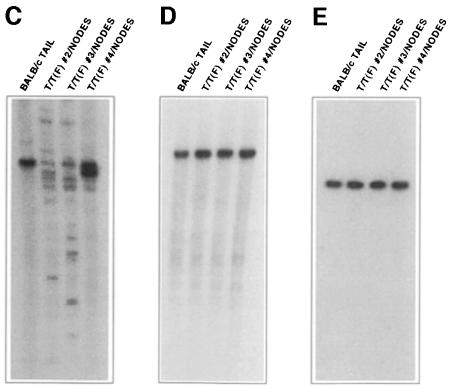
Fig. 10. The leukemia and fibrosarcoma variants of the TEL–TRKC fusion protein cause distinct diseases upon retroviral gene transfer into mouse bone marrow. (A) Spleen cells from mice that received bone marrow transduced with retroviruses encoding TEL–TRKC fusion proteins were stained with biotinylated anti-Gr-1 followed by allophycocyanin-conjugated streptavidin. Gr-1 is an antigen expressed on mature myeloid lineage cells in mice, including granulocytes and monocytes. Expression of the T/T(L) and T/T(L)Y820F forms of the fusion protein led to rapid development of a myeloproliferative process associated with large numbers of Gr-1+ cells in the spleen, which were also positive for EGFP. Only a small percentage of Gr-1+ cells were found in the spleens of mice receiving marrow transduced with the T/T(L)KI mutant construct. A mild increase in Gr-1+, EGFP+ cells is evident in the spleen of a mouse receiving marrow transduced with the T/T(F) construct, but most of the EGFP+ cells in the spleen were negative for the Gr-1 marker. (B) Spleen cells from a mouse that received bone marrow transduced with the MSCV-T/T(F)-EGFP construct were stained with phycoerythrin-conjugated antibody to B220 (CD45R) or biotinylated antibody to Gr-1, CD3 or BP-1 (Ly-51). The major EGFP+ population in the spleen consisted of B cells expressing the pan-B-cell marker B220 and the immature B-cell marker BP-1. (C–E) Immunoglobulin gene rearrangement in tumors from T/T(F)-transplanted mice. Southern blot analysis of T/T(F)-transplanted tumor samples showed gene rearrangements on the immunoglobulin heavy chain locus, but not on the κ light chain locus or βTCR locus, suggesting that the disease in T/T(F)-transplanted mice was a pre-B-cell lymphoma, consistent with the flow cytometric analysis. (C) EcoRI digestion, µVJ probe; (D) EcoRI + BamHI digestion, kC probe; (E) HindIII digestion, βTCR probe. Genomic tail DNA from an unaffected littermate was used as a control.
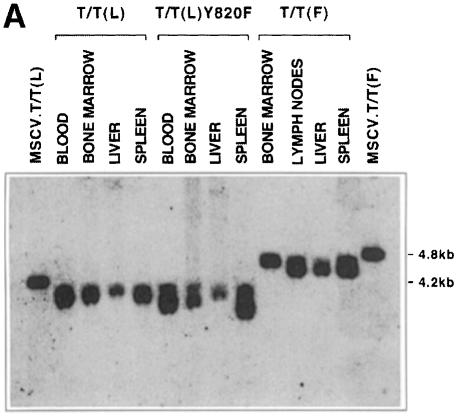
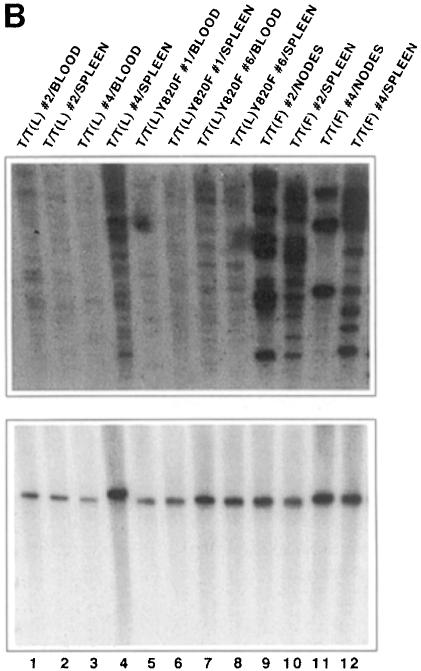
The presence of the T/T(L)-containing provirus was demonstrated by Southern blot analysis of affected tissues using green fluorescent protein (GFP) as a probe. DNA was digested with XbaI, which cleaves at two flanking sites within the retrovirus. The expected band size of ∼4.2 kb in was seen in blood, bone marrow, liver and spleen (Figure 11A). Clonality of the tumor was then assessed using the restriction endonuclease HpaI, which cleaves once within the provirus and again in flanking genomic DNA. Thus, when GFP is used as a probe, discrete bands obtained by Southern blot analysis reflect distinct proviral integration events. As shown in the first eight lanes of Figure 11B, the smeared pattern observed in tissues derived from animals transplanted with either T/T(L) or T/T(L)Y820F indicates oligoclonal or polyclonal disease. The smeared pattern indicates polyclonality rather than incomplete digestion of DNA, as demonstrated by stripping and reprobing the blot with a probe for the two-copy gene NFE2 shown in the bottom panel of Figure 11B.
