Abstract
Purpose
To determine the relative efficacy and complications of the Ahmed FP7 Glaucoma Valve (AGV) and the Baerveldt 101–350 Glaucoma Implant (BGI) in refractory glaucoma.
Design
Multicenter randomized controlled clinical trial.
Participants
276 patients, including 143 in the AGV group and 133 in the BGI group.
Methods
Patients aged 18–85 years with refractory glaucoma with intraocular pressure (IOP) greater than or equal to 18 mm Hg in whom an aqueous shunt was planned were randomized to undergo implantation of either an AGV or a BGI.
Main Outcome Measures
Primary outcome was failure, defined as IOP > 21 mm Hg or not reduced by 20%, IOP ≤ 5 mm Hg, reoperation for glaucoma or removal of implant, or loss of light perception vision. Secondary outcomes included mean IOP, visual acuity, use of supplemental medical therapy, and complications.
Results
Preoperative IOP (mean ± standard deviation, SD) was 31.2 ± 11.2 in the AGV group and 31.8 ± 12.5 in the BGI group (p = 0.71). At 1 year, IOP was 15.4 ± 5.5 mm Hg in the AGV group and 13.2 ± 6.8 mm Hg in the BGI group (p = 0.007). The number of glaucoma medications (mean ± SD) was 1.8 ± 1.3 in the AGV group and 1.5 ± 1.4 in the BGI group (p = 0.071). The cumulative probability of failure was 16.4% (standard error, SE = 3.1%) in the AGV group and 14.0% (SE = 3.1%) in the BGI group at 1 year (p = 0.52). More patients experienced early postoperative complications in the BGI group (n = 77, 58%) compared to the AGV group (n = 61, 43%, p = 0.016). Serious postoperative complications associated with reoperation and/or vision loss of ≥ 2 Snellen lines occurred in 29 patients (20%) in the AGV group and 45 patients (34%) in the BGI group (p = 0.014).
Conclusions
Although the average IOP after one year was slightly higher in patients who received an AGV, there were fewer early and serious postoperative complications associated with the use of the AGV than the BGI.
INTRODUCTION
Aqueous shunts have been increasingly utilized in the management of glaucoma considered refractory to trabeculectomy. These would include eyes with previous incisional eye surgery causing scarring of the conjunctiva (e.g., cataract extraction and trabeculectomy) and secondary glaucomas that are known to have poor success rates with trabeculectomy (e.g., neovascular glaucoma and uveitic glaucoma). Data from the United States Medicare database for glaucoma procedures performed between 1995 and 2004 demonstrate a 184% increase in the number of aqueous shunt procedures.1 The number of trabeculectomies performed in eyes with previous surgery or trauma, most closely resembling the types of patients in which aqueous shunts have been traditionally performed, increased only 9% in this same period.1 In addition, two surveys of the surgical practice patterns of the membership of the American Glaucoma Society, one performed in 1996 and a follow-up survey performed in 2002, demonstrated a marked and statistically significant increase in the use of aqueous shunts in patients who had undergone prior surgery or who had neovascular or uveitic glaucoma compared to trabeculectomy with mitomycin-C.2, 3 In addition, the ongoing Tube versus Trabeculectomy Study, which showed a higher success rate using a Baerveldt glaucoma implant (BGI) than trabeculectomy with mitomycin C in patients with prior failed filtration surgery and/or cataract surgery, has stimulated interest in aqueous shunt implantation in similar patient groups.4 – 6
Commonly used aqueous shunts include the Ahmed glaucoma valve (AGV, New World Medical, Cucamonga, CA), the BGI (Abbott Medical Optics, Abbott Park, IL), the Eagle Vision glaucoma drainage device (Eagle Vision, Memphis, TN) and the Molteno implant (Molteno Ophhtalmic Limited, Dunedin, New Zealand). These implants share a common design consisting of a tube that shunts aqueous humor from the anterior chamber to an end plate located at the equatorial region of the globe. Aqueous shunts differ in terms of materials and design features, including the presence or absence of a valve that limits aqueous flow through the device if the intraocular pressure (IOP) becomes too low. Surgeons choose specific aqueous shunts for various reasons, including perceived efficacy in controlling IOP, perceived risks of complications, and ease of implantation.
