Abstract
Two immunologically-different mouse strains, C57BL/6 and SNF1, were exposed to a mid-gestation dose of TCDD. The C57BL/6 mouse has a high-affinity aryl hydrocarbon receptor (AhR) and is sensitive to TCDD. The SNF1 mouse has a low-affinity AhR but spontaneously develops autoimmune nephritis. Autoreactive Vβ+CD4+17a and Vβ+CD3+ T cells were increased at 24-weeks-of-age in offspring of C57BL/6 mice, more so in females than males. The cytokine IFN-γ was elevated in the females, while IL-10 was elevated in males. Phenotypic changes in B-lineage cells were present in bone marrow and spleen, and circulating autoantibodies were increased after prenatal TCDD. Kidneys of males showed significant anti-IgG and anti-C3 deposition, suggesting early-stage autoimmune disease. The SNF1 offspring similarly showed increased peripheral Vβ+ cells in the females, increased autoantibody production in both sexes, and increased IFN-γ production in females. Male SNF1 mice had increased anti-IgG and anti-C3 deposition in kidneys. Both mouse models therefore showed clear signatures of enhanced autoimmunity after prenatal TCDD.
Keywords: TCDD, autoimmunity, developmental, C57BL/6, SNF1, lupus nephritis
1.1 Introduction
Reports evaluating the developmental immunotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin; TCDD) began appearing in the literature about 1977, when perinatal exposure of mice was found to produce a more severe thymic atrophy than a similar exposure in the adult [1]. Subsequent studies in mice described transient postnatal T-lineage phenotypic changes and suppressed cytotoxic T lymphocyte responses, which were reported to resolve by 8–10 weeks after birth [2,3]. Gehrs and Smialowicz [4] later reported permanent suppression of the delayed-type hypersensitivity (DTH) response in rats after prenatal exposure to TCDD. In the latter studies, F344 rats were dosed on gestation day 14 with 0.1 µg/kg TCDD, and the male offspring displayed significant inhibition of DTH responses as late as 14 months of age, the last endpoint examined. The female offspring also showed depressed DTH responses at 14 months, but were not as sensitive as males, requiring a 3-fold higher (0.3 µg/kg) maternal TCDD dose.
Beyond immune suppression, a progression of reports in the literature suggests TCDD may increase risk of autoimmune responses by altering T cell selection. Greenlee et al. [5] and Schuurman et al. [6] described thymic epithelial targeting by TCDD, which led both to propose that TCDD may have potential to alter epithelium-dependent deletion of autoreactive thymocytes. DeWaal et al. [7] observed altered thymic epithelial distribution of major histocompatability complex (MHC) class II molecules in TCDD-treated mice, leading these authors to also suggest possible altered thymocyte deletion. Fisher et al. [8] later reported enhanced thymic negative selection of T cells and increased autoreactive T cell release from the thymus of mice treated with TCDD. Given these observations, it may be noteworthy that similar patterns of inhibited thymic T cell differentiation occur spontaneously in some autoimmune mouse models, in mice treated in vivo with monoclonal antibodies to the thymic MHC class I and class II molecules that govern selection, and in TCDD-treated mice [9]. We therefore hypothesized that prenatal exposure of mice to TCDD may, in addition to selective immunosuppression, produce immune modulation suggestive of enhanced autoimmunity.
1.2 C57BL/6 and SNF1 mouse studies
The present studies used two immunologically different mouse models in an attempt to mimic humans who may display both a high susceptibility to TCDD, and a genetic predisposition for developing a lupus-like autoimmune disease. The C57BL/6 mouse has a high affinity aryl hydrocarbon receptor (AhR), and as such is highly sensitive to TCDD and related ligands of the AhR. The SNF1 mouse has a low affinity AhR (Ahdd), making it less sensitive to TCDD, but spontaneously develops lupus-like autoimmune nephritis. Thus, if C57 mice showed an enhanced autoimmune profile at low doses of TCDD, and SNF1 mice did the same at higher doses of TCDD, this outcome would suggest an individual who has both a high-affinity AhR and genetic programming toward autoimmune disease, may be at higher risk for immune modulation than an individual showing only one of these predispositions.