Fig. 11. Integration of MSCV-TEL-TRKC-IRES-EGFP retrovirus into genomic DNA of transplanted mice. (A) Southern blot analysis of genomic DNA (10 µg) from various organs digested with XbaI, which cleaves once within each flanking long terminal repeat. Proviral integration was demonstrated using an 800 bp probe for GFP. Two plasmid lanes were added as a control. (B) Upper panel: Southern blot analysis of DNA digested with HpaI, which cleaves once in the MSCV.EGFP-TEL-TRKC provirus. Blotting with the GFP probe thus identifies unique integration events. All samples from T/T(L) and its variants showed polyclonal disease, and samples from T/T(F) showed oligoclonal disease. Bottom panel: the same membrane was stripped and reprobed with a 1.1 kb p45-NFE2 probe (nuclear factor erythroid 2) to demonstrate the integrity of the DNA.
T/T(F) causes a long-latency, B-cell lymphoblastic lymphoma. Mice that were transplanted with the T/T(F) construct developed hematopoietic malignancy with a prolonged latency of ∼50 days, in contrast with a latency of 20 days for mice transplanted with either the T/T(L) or T/T(L)Y820F constructs. Furthermore, there were striking phenotypic differences in the T/T(F)-transplanted animals. Three out of three animals analyzed had enlarged lymph nodes and splenomegaly, with low peripheral WBC (1–3 × 106/ml; Table I). Two of these animals demonstrated either large posterior mediastinal or parasacral soft tissue masses, and all animals had hind limb motor abnormalities. Microscopic examination of these tissues revealed infiltration by a high-grade lymphoid neoplasm comprised of immature blast-like cells with irregular nuclear contours, dispersed chromatin and scant cytoplasm (Figure 9G and H). In the animal with the large parasacral mass, extensive infiltration of the musculature of this region was seen, which disrupted the normal architecture of the muscle bundles (Figure 9K and L). Neoplastic blast-like cells were seen surrounding, but not directly invading, nerve structures. The disruption of the hind limb musculature and impingement on the peripheral nerves presumably resulted in the observed hind leg motor abnormalities. Mitotic activity and cellular apoptosis were frequently observed (Figure 9H). Both splenic white and red pulp, as well as lymphoid tissues, were infiltrated by these immature neoplastic cells. Bone marrow involvement was focally present (Figure 9J), and rare atypical lymphoid cells were seen in some animals in the peripheral blood (Figure 9I). Small clusters of malignant lymphoid cells were seen in the livers of these mice, primarily in a perivenular distribution (data not shown).
In contrast with animals transplanted with either the T/T(L) or T/T(L)Y820F constructs, flow cytometric analysis of single-cell suspensions from the spleens of T/T(F)-transplanted animals showed few Gr-1+ cells (Figure 10A). Instead, there was a major population of GFP-positive B cells that marked with the pan-B-cell marker B220 and the immature B-cell marker BP-1 (Figure 10B). Cells were negative for the T-cell marker CD3 (Figure 10B). Immunoglobulin gene rearrangement studies showed rearrangement of the immunoglobulin heavy chain locus (Figure 10C), but no rearrangement of the κ light chain locus or of the βTCR locus (Figure 10D and E, respectively). The flow cytometric analysis and gene rearrangement studies were thus most consistent with a pre-B-cell lymphoblastic lymphoma.
Proviral integration was demonstrated by Southern blot analysis of affected tissues, which showed that T/T(F) provirus was in bone marrow, lymph nodes, liver and spleen (Figure 11A). As expected, the T/T(F) virus was slightly larger than the T/T(L) virus due to the larger size of the T/T(F) fusion cDNA. Southern blot analysis for clonality demonstrated that these tumors were oligoclonal (Figure 11B, lanes 9–12), an observation that was consistent with oligoclonality of some tumors seen in the immunoglobulin gene rearrangement studies (see, for example, Figure 10C, lanes 2 and 3).