In the United States, the AGV and BGI implants are currently the two most widely used aqueous shunts. A 2008 unpublished survey of the American Glaucoma Society membership demonstrated that approximately half of the respondents favor the AGV and half prefer the BGI when operating on patients with previous incisional eye surgery or refractory glaucoma (Manishi Desai, MD, personal communication, August 2, 2009). Several retrospective studies comparing the Ahmed and Baerveldt implants have been inconclusive as to the relative success rates and complications of these two types of implants in refractory glaucomas7–11 and suffer from selection bias. The purported advantage of the Ahmed implant is in its early postoperative IOP control and reduced risk of hypotony due to a restrictive valve-like mechanism. The suggested advantage of the Baerveldt implant is its larger surface area, 350 mm2 versus 184 mm2 for the Ahmed, which could result in lower long-term IOP if one accepts the premise that the level of IOP is dependent on the surface area of the drainage plate.12 Success rates reported for the Ahmed implant range from 68 to 100% and for the Baerveldt implant from 43 – 100% and are highly dependent upon the length of follow-up, type of glaucoma, and success criteria.13 A recent Ophthalmic Technology Assessment report by the American Academy of Ophthalmology states that “Too few high-quality direct comparisons of various available shunts have been published to assess the relative efficacy or complication rates of specific devices…”14, highlighting the need for randomized clinical trials in this area.
The Ahmed Baerveldt Comparison (ABC) Study was designed to prospectively compare the safety and efficacy of these two commonly implanted glaucoma drainage devices. Patients with uncontrolled glaucoma who had prior incisional surgery or other glaucoma diagnoses known to be poor candidates for trabeculectomy were enrolled in this multicenter clinical trial and randomized to placement of an Ahmed FP7 Glaucoma Valve or a Baerveldt 101–350 Glaucoma Implant.
METHODS
The Institutional Review Board at each Clinical Center approved the study protocol before recruitment was started, and each patient gave informed consent. The study was registered at www.clinicaltrials.gov. The design and methods of the ABC Study are described in detail in a companion paper,15 and are summarized as follows.
Randomization and Treatment
The ABC Study was conducted at 16 Clinical Centers. Eligibility, as described in the accompanying baseline paper,15 was independently confirmed at the Statistical Coordinating Center (SCC) at the Bascom Palmer Eye Institute. Individuals enrolled in the study were randomized to placement of an Ahmed FP7 glaucoma valve or a Baerveldt 350-mm2 glaucoma implant. Randomization was performed after informed consent was obtained for participation in the study at the SCC using a permuted block design stratified by Clinical Center and glaucoma diagnosis. Since this was a surgical study and proper surgical informed consent was necessary, neither the subject nor the investigator could be masked to the randomization assignment. Details of the surgical procedures for Ahmed and Baerveldt implantation used in this study are described in detail in an accompanying paper.15
Patient Visits
Follow-up visits were scheduled 1 day, 1 week, 1 month, 3 months, 6 months, 1 year, 18 months, 2 years, 3 years, 4 years, and 5 years postoperatively. Additional information about data obtained at baseline and follow-up visits is contained in the accompanying baseline paper.
Primary and Secondary Outcome Measures
The primary outcome measure was failure, which was prospectively defined as IOP > 21mm Hg or less than a 20% reduction below baseline on 2 consecutive study visits after 3 months, IOP ≤ 5 mm Hg on 2 consecutive study visits after 3 months, reoperation for glaucoma, loss of light perception vision, or removal of the implant for any reason. Eyes with successfully controlled intraocular pressures (≤ 21 mm HG and >5 mmHg and reduced by at 20% from baseline) were considered complete successes if medications were not used at either the six or twelve month visits and were considered qualified successes otherwise. Reoperation for glaucoma was defined as additional glaucoma surgery requiring a return to the operating room, such as placement of an additional aqueous shunt. Cyclodestruction was also counted as a reoperation for glaucoma. Interventions performed at the slit lamp, such as needling procedures, removal of occluding stents, or laser suture lysis, were not considered glaucoma reoperations.
IOP and the rate of surgical complications were secondary outcome measures in the ABC Study. Early complications were those that were recorded by the 3 months follow-up visit whereas later complications were those that were experienced after the 3 month follow-up visit. A serious complication was defined as any complication, early or late, that was associated with a two line Snellen acuity decrease and/or a major surgery (reoperation in the operating room) to manage the complication. If a patient lost 2 or more lines of Snellen visual acuity compared to baseline, the investigator was asked to determine the cause of the visual loss. A revision to manage an occluded tube was considered a reoperation for a complication. The Snellen visual acuity decrease was assessed at the one year visit or, if that visit was missed, at the six month visit. If the patient did not have either a 6 or 12 month visit then their complications (n=8) could not be categorized as serious by vision loss, but could by virtue of reoperation.