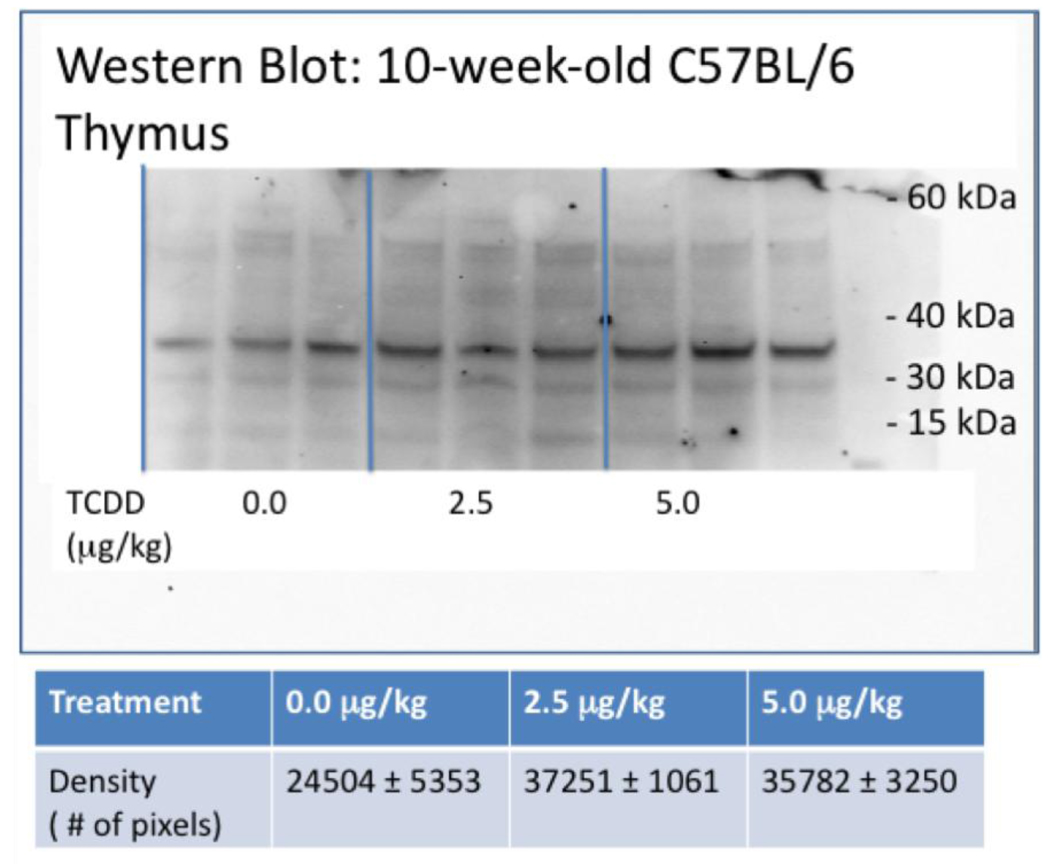
The thymus was collected from C57BL/6 mice that were prenatally-exposed to 0, 2.5 or 5.0 mg/kg TCDD, and screened MHC class II antigen protein expression. This analysis was performed by Western blot using an affinity-purified anti-mouse MHC class II antibody tagged with a secondary HRP goat anti-rat IgG. The MHC antigen expression numerically and visibly increased in the mice that received developmental TCDD exposure (Fig. 1). Western blot analyses of protein from TCDD-treated thymic stromal tissue at 10, 24 and 36 weeks yielded the same trend toward increasing expression of MHC Class II (data not shown). These data contribute to the above reports suggesting the possibility of altered thymic selection in mice developmentally exposed to TCDD.
Figure 1.
Representative western blot of the thymus. Thymic stromal tissue was collected from 10-week-old C57BL/6 mice prenatally exposed to 0.0, 2.5 or 5.0 µg/ml TCDD, and stored at −80°C in RNAlater. Protein was isolated from each tissue, quantitated and normalized across all samples. The protein samples were separated on a NuPage Novex Bis-Tris mini gel, blot transferred, probed with an affinity-purified anti-mouse MHC class II antibody and tagged by a secondary HRP goat anti-rat IgG. (n=3 mice/treatment)
The relative percent expression of specific autoreactive T cell clones was next examined in the spleen and thymus of the study mice, to detect possible altered T cell selection. The autoreactive phenotypes chosen for evaluation were CD4+ 17a variable beta (Vβ) and CD3+ Vβ T cells. Silverstone et al. [10] previously found increased numbers of these T cell phenotypes in the livers of young adult mice dosed with TCDD, which was the basis for the present selection. Table 1 shows that both Vβ phenotypes were increased in the periphery of adult C57BL/6 mice that were prenatally exposed to TCDD. These data exhibit a dose-response pattern almost in every case, with the females being more affected than males. There was as much as a 4-fold increase of autoreactive cells in the spleen of treated female mice. This could be an important observation, in that a small number of these cells may be required to manifest an autoimmune response, if activated. The female tendency for increased autoreactive T cells is in contrast to the reduced male response in DTH activity, reported by Gehrs and Smialowicz [4], but is consistent with the general increased tendency of females to express autoimmune diseases.
Table 1.
Variable-beta positive T cells in the spleen and lymph nodes of C57BL/6 mice following prenatal exposure to TCDD. Modified from Mustafa et al., 2008 [23].