Discussion
The constitutive activation of tyrosine kinases has emerged as an important theme in the pathogenesis of human cancers. In hematopoietic malignancies, a common mechanism for activation of tyrosine kinases is fusion of the catalytic domain of the tyrosine kinase to an N-terminal partner that contains a dimerization or oligomerization domain as a consequence of balanced chromosomal translocations. Examples in hematopoietic malignancy include the t(9;22)(q34;q11) translocation that gives rise to the BCR–ABL fusion molecule associated with chronic myeloid and acute lymphoblastic leukemias (Daley et al., 1987, 1990; Lugo and Witte, 1989; Pendergast et al., 1993; Witte, 1993; Puil et al., 1994). Similar mechanisms generate the leukemogenic fusion proteins TEL–ABL (Papadopoulos et al., 1995; Golub et al., 1996; Okuda et al., 1996), TEL–PDGFβR (Golub et al., 1994; Carroll et al., 1996, 1997; Jousset et al., 1997), TEL–JAK2 (Lacronique et al., 1997; Peeters et al., 1997; Schwaller et al., 1998), CEV14–PDGFβR (Abe et al., 1997), HIP1–PDGFβR (Ross et al., 1998; Ross and Gilliland, 1999), ZNF198–FGFR (Reiter et al., 1998; Xiao et al., 1998) and NPM–ALK (Morris et al., 1994; Kuefer et al., 1997; Kadin and Morris, 1998) fusions associated with hematological malignancies. Similarly, activated or overexpressed tyrosine kinases have been reported in association with non-hematopoietic solid tumors, such as overexpression of HER-2/NEU in breast cancer (Ross and Fletcher, 1999), and other cancers.
The TEL–TRKC fusion is unusual in that it has been associated with the solid tumors congenital fibrosarcoma and mesoblastic nephroma, as well as with the hematological malignancy AML (Knezevich et al., 1998a,b; Rubin et al., 1998; Eguchi et al., 1999). The TEL–TRKC variant associated with AML includes exons 1–4 of TEL and lacks a 42 bp alternatively spliced exon in the TRKC moiety. The lack of this exon in the context of the native receptor enhances tyrosine kinase activity (Lamballe et al., 1993; Valenzuela et al., 1993; Guiton et al., 1995; Tsoulfas et al., 1996). Because the TEL–TRKC fusion is rare in AML, it is not known whether all leukemias express this splice variant. However, the TEL–TRKC fusion reported in association with all fibrosarcoma cases reported to date includes TEL exons 1–5, and contains the 42 bp exon in the TRKC moiety, which decreases tyrosine kinase activity in the context of the native receptor, and impairs signal transduction mediated by SHC and PLCγ (Guiton et al., 1995).
Both TEL–TRKC variants are constitutively tyrosine phosphorylated, and transform Ba/F3 cells to IL-3-independent growth. Inactivation of the TRKC kinase by point mutation abrogates transformation in cultured hematopoietic cells and in the murine BMT assay. However, there are significant differences in the transformation properties of the two TEL–TRKC fusion variants. Ba/F3 and NIH 3T3 cells transformed with T/T(F) grow more slowly than those transformed with T/T(L). Furthermore, mice transplanted with T/T(L) rapidly develop a lethal MPD, whereas T/T(F) causes a longer latency pre-B-cell lymphoma involving the spine and paraspinal musculature.
TEL–TRKC mutants were prepared to determine which signal transduction pathways were activated by the two variants. The Y820 binding site for PLCγ on native TRKC (Knezevich et al., 1998a,b; Rubin et al., 1998; Eguchi et al., 1999) is retained in the TEL–TRKC fusion proteins. PLCγ was strongly tyrosine phosphorylated in Ba/F3 cells stably transduced with T/T(L), but not T/T(F). A T/T(L)Y820F mutant was prepared to determine whether the marked disparity in association and tyrosine phosphorylation of PLCγ between T/T(L) and T/T(F) might explain the differences in transforming properties of the variants. As expected, T/T(L)Y820F does not associate with, or tyrosine phosphorylate, PLCγ. However, the T/T(L)Y820F mutant was indistinguishable from the T/T(L) fusion in Ba/F3 transformation assays, and caused a lethal MPD in the murine BMT model. These data indicate that association and tyrosine phosphorylation of PLCγ are dispensable for transformation and the induction of MPD.
Neither of the TEL–TRKC fusion proteins activated Stat5. This result stands in contrast to all other tyrosine kinase fusions examined to date, including BCR–ABL, TEL–ABL, TEL–PDGFβR, TEL–JAK2 and HIP1–PDGFβR, each of which activates Stat5 (Ilaria and Van Etten, 1996; Okuda et al., 1996; Lacronique et al., 1997; Schwaller et al., 1998; Ilaria et al., 1999; Ross and Gilliland, 1999; Sternberg et al., 1999). Thus, TEL–TRKC-mediated transformation of Ba/F3 cells, and development of MPD in murine BMT assays, do not require activation of Stat5. Further analysis of transformation of TEL–TRKC in Stat5a/b-deficient backgrounds should allow for definitive understanding of the role, if any, of Stat5 in TEL–TRKC transformation.