Sample Size Calculations
A recent retrospective comparison from Singapore reported an 83% success rate for the Baerveldt and 67% for the Ahmed. This study was powered to detect a true difference in success rates of this size. Setting the power at 80% and alpha at 5%, 125 patients in each group were required to detect this difference. The overall study size of 275 was determined to allow for a 10% dropout rate.
Statistical Analysis
Snellen VA measurements were converted to logarithm of minimum angle of resolution (logMAR) equivalents for the purpose of data analysis, as reported previously.16 The time to failure was defined either as the time from surgical treatment to reoperation for glaucoma, loss of acuity to no light perception in the study eye, or as the time from surgical treatment to the first of two consecutive follow-up visits after 3 months in which the patient had persistent hypotony (IOP ≤ 5 mm Hg) or inadequately controlled IOP (IOP > 21 mm Hg or not reduced by 20%). Data on intraocular pressure and number of glaucoma medications were censored once a subject underwent a reoperation for glaucoma, explantation of the implant for a complication, or loss of light perception vision, but not after failure due to high IOP, hypotony, or reoperation for complication. There was no censoring of visual acuity results. Univariate comparisons between treatment groups were performed using the two-sided Student t-test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables. Risk factors for treatment failure were assessed for statistical significance with the Kaplan-Meier survival analysis log-rank test. Multivariate analysis was performed using Cox proportional hazard regression analysis with forward stepwise elimination. Patients’ data were analyzed in the group to which they were assigned during randomization (intent to treat analysis). A p-value of .05 or less was considered statistically significant.
RESULTS
Recruitment and Retention
A total of 276 patients were enrolled in the ABC Study between October 2006 and April 2008. Randomization assigned 143 patients (52%) to placement of an Ahmed FP7 implant and 133 (48%) to a Baerveldt 350-mm2 glaucoma implant. Protocol violations are described in the accompanying baseline paper.15 All patients were analyzed in the group to which they were originally assigned according to the intent to treat protocol.
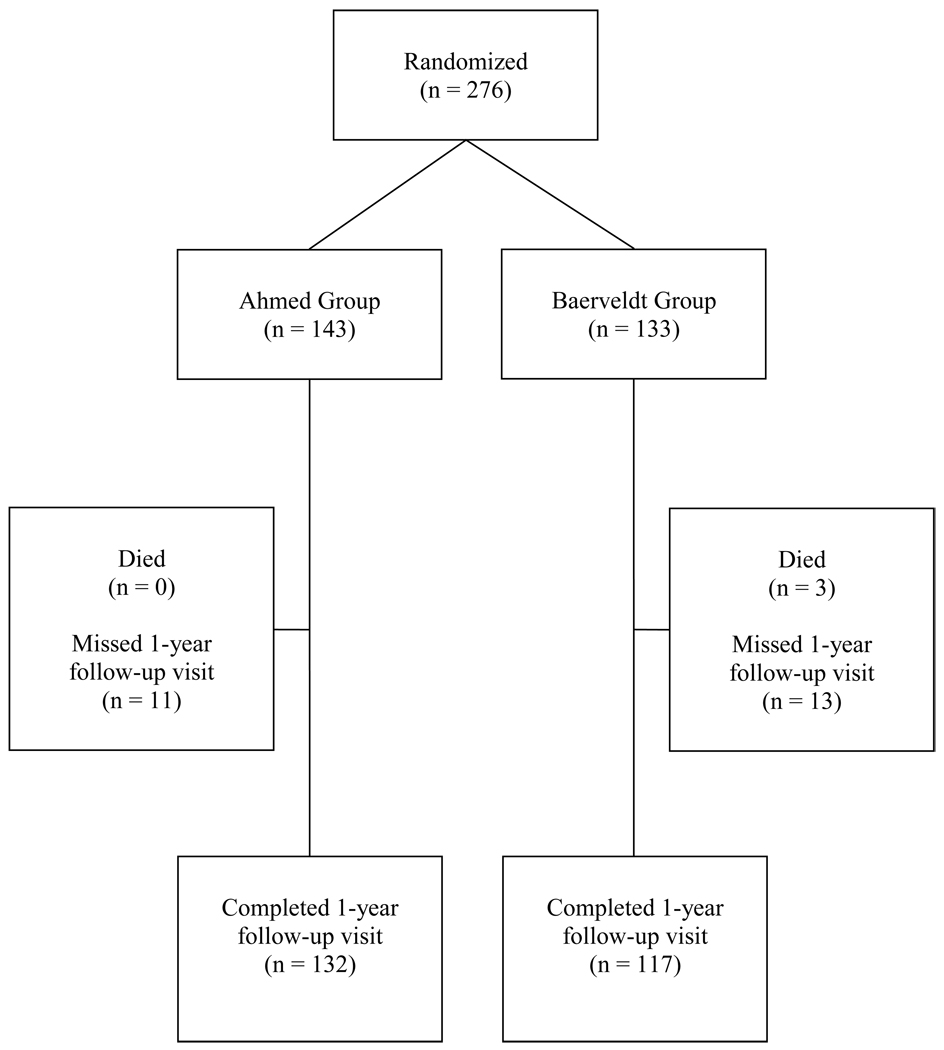
The progress of patients in the study is shown in Figure 1. In the overall study group, 260 (94.2%) patients had at least one year of follow-up. There were 132 (92%) patients in the AGV group and 117 (88%) in the BGI group who had 1-year follow-up data available. Eleven patients who missed the 1-year follow-up visit window did have a study visit subsequent to the 1-year visit, accounting for the discrepancy in the number of patients with at least 1-year follow-up (260) and the number of patients with follow-up at one year (149). Eighty-eight percent of patients in the AGV group completed both the 6-month and 12-month visits compared to 86% in the BGI group. This is important because these were the proportion of patients who would have been able to fail by the IOP criterion at the one-year visit. The number of patients completing each follow-up visit is shown in Table 1.