| Splenic T cells | |||
|---|---|---|---|
| 0 µg/kg | 2.5 µg/kg | 5.0 µg/kg | |
| Vβ17a+ (%) | ♀ 2.76±0.54 ♂3.67±0.58 |
5.44±1.25 4.82±0.48 |
7.83±1.94* 4.76±0.29 |
| Vβ3+ (%) | ♀2.02±0.50 ♂2.92±0.71 |
4.56±0.73 5.02±0.96 |
10.08±2.49* 5.32±0.54 |
| Lymph node T cells | |||
| Vβ17a+ (%) | ♀6.05±0.28 ♂3.55±0.28 |
7.25±0.90 4.93±1.02 |
8.73±0.84* 5.35±0.54 |
| Vβ3+ (%) | ♀5.07±0.40 ♂5.23±0.41 |
6.35±1.04 6.35±1.04 |
8.24±1.23* 8.08±1.03* |
Data are mean±SE for N=5 mice/treatment/sex;
p≤0.05, Dunnett’s t-test
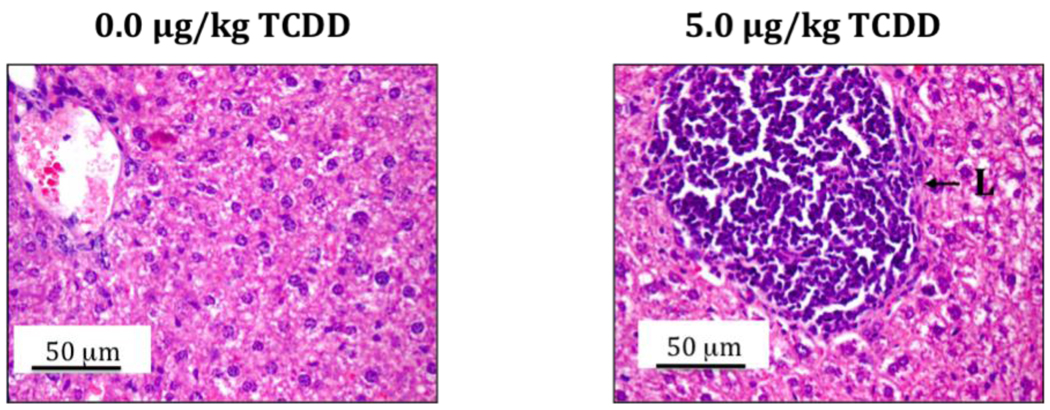
An important question that remains to be answered is the origin of increased peripheral Vβ+ T cells in the 24-week-old study mice that were prenatally exposed to TCDD. Given the above-described effects of TCDD on thymic epithelium and MHC antigen expression, a persistent effect on thymocyte negative selection may exist and result in elevated peripheral Vβ+ cells. Alternately, Silverstone et al. [10] hypothesized that TCDD may drive T cell development, including negative selection, into extrathymic compartments where deletion of autoreactive cells is inefficient. With this in mind, routine histopathology in the present C57BL/6 mice detected atypical centers of lymphocytic infiltration in the livers at adulthood (Fig. 2). It is not yet known if these centers may include extrathymic T lymphopoiesis.
Figure 2.
The livers from 24-week-old C57BL/6 mice that were prenatally exposed to 0.0 or 5.0 µg/kg TCDD were collected, fixed, sectioned and H&E stained. The right image shows a representative lymphocytic center of infiltration (L) in the TCDD group.
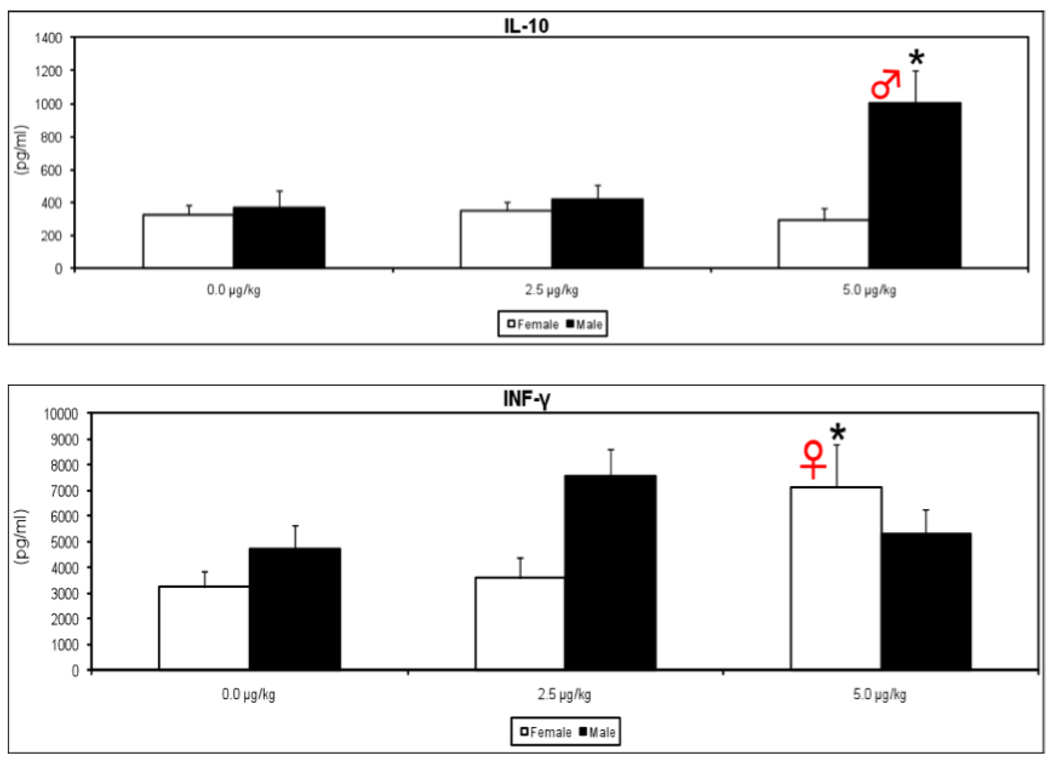
The presence of phenotypic differences in T cells at 24 weeks in the prenatal TCDD-exposed mice suggested possible co-existing functional differences in these cells. Also, a skewed Th1/Th2 cytokine balance has been associated with pathology in several autoimmune disorders. Therefore select cytokines were examined in Con A-activated splenic T cells from prenatally exposed mice. Interferon-gamma (IFN-γ) was increased in the adult female offspring but not in the males (Fig. 3), suggesting a sex-dependent skewing toward Th1 activity. Increased IFN-γ has been linked to disease development in several models of autoimmunity, including lupus nephritis, autoimmune insulitis, Sjogren’s syndrome, and autoimmune arthritis [11–13].