PLCγ and Stat5 activation are not required for transformation. However, T/T(L), T/T(L)Y820F and T/T(F) all activate the MAP kinase pathway, suggesting that this pathway may be critical for the transformation. Activation of the MAP kinase cascade plays a central role in NGF-induced PC12 cell differentiation by TRKA, and expression of a constitutively activated form of MEK1 is sufficient to induce neurite outgrowth of PC12 cells (Cowley et al., 1994). Furthermore, expression of a dominant-negative form of MEK1 blocks NGF-induced outgrowth of neurites, indicating that activation of MEK1 is necessary for certain aspects of PC12 differentiation (Cowley et al., 1994). More recent evidence suggests that sustained activation of the RAS–MAPK pathway may not be sufficient for some physiological responses of PC12 cells or primary neurons to neurotrophic factors (reviewed in Kaplan and Miller, 1997). It is interesting to note in this context that although T/T(F) is an effective activator of the MAP kinase pathway, it is incapable of inducing an MPD in the murine BMT assay. These experiments demonstrate that activation of the MAP kinase pathway alone is not sufficient to cause a myeloproliferative syndrome. MAP kinase-specific inhibitors or dominant-negative MEK1 will be useful to address the necessity and sufficiency of MAP kinase activation in transformation.
In summary, these studies have provided insights into the molecular mechanisms of transformation mediated by TEL–TRKC in human acute myeloid leukemia, congenital fibrosarcoma and mesoblastic nephroma. The TEL–TRKC fusion variants are oncoproteins that do not require activation of PLCγ or Stat5 for a myeloproliferative phenotype. Furthermore, although the TEL–TRKC variants activate the MAP kinase pathway, activation of additional pathways is required for efficient induction of MPD in the murine BMT model.
Materials and methods
Cell lines
Murine Ba/F3 cells (a gift from A.D’Andrea, Dana Farber Cancer Institute, Boston, MA) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 0.5–1.0 ng/ml recombinant IL-3 (R&D Systems, Minneapolis, MN) in a 5% CO2 incubator at 37°C. NIH 3T3 cells were kept in Dulbecco's modified Eagle's medium (DMEM) with 10% newborn calf serum, and 293T cells in DMEM with 10% FBS.
DNA constructs
Human TRKC and TRKC(K14) cDNAs were obtained by PCR from a human brain cDNA library (Clontech, Palo Alto, CA) and subcloned into pGEM-T easy vector (Promega, Madison, WI). TEL–TRKC, TEL–TRKC delPNT, TEL–TRKC(K14) and TEL–TRKC(K14) delPNT were constructed by PCR using the unique BsgI site in TRKC and TRKC(K14) near the breakpoint. Site-directed mutagenesis was carried out using the GeneEditor in vitro site-directed mutagenesis system (Promega, Madison, WI). All the constructs were confirmed by DNA sequencing.
Retrovirus production and retroviral transduction of mammalian cell lines
For transduction of genes into Ba/F3 and NIH 3T3 cells, the cDNA constructs were subcloned into MSCVneo vector (kindly provided by R.Hawley, University of Toronto, Toronto, Canada). Retroviral stocks were generated by transient co-transfection of 293T cells with the MSCVneo constructs with packaging DNA using Superfect (Qiagen, Valencia, CA) as described previously. Ba/F3 or NIH 3T3 cells (1 × 106) were infected with retrovirus with the addition of 10 µg/ml polybrene. After 48 h, the Ba/F3 cells were split, washed three times in phosphate-buffered saline (PBS) and either placed in medium containing 1 mg/ml G418 for selection or in IL-3-free medium for assays of transformation. For transformation assays in NIH 3T3 cells, cells were split 48 h after retroviral transduction, and subjected to 0.5 mg/ml G418 selection for up to 2 weeks. Transformed clusters of cells were quantified with low-magnification microscopy. Transformation efficiency was calculated by dividing the number of transformed clusters by the viral titer (the number of G418-resistant colonies) and expressing the ratio as a percentage.
Immunoprecipitation, Western blotting, MAP kinase assays and EMSAs
Parental Ba/F3 cells and cells expressing fusion proteins were washed three times with ice-cold PBS, lysed in lysis buffer (10 mM Tris pH 7.5, 130 mM NaCl, 1% Triton X-100, 10 mM NaF, 10 mM sodium phosphate, 10 mM sodium pyrophosphate, 10 mM EDTA, 1 mM sodium vanadate and protease inhibitor cocktail tablets) and clarified by centrifugation. The protein concentration was measured using a Protein Assay Kit (Bio-Rad Laboratories, CA). Lysates (1 mg protein) were subjected to immunoprecipitation with the indicated antibodies. Western blotting was conducted with the indicated primary antibodies using the manufacturer’s protocols and horseradish peroxidase-linked secondary antibodies from Amersham Life Science, followed by visualization by ECL (Amersham, Arlington Heights, IL).
The p44/42 MAP Kinase Assay Kit (New England Biolabs, Beverly, MA) was used to measure p44/42 MAP kinase activity in the cell using Elk-1 protein as a substrate. Ba/F3 cells expressing TEL–TRKC fusion proteins were grown in medium without IL-3. Cells were treated with 90 µM PD98059 (BIOMOL Research Laboratories, Inc., Plymouth Meeting, PA) for 90 min, lysed, and the in vitro kinase assay was performed according to the manufacturer’s protocol. Elk-1 phosphorylation was detected using Phospho-Elk-1 (Ser383) antibody.