Figure 1.
Flowchart of subject progress in the Ahmed Baerveldt Comparison (ABC) Study.
Table 1.
Intraocular Pressure and Medical Therapy at Baseline and Follow-up in the Ahmed Baerveldt Comparison Study*
| Ahmed Group | Baerveldt Group | P-value† | |
|---|---|---|---|
| Baseline | |||
| IOP (mm Hg) | 31.2 ± 11.2 | 31.8 ± 12.5 | .71 |
| Glaucoma medications | 3.4 ± 1.1 | 3.5 ± 1.1 | .34 |
| N | 143 | 133 | |
| 1 day | |||
| IOP (mm Hg) | 10.0 ± 7.9 | 18.6 ± 13.7 | < 0.001 |
| N followed (% of baseline) | 142 (99%) | 130 (98%) | |
| 1 week | |||
| IOP (mm Hg) | 10.6 ± 5.6 | 17.2 ± 12.0 | < 0.001 |
| Glaucoma medications | 0.2 ± 0.7 | 0.9 ± 1.4 | < 0.001 |
| N followed (% of baseline) | 140 (98%) | 118 (89%) | |
| 1 month | |||
| IOP (mm Hg) | 20.7 ± 9.7 | 18.0 ± 10.0 | 0.024 |
| Glaucoma medications | 0.5 ± 1.0 | 1.3 ± 1.5 | < 0.001 |
| N followed (% of baseline) | 139 (97%) | 130 (98%) | |
| 3 months | |||
| IOP (mm Hg) | 18.8 ± 8.3 | 16.7 ± 8.2 | 0.043 |
| Glaucoma medications | 1.4 ± 1.3 | 1.2 ± 1.3 | 0.32 |
| N followed (% of baseline) | 133 (93%) | 125 (94%) | |
| 6 months | |||
| IOP (mm Hg) | 15.7 ± 5.3 | 14.8 ± 6.8 | 0.26 |
| Glaucoma medications | 1.7 ± 1.4 | 1.3 ± 1.3 | 0.012 |
| N followed (% of baseline) | 131 (92%) | 125 (94%) | |
| 1 year | |||
| IOP (mm Hg) | 15.4 ± 5.5 | 13.2 ± 6.8 | 0.007 |
| Glaucoma medications | 1.8 ± 1.3 | 1.5 ± 1.4 | 0.071 |
| N followed (% of baseline) | 132 (92%) | 117 (88%) | |
Data are presented as mean ± standard deviation.
IOP - Intraocular pressure
IOP censored after treatment failure by no light perception vision, reoperation for glaucoma, explantation for complication
Student t-test
Baseline Characteristics
The baseline characteristics of the study population are provided in an accompanying publication.15 There were no differences in baseline demographic or clinical characteristics between the two groups.