Figure 3.
Supernatants were collected from splenocytes of 48-week-old mice that were prenatally exposed to 0.0, 2.5 or 5.0 µg/kg TCDD, after culturing for 48h with Con A (10 µg/mL). The levels of IL-10 and INF-γ were determined using a commercially-available murine cytokine ELISA kit. (n=5 mice/treatment/gender, *p ≤ 0.05, Dunnett's t-test).
In the male but not female mice, interleukin-10 (IL-10) was also elevated at 24-weeks-of-age (Fig. 3). This cytokine has been considered the hallmark systemic lupus erythematosus (SLE) marker; serum levels of IL-10 are elevated in SLE patients and increased IL-10 correlates well with disease activity [14]. These IFN-γ and IL-10 data infer that a permanent dysregulation of cytokine production was imprinted into T cells by the prenatal TCDD, in a direction that indicates increased risk of disease development.
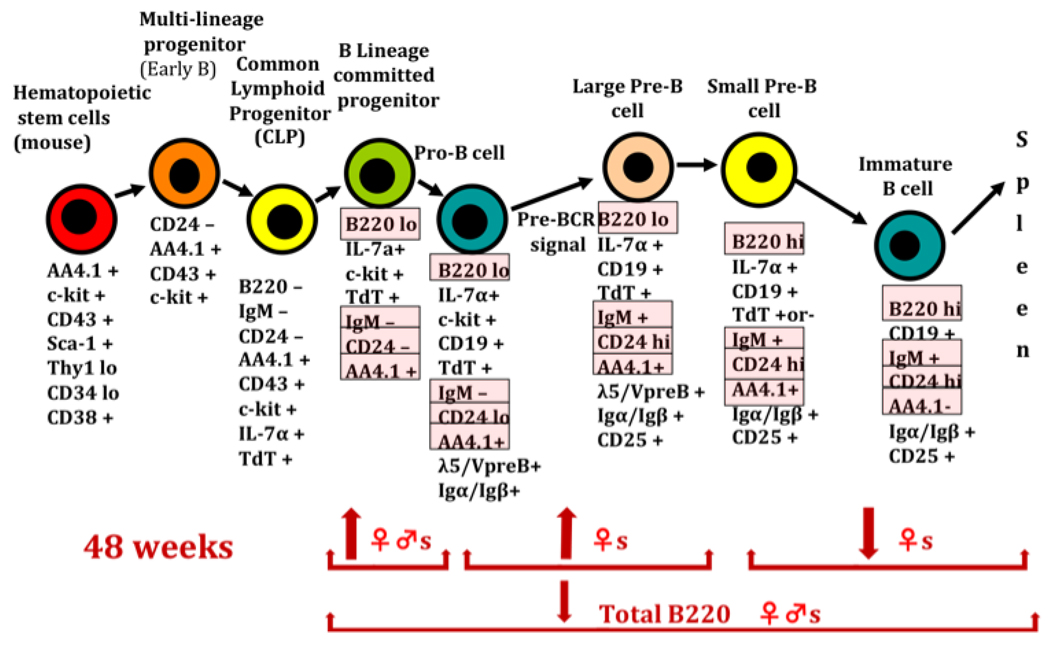
Very limited information is available in the literature regarding transient or persistent B cell effects from prenatal exposure to dioxin. However, B cells may contribute to autoimmunity by several mechanisms, including autoantibody production, altered antigen presentation, or modulation of other immune cells through inappropriate cytokine production. Four surface markers, B220/CD45R, IgM, CD24, and AA4.1, were selected to provide a preliminary view of B lymphopoiesis in the adult bone marrow. When combined these 4 surface markers allow for the characterization of the multi-lineage and common lymphoid progenitor cells (B220−, CD24−, IgM−, AA4.1+), the B lineage progenitor and Pro B Cells (B220lo, CD24lo, IgM−, AA4.1+), large Pre-B cells (B220lo, CD24hi, IgM+, AA4.1+) small Pre-B cells (B220hi, CD24hi, IgM+, AA4.1+) and immature B cells (B220hi, CD24hi, IgM+, AA4.1−) [23–24]. In females, prenatal TCDD significantly increased B lineage committed progenitor cells, Pro-B cells, and large Pre-B cells, while decreasing the more mature small Pre-B and immature B cell populations. Males were less affected, showing an increase only in B lineage committed progenitors (Fig. 4). These data suggest a permanent change in immature B cell development, including possible inhibited differentiation of these cells toward more mature phenotypes. This profile of effect has some similarities to that seen with T cells, where bone marrow T progenitors (prothymocytes) are decreased [15] and thymic T precursors (thymocytes) are both diminished and display inhibited differentiation [16]. Similar to T cells, critical steps in B cell selection and receptor editing occur during these stages of differentiation, including deletion of lambda or kappa light chains from the B cell receptor [17].
Figure 4.
B-cell lymphopoiesis in the bone marrow. The cell-surface markers highlighted under the specific cell types were the ones evaluated in this study. Arrows pointing up indicate significantly increased, and arrows pointing down indicate significantly decreased (n=5 mice/treatment/gender, *p ≤ 0.05, Dunnett's t-test). Modified from Mustafa et al., 2008 [23].