EMSAs were performed as described previously (Ilaria and Van Etten, 1996). In brief, nuclear extracts (6 µg) from control cells or cells expressing fusion proteins were incubated (20 min) with a 32P-labeled and purified double-stranded oligonucleotide probe recognized by activated Stat protein complexes [γ-interferon activated sequence (GAS) based on the FcγRI gene promoter]. For supershift analysis, anti-Stat antibodies (Santa Cruz, CA) were added. The DNA–protein complexes were resolved on 4% TAE–polyacrylamide gel and detected by autoradiography.
Murine bone marrow transplantation
For BMT, an MSCV variant (MSCV-EGFP, kindly provided by W.Pear, University of Pennsylvania, PA) containing an IRES–EGFP expression cassette was used. The retrovirus-containing supernatant was produced as described above. The viral titer was estimated as described previously (Limon et al., 1997) with minor modifications. Briefly, 2 × 106 Ba/F3 cells were infected with different volumes of viral supernatant (range 2–1000 µl). After 24 h, the cells were diluted 1:5. At 48 h post-transduction, the cells were collected and washed twice with PBS, and the percentage of infected cells was determined by counting EGFP-positive cells using flow cytometry, and plotting increasing volumes of supernatant against the percentage of EGFP-positive cells. The viral titer was calculated using the following formula:
viral titer = (Ba/F3 cell no. × % of EGFP-positive cells)/volume of supernatant (ml)
Viral titers of constructs used for murine BMT were T/T(L) = 0.80 × 106/ml, T/T(L)Y820F = 0.96 × 106/ml, T/T(F) = 1.00 × 106/ml, T/T(L)KI = 1.00 × 106/ml and T/T(F)KI = 0.86 × 106/ml. Primary murine bone marrow was infected as described previously (Schwaller et al., 1998). Briefly, 6- to 8-week-old male BALB/c mice (Taconic, Germantown, NY) were primed with 150 mg/kg 5-fluorouracil (5-Fu; Sigma, St Louis, MO) beginning 6 days prior to harvest. Two days before transplantation, donor mice were killed by CO2 asphyxiation followed by cervical dislocation. The femurs and tibias were removed, and the bone marrow was flushed with RPMI 1640 medium. After treatment with red blood lysis solution (150 mM NH4Cl, 0.1 mM EDTA, 10 mM KHCO3 pH 7.4) for 5 min, the cells were counted and incubated in 5 ml of transplant medium [RPMI 1640 medium supplemented with 6 U/ml recombinant murine IL-3 (Genzyme, Cambridge, MA), 5 U/ml recombinant murine stem cell factor (Genzyme, Cambridge, MA), 10 000 U/ml recombinant murine IL-6 (Peprotech), 20% FBS and 100 U/ml penicillin/streptomycin] in a Retronectin (Tanaka-Shuzo, Japan)-coated Petri dish. One milliliter of viral supernatant (viral titer range 0.80–1.00 × 106) was added to the dish, and after 24 h another 1 ml of viral supernatant as well as 1 ml of transplant medium were added again. After an additional 24 h, the cells were harvested, washed in PBS and injected (0.5 × 106 in 500 µl HBSS) into the tail vein of each previously sublethally irradiated (2 × 450 cGy) female syngeneic recipient mouse. The recipient mice were maintained in microisolator cages with acidified water and autoclaved chow.
Southern blot analysis
Genomic DNA was isolated from various murine tissues using a PUREGENE DNA isolation kit (Gentra Systems, Minneapolis, MN). Ten micrograms of DNA were digested with restriction enzymes, separated on a TBE–agarose gel and transferred to a nylon membrane (Hybond N+; Amersham, Arlington Heights, IL). The membranes were then hybridized to [32P]dCTP-labeled DNA probe at 65°C.
Histopathological analysis of murine tissues
Histopathological techniques were performed as described previously (Schwaller et al., 1998). Briefly, murine tissues were fixed for at least 24 h in 10% neutral buffered formalin and embedded in paraffin. Femurs were subjected to an additional decalcification step in RDO (Apex Engineering Products, Plainfield, IL) for 1–2 h prior to processing. Sections (3 µm) were deparaffinized and stained with hematoxylin and eosin.