Adult B cell differentiation in the spleen was also altered by prenatal TCDD. Females displayed increased transitional 1 (T1) B cells, while in males, T2 B cells were increased. T1 cells represent recent immigrants from the bone marrow, are believed to be direct precursors of T2 B cells, and are commonly increased in SLE patients [18]. Both sexes also displayed enhanced shifting of B cells to the marginal zone (MZ), identified as B220hi, IgDlo, CD23−, CD21hi, and CD1dhi. This splenic phenotype is characterized by a lower activation threshold, and is enhanced in murine models of SLE [19].
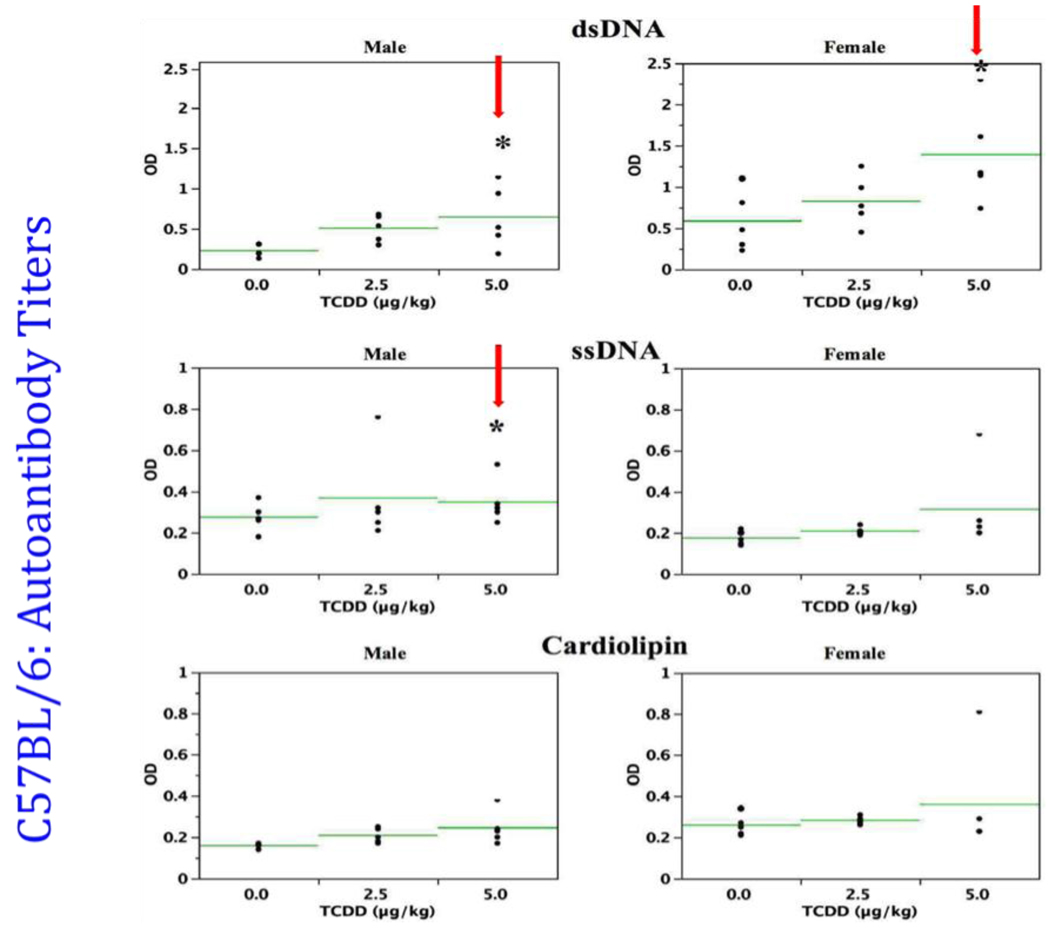
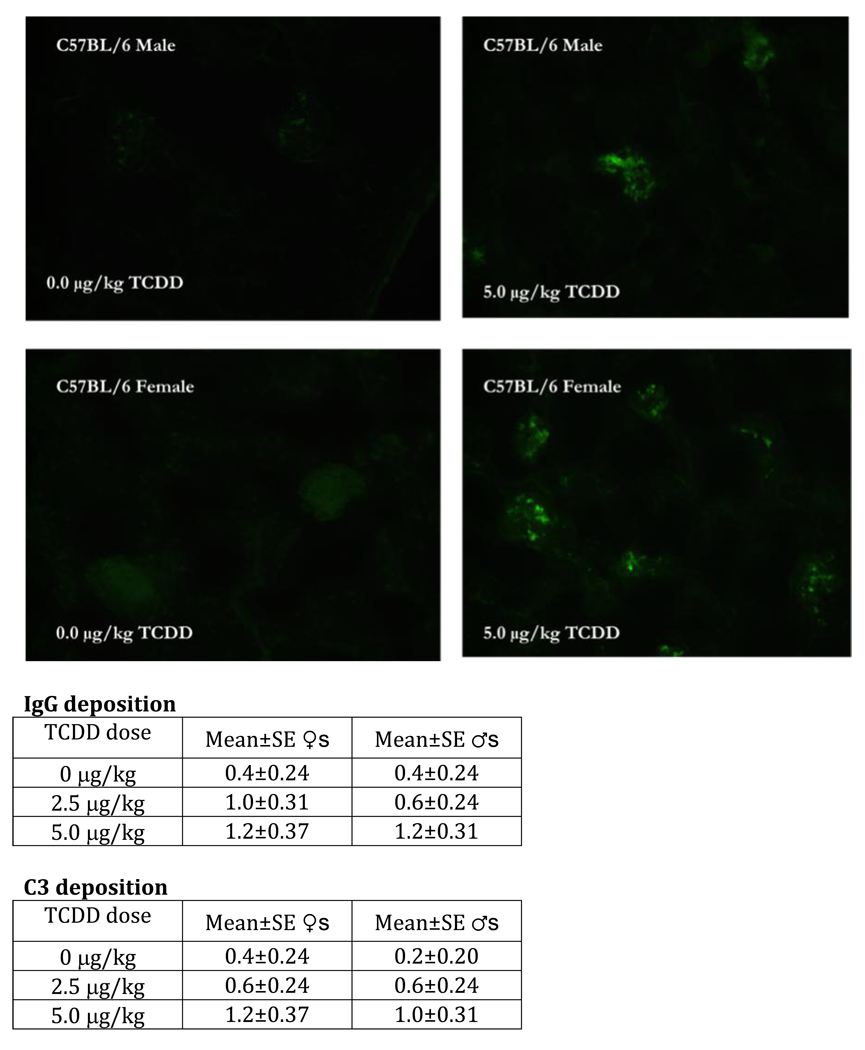
Thus, several changes in B cells, after prenatal TCDD, suggested possible enhanced self-directed activity in these cells. We therefore examined the profile of autoantibody production in the 24-week-old mice. In all cases these mice either showed trends toward higher serum autoantibodies against double stranded DNA, single stranded DNA, and cardiolipin, or produced significantly higher levels (Fig. 5). Since immune complex deposition in the kidney is a common signalment in mouse lupus models, immunofluorescence staining to elucidate IgG and C3 deposition was next performed. Female and male mice that received prenatal TCDD showed increasing, dose-related trends for both autoantibody IgG and C3 immune complex deposition at 24-weeks-of-age (Fig. 6). By 48-weeks-of-age, IgG and C3 immune complex deposition were significantly increased in the male offspring, at both the 2.5 and 5.0 µg/kg prenatal TCDD doses (unpublished data). Such deposition is not a normal observation in C57BL/6 mice, and is visual evidence of initiation of autoimmunity. In particular, autoantibodies produced by B cells tend to display strong polyreactivity to DNA and glomerular substrate, and are frequently localized as immune deposits in kidneys [20].
Figure 5.
Sera from 24-week-old C57BL/6 mice that were prenatally exposed to 0.0, 2.5 and 5.0 µg/kg TCDD were analyzed for the presence of autoantibodies to dsDNA (A), ssDNA (B) and cardiolipin (C), (n=5/gender; *p ≤ 0.05, Dunnett's t-test). Each horizontal line represents the arithmetic mean for each treatment group. Modified from Mustafa et al., 2008 [23].
Figure 6.
The kidneys from 24-week-old C57BL/6 mice that were prenatally exposed to 0.0 or 5.0 µg/kg TCDD were collected, fixed, section and stained with FITC-labeled anti-IgG. The above figures are representative of kidneys stained with FITC-anti-IgG from control female (bottom left) and male (top left) or 5.0 µg/kg TCDD-exposed female (bottom right) and male (top right) mice. The table data show the mean±SEM of the IgG and C3 disposition scores of 5 mice/treatment/gender. Modified from Mustafa et al., 2008 [23].
Therefore, C57BL/6 mice that received a single prenatal dose of TCDD displayed several signs of autoimmunity in adulthood. These included increased autoreactive Vβ T cells in the periphery, altered cytokine profile suggestive of enhanced immune reactivity, increased autoantibody production by B cells, and signs of an immune-mediated autoimmune disease or glomerulonephritis in a murine strain not normally known to express this condition. Given these observations, prenatal TCDD exposure was next evaluated in SNF1 hybrid mice.
1.3 Prenatal TCDD exposure in autoimmune SNF1 mice
The incidence of nephritis is low in autoimmune NZB mice, but when this strain is crossed with normal SWR mice, the female SNF1 hybrids develop glomerulonephritis that is fatal by about 8 months of age. Onset of clinical disease is much slower in the male offspring, beginning at about 1 year of age. The progression of disease in both sexes is then critically dependent on accelerated antibody production subsequent to inappropriate activity of CD4+ T helper cells, and histopathologically characterized by IgG deposition in kidney glomeruli [21]. This genetic predisposition to a disease that showed signs of being induced in non-autoimmune C57BL/6 mice after prenatal TCDD, made the SNF1 model of value for parallel developmental studies.