Flow cytometric analysis
Single-cell suspensions of bone marrow, spleen and lymph nodes were prepared as described previously (Schwaller et al., 1998). Briefly, red blood cells were lysed in red blood lysis solution (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA pH 7.4) for 5 min at room temperature. The cells were washed in PBS with 0.1% NaN3 and 0.1% bovine serum albumin (staining buffer). To block non-specific Fc receptor-mediated binding, the cells were pre-incubated with supernatant from the 2.4G2 hybridoma line (anti-CD16/CD32; cell line obtained from the American Type Culture Collection, Rockville, MD) for 20 min on ice. Aliquots of 0.5–1.0 × 106 cells were stained for 20 min on ice with monoclonal antibodies to Gr-1, CD3, B220 (CD45R) or BP-1 (Ly-51) purchased from PharMingen (San Diego, CA). The antibody to B220 was directly conjugated to phycoerythrin, and antibodies to Gr-1, CD3 and BP-1 were purchased as biotin conjugates. The binding of these biotinylated primary antibodies was detected using allophycocyanin-conjugated streptavidin (Caltag Laboratories, Burlingame, CA). Cells were washed once in staining buffer and multicolor flow cytometric analysis was carried out with a FACSort cytometer (Becton-Dickinson) equipped for four-color analysis. A minimum of 10 000 events were acquired and the data were analyzed using CellQuest software (Version 3.1). The fluorescence of viable cells gated on the basis of forward and side scatter signals is presented as dot plots showing EGFP fluorescence (detected in the FL1 channel) on the horizontal axis and either phycoerythrin (detected in the FL2 channel) or allophycocyanin (detected in the FL4 channel) fluorescence on the vertical axis. The percentages of gated cells located in the upper right and upper left quadrants are indicated in those quadrants on the dot plot.
Acknowledgments
Acknowledgements
We gratefully acknowledge the administrative assistance of Francesca Garcia. We thank R.Segal for helpful discussions, as well as D.Sternberg, M.Tomasson, J.Franstve, W.J.Song and other members of the Gilliland laboratory. We thank R.A.Van Etten, T.R.Golub and S.L.Lessnick for critical review of this manuscript. This work was supported in part by NIH grants PO1 DK50654 and PO1 66996 and the MarJo Foundation (D.G.G.). J.S. was supported by the Leukemia Society of America. Q.L. is an Associate and D.G.G. an Associate Investigator of the Howard Hughes Medical Institute.
References
- Abe A., Emi,N., Tanimoto,M., Terasaki,H., Marunouchi,T. and Saito,H. (1997) Fusion of the platelet-derived growth factor receptor β to a novel gene CEV14 in acute myelogenous leukemia after clonal evolution. Blood, 90, 4271–4277. [PubMed] [Google Scholar]
- Berkemeier L.R., Winslow,J.W., Kaplan,D.R., Nikolics,K., Goeddel,D.V. and Rosenthal,A. (1991) Neurotrophin 5: a novel neurotrophic factor that activates trk and trkB. Neuron, 7, 857–866. [DOI] [PubMed] [Google Scholar]
- Carroll M., Tomasson,M.H., Barker,G.F., Golub,T.R. and Gilliland,D.G. (1996) The TEL/platelet-derived growth factor β receptor (PDGFβR) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGFβR kinase-dependent signaling pathways. Proc. Natl Acad. Sci. USA, 93, 14845–14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M., Ohno-Jones,S., Tamura,S., Buchdunger,E., Zimmermann,J., Lydon,N.B., Gilliland,D.G. and Druker,B.J. (1997) CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR–ABL, TEL–ABL and TEL–PDGFR fusion proteins. Blood, 90, 4947–4952. [PubMed] [Google Scholar]
- Cowley S., Paterson,H., Kemp,P. and Marshall,C.J. (1994) Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation. Cell, 77, 841–852. [DOI] [PubMed] [Google Scholar]
- Daley G.Q., McLaughlin,J., Witte,O.N. and Baltimore,D. (1987) The CML-specific P210 bcr/abl protein, unlike v-abl, does not transform NIH/3T3 fibroblasts. Science, 237, 532–535. [DOI] [PubMed] [Google Scholar]
- Daley G.Q., Van Etten,R.A. and Baltimore,D. (1990) Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science, 247, 824–830. [DOI] [PubMed] [Google Scholar]
- Eguchi M. et al. (1999) Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25). Blood, 93, 1355–1363. [PubMed] [Google Scholar]
- Golub T.R., Barker,G.F., Lovett,M. and Gilliland,D.G. (1994) Fusion of PDGF receptor β to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell, 77, 307–316. [DOI] [PubMed] [Google Scholar]