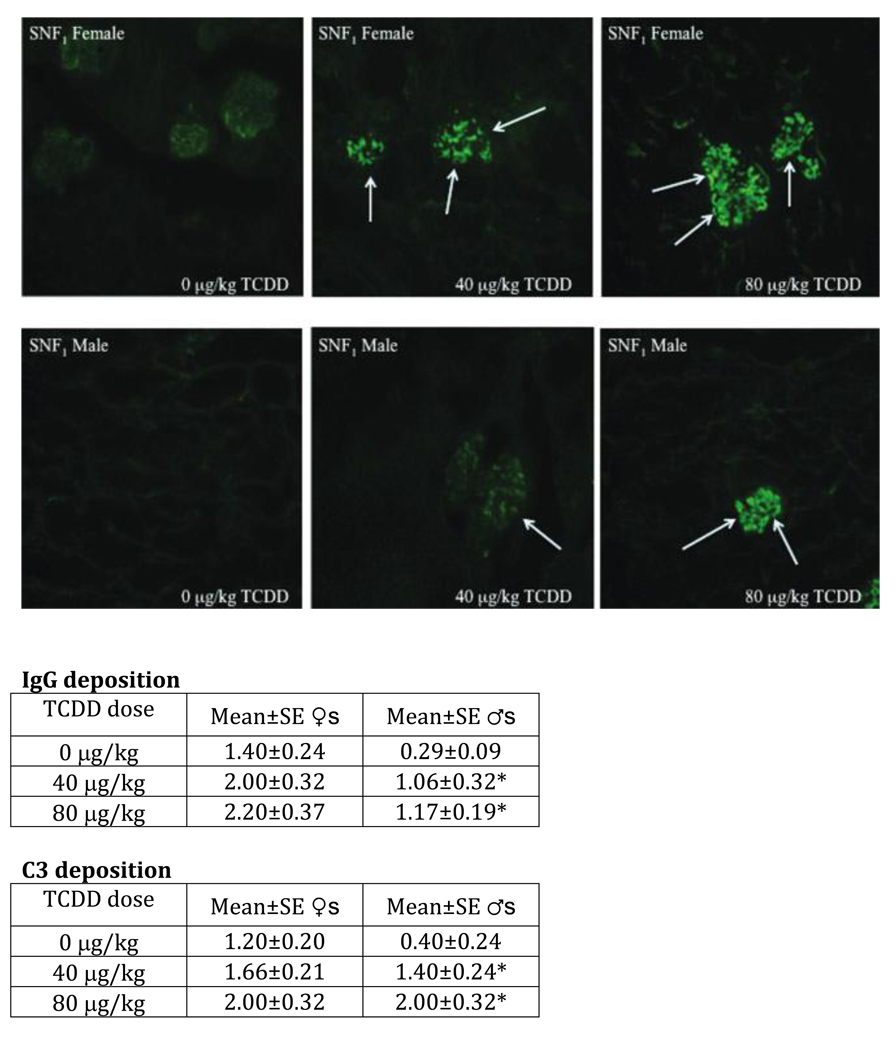
Using the same study design as for the C57BL/6 mice, pregnant female SWR × NZB:F1 (SNF1) mice were administered a single oral dose of 40 or 80 mg/kg TCDD on gestation day 12. The TCDD-exposed SNF1 offspring showed a number of noteworthy immune changes at 24 weeks of age, including increased peripheral Vβ+ T cells in the females, increased autoantibody production in both sexes, and increased IFN-γ production by T cells in females [22]. Histopathologically, the control female mice were in early stages of autoimmune nephritis, while control males showed almost no autoantibody IgG or C3 immune complex deposition, consistent with normal progression of disease in these mice (Fig. 7). The female mice showed a visual suggestion of increased deposition, and also numeric, but non-significant, increased deposition of both immune complexes. For males, both autoantibody IgG or C3 immune complex deposition showed dose-dependent and significant increases. These results suggest prenatal TCDD may exacerbate autoimmune nephritis in SNF1 lupus-like female mice, and induce early expression of disease in the males. This pattern of result was almost identical to that displayed by the C57BL/6 mice, where females showed trends toward increased nephritis and males showed significantly enhanced disease.
Figure 7.
Kidneys from 24-week-old SNF1 mice that were prenatally exposed to 0, 40, or 80 µg/kg TCDD were collected, fixed, sectioned, and stained with FITC-labeled anti-IgG or anti-C3. The above are representative images of kidneys stained with FITC-anti-IgG based on treatment and sex. Arrows indicate regions of heavy glomerular IgG deposition. The data are based on five mice per treatment per sex *p ≤ 0.05, Dunnett's t-test). Modified from Mustafa et al., 2009b [24].
1.4 Conclusions
As initially stated, the C57BL/6 and the SNF1 mouse models were selected to represent TCDD-sensitive and genetically autoimmune segments of the human population, respectively. Both models showed clear signatures of enhanced autoimmunity after prenatal TCDD. This outcome suggests that prenatal exposure to TCDD or other environmental AhR ligand, during development of the immune system, may elevate risk of autoimmune responses in individuals who express the combination of a high-affinity AhR and a genetic predisposition for autoimmune disease.
Acknowledgement
Supported by NIHR21-PAR-03-121
Abbreviations
- AhR
aryl hydrocarbon receptor
- DTH
delayed-type hypersensitivity
- IFN-γ
interferon-gamma
- IL
interleukin
- MHC
major histocompatability complex
- SLE
systemic lupus erythematosus
- T1
transitional 1
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- Th
T helper
- Vβ
variable beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faith RE, Moore JA. Impairment of thymus-dependent immune functions by exposure of the developing immune system to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Toxicol Envir Health. 1997;3:451–464. doi: 10.1080/15287397709529578. [DOI] [PubMed] [Google Scholar]
- 2.Fine JS, Gasiewicz T, Silverstone AE. Lymphocyte stem cell alterations following perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Pharmacol. 1989;35:18–28. [PubMed] [Google Scholar]
- 3.Holladay SD, Lindstrom P, Blaylock BL, Comment CE, Germolec DR, Heindel JJ, Luster MI. Perinatal thymocyte antigen expression and postnatal immune development altered by gestational exposure to tetrachlorodibenzo-p-dioxin (TCDD) Teratology. 1991;44:385–393. doi: 10.1002/tera.1420440405. [DOI] [PubMed] [Google Scholar]
- 4.Gehrs BC, Smialowicz RJ. Persistent suppression of delayed-type hypersensitivity in adult F344 rats after perinatal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. 1999;134:79–88. doi: 10.1016/s0300-483x(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee WF, Dold KM, Irons RD, Osborne R. Evidence for direct action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on thymic epithelium. Toxicol Appl Pharmacol. 1985;79:112–120. doi: 10.1016/0041-008x(85)90373-4. [DOI] [PubMed] [Google Scholar]
- 6.Schuurman H-J, Van Loveren H, Rozing J, Vos JG. Chemicals trophic for the thymus: Risk for immunodeficiency and autoimmunity. Int J Immunopharm. 1992;14:369–375. doi: 10.1016/0192-0561(92)90166-i. [DOI] [PubMed] [Google Scholar]
- 7.DeWaal EJ, Schuurman H-J, Loeber JG, Van Loveren H, Vos JG. Alterations in the cortical thymic epithelium of rats after in vivo exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): An (immuno)histological study. Toxicol Appl Pharmacol. 1992;115:80–88. doi: 10.1016/0041-008x(92)90370-8. [DOI] [PubMed] [Google Scholar]
- 8.Fisher MT, Nagarkatti M, Nagarkatti PS. 2,3,7,8-tetrachlorodibenzo-p-dioxin enhances negative selection of T cells in the thymus but allows autoreactive T cells to escape deletion and migrate to the periphery. Mol Pharmacol. 2005;67:327–335. doi: 10.1124/mol.104.005868. [DOI] [PubMed] [Google Scholar]
- 9.Blaylock BL, Ahmed SA, Holladay SD. “Perinatal immunotoxicant exposure and postnatal autoimmune disease.”. In: Holladay SD, editor. Developmental Immunotoxicology. New York: CRC Press; 2005. pp. 215–228. [Google Scholar]
- 10.Silverstone AE, Frazier DE, Jr, Gasiewicz TA. Alternate immune system targets for TCDD: lymphocyte stem cells and extrathymic T-cell development. Exp Clin Immunogenet. 1994;11:94–101. doi: 10.1159/000424198. [DOI] [PubMed] [Google Scholar]
- 11.Meyer O. Interferons and autoimmune disorders. Joint Bone Spine. 2009;76:464–473. doi: 10.1016/j.jbspin.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F, Marchetti P, Dotta F. Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol. 2010;159:338–343. doi: 10.1111/j.1365-2249.2009.04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimohata H, Yamada A, Yoh K, Ishizaki K, Morito N, Yamagata K, Takahashi S. Overexpression of T-bet in T cells accelerates autoimmune glomerulonephritis in mice with a dominant Th1 background. J Nephrol. 2009;22:123–129. [PubMed] [Google Scholar]
- 14.Wang Y, Ito S, Chino Y, Goto D, Matsumoto I, Murata H, Tsutsumi A, Hayashi T, Uchida K, Usui J, Yamagata K, Sumida T. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clin Exp Immunol. 2010;159:1–10. doi: 10.1111/j.1365-2249.2009.04031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine JS, Silverstone AE, Gasiewicz TA. Impairment of prothymocyte activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 1990;144:1169–1176. [PubMed] [Google Scholar]
- 16.Holladay SD, Smialowicz RJ. Development of the immune system: Differential effects depending on time of exposure. Environmental Health Perspectives. 2000;108 Supl. 3:463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naïve B cell population. J Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 19.Zandvoort A, Timens W. The dual function of the splenic marginal zone: essential for initiation of anti-T1-2 responses but also vital in the general first-line defense against blood-borne antigens. Clin Exp Immunol. 2002;130:4–11. doi: 10.1046/j.1365-2249.2002.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim BJ, Hong SW, Jeong HJ. IgG nephropathy - confusion and overlap with Cq nephropathy. Clin Nephrol. 2009;72:360–365. doi: 10.5414/cnp72360. [DOI] [PubMed] [Google Scholar]
- 21.Mohan C, Adams S, Stanik V, Datta SK. Mucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustafa A, Holladay SD, Goff M, Witonsky S, Kerr R, Reilly C, Sponenberg P, Gogal RM., Jr Developmental exposure to 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) alters postnatal T cell phenotypes and exacerbates autoimmune lupus in 24-week-old SNF1 mice. Birth Defects Research A: Clinical and Molecular Teratology. 2009a;85:828–836. doi: 10.1002/bdra.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mustafa A, Holladay SD, Goff M, Witonsky S, Kerr R, Reilly C, Sponenberg P, Gogal RM., Jr An enhanced postnatal autoimmune profile in 24 week-old C57BL/6 mice developmentally exposed to TCDD. Toxicol Appl Pharmacol. 2008;232:51–59. doi: 10.1016/j.taap.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustafa A, Holladay SD, Goff M, Witonsky S, Kerr R, Reilly C, Sponenberg P, Weinstein DA, Karpuzoglu-Belgin E, Gogal RM., Jr Prenatal exposure to 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) alters adult B cell lymphopoiesis and exacerbates autoimmune lupus in 24-week-old SNF1 mice. Toxicological Sci. 2009b;112:133–143. doi: 10.1093/toxsci/kfp177. [DOI] [PMC free article] [PubMed] [Google Scholar]