- Golub T.R., Goga,A., Barker,G., Afar,D., McLaughlin,J., Bohlander,S., Rowley,J., Witte,O. and Gilliland,D.G. (1996) Oligomerization of the ABL tyrosine kinase by the ETS protein TEL in human leukemia. Mol. Cell. Biol., 16, 4107–4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub T.R., Barker,G.F., Stegmaier,K. and Gilliland,D.G. (1997) The TEL gene contributes to the pathogenesis of myeloid and lymphoid malignancies by diverse molecular genetic mechanisms. In Rauscher,F.J.I. and Vogt,P.K. (eds), Current Topics in Microbiology and Immunology. Springer, New York, NY, pp. 67–79. [DOI] [PubMed] [Google Scholar]
- Guiton M., Gunn-Moore,F.J., Glass,D.J., Geis,D.R., Yancopoulos,G.D. and Tavare,J.M. (1995) Naturally occurring tyrosine kinase inserts block high affinity binding of phospholipase C γ and Shc to TrkC and neurotrophin-3 signaling. J. Biol. Chem., 270, 20384–20390. [DOI] [PubMed] [Google Scholar]
- Ilaria R.L.J. and Van Etten,R.A. (1996) P210 and P190 (BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J. Biol. Chem., 271, 31704–31710. [DOI] [PubMed] [Google Scholar]
- Ilaria R.L.J., Hawley,R.G. and Van Etten,R.A. (1999) Dominant negative mutants implicate STAT5 in myeloid cell proliferation and neutrophil differentiation. Blood, 93, 4154–4166. [PubMed] [Google Scholar]
- Jing S., Tapley,P. and Barbacid,M. (1992) Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron, 9, 1067–1079. [DOI] [PubMed] [Google Scholar]
- Jousset C. et al. (1997) A domain of TEL conserved in a subset of ETS proteins defines a specific oligomerization interface essential to the mitogenic properties of the TEL-PDGFR β oncoprotein. EMBO J., 16, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadin M.E. and Morris,S.W. (1998) The t(2;5) in human lymphomas. Leuk. Lymphoma, 29, 249–256. [DOI] [PubMed] [Google Scholar]
- Kaplan D.R. and Miller,F.D. (1997) Signal transduction by the neurotrophin receptors. Curr. Opin. Cell Biol., 9, 213–221. [DOI] [PubMed] [Google Scholar]
- Kaplan D.R., Hempstead,B.L., Martin-Zanca,D., Caho,M.V. and Parada,L.F. (1991) The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science, 252, 554–558. [DOI] [PubMed] [Google Scholar]
- Kim J. et al. (1999) Activation of neurotrophin-3 receptor TrkC induces apoptosis in medulloblastomas. Cancer Res., 59, 711–719. [PubMed] [Google Scholar]
- Klein R. et al. (1991) The trkB tyrosine kinase is a receptor for grain-derived neurotrophic factor and neurotrophin-3. Cell, 66, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Silos-Santiago,I., Smeyne,R.J., Lira,S.A., Brambilla,R., Bryant,S., Zhang,L., Snider,W.D. and Barbacid,M. (1994) Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature, 368, 249–251. [DOI] [PubMed] [Google Scholar]
- Knezevich S.R., Garnett,M.J., Pysher,T.J., Beckwith,J.B., Grundy,P.E. and Sorensen,P.H. (1998a) ETV6–NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res., 58, 5046–5048. [PubMed] [Google Scholar]
- Knezevich S.R., McFadden,D.E., Tao,W., Lim,J.F. and Sorensen,P.H. (1998b) A novel ETV6–NTRK3 gene fusion in congenital fibrosarcoma. Nature Genet., 18, 184–187. [DOI] [PubMed] [Google Scholar]
- Kuefer M.U., Look,A.T., Pulford,K., Behm,F.G., Pattengale,P.K., Mason,D.Y. and Morris,S.W. (1997) Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood, 90, 2901–2910. [PubMed] [Google Scholar]
- Lacronique V. et al. (1997) A TEL–JAK2 fusion protein with constitutive kinase activity in human leukemia. Science, 278, 1309–1312. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Klein,R. and Barbacid,M. (1991) trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell, 66, 967–979. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Tapley,P. and Barbacid,M. (1993) trkC encodes multiple neurotrophin-3 receptors with distinct biological properties and substrate specificities. EMBO J., 12, 3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G.R. and Barde,Y.A. (1996) Physiology of the neurotrophins. Annu. Rev. Neurosci., 19, 289–317. [DOI] [PubMed] [Google Scholar]
- Limon A. et al. (1997) High-titer retroviral vectors containing the enhanced green fluorescent protein gene for efficient expression in hematopoietic cells. Blood, 90, 3316–3321. [PubMed] [Google Scholar]
- Lugo T.G. and Witte,O.N. (1989) The BCR-ABL oncogene transforms Rat-1 cells and cooperates with v-myc. Mol. Cell. Biol., 9, 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.W., Kirstein,M.N., Valentine,M.B., Dittmer,K.G., Shapiro,D.N., Saltman,D.L. and Look,A.T. (1994) Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science, 263, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Obermeier A., Halfter,H., Wiesmuller,K.H., Jung,G., Schlessinger,J. and Ullrich,A. (1993) Tyrosine 785 is a major determinant of Trk–substrate interaction. EMBO J., 12, 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier A., Bradshaw,R.A., Seedorf,K., Choidas,A., Schlessinger,J. and Ullrich,A. (1994) Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC γ. EMBO J., 13, 1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Golub,T.R., Gilliland,D.G. and Griffin,J.D. (1996) p210BCR/ABL, p190BCR/ABL and TEL/ABL activate similar signal transduction pathways in hematopoietic cell lines. Oncogene, 13, 1147–1152. [PubMed] [Google Scholar]
- Papadopoulos P., Ridge,S.A., Boucher,C.A., Stocking,C. and Wiedemann,L.M. (1995) The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res., 55, 34–38. [PubMed] [Google Scholar]
- Peeters P. et al. (1997) Fusion of TEL, the ETS-variant gene 6 (ETV6), to the receptor-associated kinase JAK2 as a result of t(9;12) in a lymphoid and t(9;15;12) in a myeloid leukemia. Blood, 90, 2535–2540. [PubMed] [Google Scholar]
- Pendergast A.M., Quilliam,L.A., Cripe,L.D., Bessing,C.H., Dai,Z., Li,N., Der,C.J., Sclessinger,J. and Gishizky,M.L. (1993) BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adapter protein. Cell, 75, 175–185. [PubMed] [Google Scholar]
- Puil L., Liu,J., Gish,G., Mbamalu,G., Bowtell,D., Pelicci,P.G., Arlinghaus,R. and Pawson,T. (1994) Bcr-Abl oncoproteins bind directly to activators of the Ras signaling pathway. EMBO J., 13, 764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter A. et al. (1998) Consistent fusion of ZNF198 to the fibroblast growth factor receptor-1 in the t(8;13)(p11;q12) myeloproliferative syndrome. Blood, 92, 1735–1742. [PubMed] [Google Scholar]
- Ross J.S. and Fletcher,J.A. (1999) HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am. J. Clin. Pathol. Suppl. 1, 112, S53–S67. [PubMed] [Google Scholar]
- Ross T.S. and Gilliland,D.G. (1999) Transforming properties of the Huntingtin interacting protein 1/platelet-derived growth factor β receptor fusion protein. J. Biol. Chem., 274, 22328–22336. [DOI] [PubMed] [Google Scholar]
- Ross T.S., Bernard,O.A., Berger,R. and Gilliland,D.G. (1998) Fusion of Huntingtin interacting protein 1 to PDGFβR in chronic myelo monocytic leukemia with t(5;7)(q33;q11.2). Blood, 91, 4419–4426. [PubMed] [Google Scholar]
- Rubin B.P., Chen,C.J., Morgan,T.W., Xiao,S., Grier,H.E., Kozakewich,H.P., Perez-Atayde,A.R. and Fletcher,J.A. (1998) Congenital mesoblastic nephroma t(12;15) is associated with ETV–NTRK3 gene fusion: cytogenetic and molecular relationship to congenital (infantile) fibrosarcoma. Am. J. Pathol., 153, 1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaller J. et al. (1998) Transformation of hematopoietic cell lines to growth-factor independence and induction of a fatal myeloid and lymphoproliferative disease in mice by retrovirally transduced TEL/JAK2 fusion gene. EMBO J., 17, 5321–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal R.A. and Greenberg,M.E. (1996) Intracellular signaling pathways activated by neurotrophic factors. Annu. Rev. Neurosci., 19, 463–489. [DOI] [PubMed] [Google Scholar]
- Stephens R.M., Loeb,D.M., Copeland,T.D., Pawson,T., Greene,L.A. and Kaplan,D.R. (1994) Trk receptors use redundant signal transduction pathways involving SHC and PLC-γ 1 to mediate NGF responses. Neuron, 12, 691–705. [DOI] [PubMed] [Google Scholar]
- Sternberg D.W., Tomasson,M.H., Carroll,M., Kazlauskas,A. and Gilliland,D.G. (1999) Phosphorylation sites in the TEL/PDGFβR fusion protein that are required for STAT5 activation are essential for a lethal myeloproliferative disease in a murine BMT model. Blood Suppl. 1, 94, 389a. [Google Scholar]
- Tsoulfas P., Stephens,R.M., Kaplan,D.R. and Parada,L.F. (1996) TrkC isoforms with inserts in the kinase domain show impaired signaling responses. J. Biol. Chem., 271, 5691–5697. [DOI] [PubMed] [Google Scholar]
- Valenzuela D.M. et al. (1993) Alternative forms of rat TrkC with different functional capabilities. Neuron, 10, 963–974. [DOI] [PubMed] [Google Scholar]
- Witte O.N. (1993) Role of the BCR–ABL oncogene in human leukemia: fifteenth Richard and Hinda Rosenthal Foundation Award lecture. Cancer Res., 53, 485–497. [PubMed] [Google Scholar]
- Xiao S. et al. (1998) FGFR1 is fused with a novel zinc-finger gene, ZNF198, in the t (8;13) leukaemia/lymphoma syndrome. Nature Genet., 18, 84–87. [DOI] [PubMed] [Google Scholar]