IOP Reduction
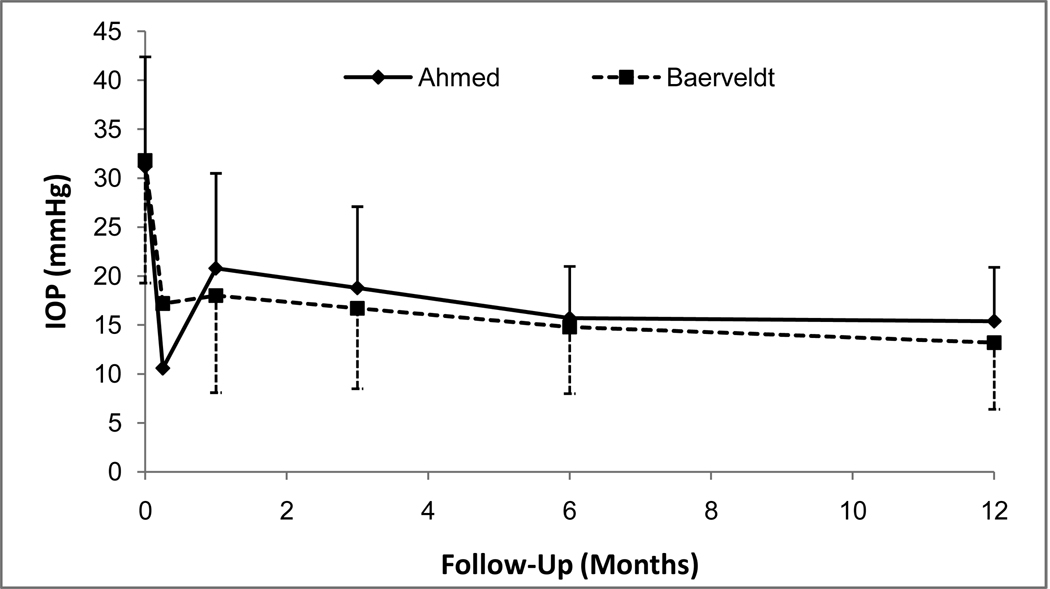
The baseline and follow-up IOPs for the two groups are reported in Table 1 and Figure 2. Patients who underwent additional glaucoma surgery were censored from analysis after the time of reoperation. Both surgical procedures produced a significant reduction in IOP. In the AGV group, IOP (mean ± standard deviation, SD) decreased from 31.2 ± 11.2 mm Hg at baseline to 15.4 ± 5.5 mm Hg at the 1-year follow-up visit (p < 0.001, paired t-test). In the BGI group, IOP (mean ± SD) was reduced from 31.8 ± 12.5 mm Hg at baseline to 13.2 ± 6.8 mm Hg at the 1-year follow-up visit (p < 0.001, paired t-test). The AGV group had a significantly lower mean IOP than the BGI group at the 1 day and 1 week follow-up visits. However, the mean IOP in the BGI group was approximately 2 mmHg lower than the AGV group IOP at the 1 month, 3 month, and 1 year visits.
Figure 2.
Mean intraocular pressures (IOP, with standard error bars) from baseline until 12-month follow-up visit in the Ahmed Baerveldt Comparison (ABC) Study.
Medical Therapy
Table 1 also shows the number of glaucoma medications in both groups at baseline and follow-up. Patients who underwent additional glaucoma surgery were censored from analysis after the time of reoperation. There was a significant reduction in the need for medical therapy in both treatment groups. The number of glaucoma medications (mean ± SD) in the AGV group decreased from 3.4 ± 1.1 at baseline to 1.8 ± 1.3 at the 1-year follow-up visit (p < 0.001, paired t-test). The number of glaucoma medications (mean ± SD) in the BGI group was reduced from 3.5 ± 1.1 at baseline to 1.5 ± 1.4 at the 1-year follow-up visit (p < 0.001, paired t-test). There was a tendency toward greater use of glaucoma medical therapy at 1 year in the Ahmed group compared with the Baerveldt group, but this difference did not reach the level of statistical significance (p = 0.071).
Primary Treatment Outcomes
Table 2 (available at http://aaojournal.org) compares the outcomes and reasons for failure of randomized patients, unadjusted for follow-up time. All patients who were seen at the 1-year follow-up visit and/or failed during the first year of the study were included in this analysis. There was no significant difference in failure rates at 1 year between the two treatment groups. At 1 year, treatment failure had occurred in 23 (16%) patients in the AGV group and 18 (14%) patients in the BGI group (p = 0.61, chi-square test). An additional analysis was performed using the same primary failure criteria (with IOP greater than 21 as the cutoff) but defining complete success as eyes that had not failed and were not on supplemental medical therapy and qualified success as eyes that had not failed but required supplemental medical therapy. In the AGV group, 27 (23%) successful patients were classified as complete successes and 92 (77%) patients were qualified successes. In the BGI group, 41 (36%) successful patients were complete successes and 73 (64%) patients were qualified successes. While there was no difference in overall success rates after one year of follow-up, the BGI group had more complete successes (p = 0.031, Fisher's Exact test).
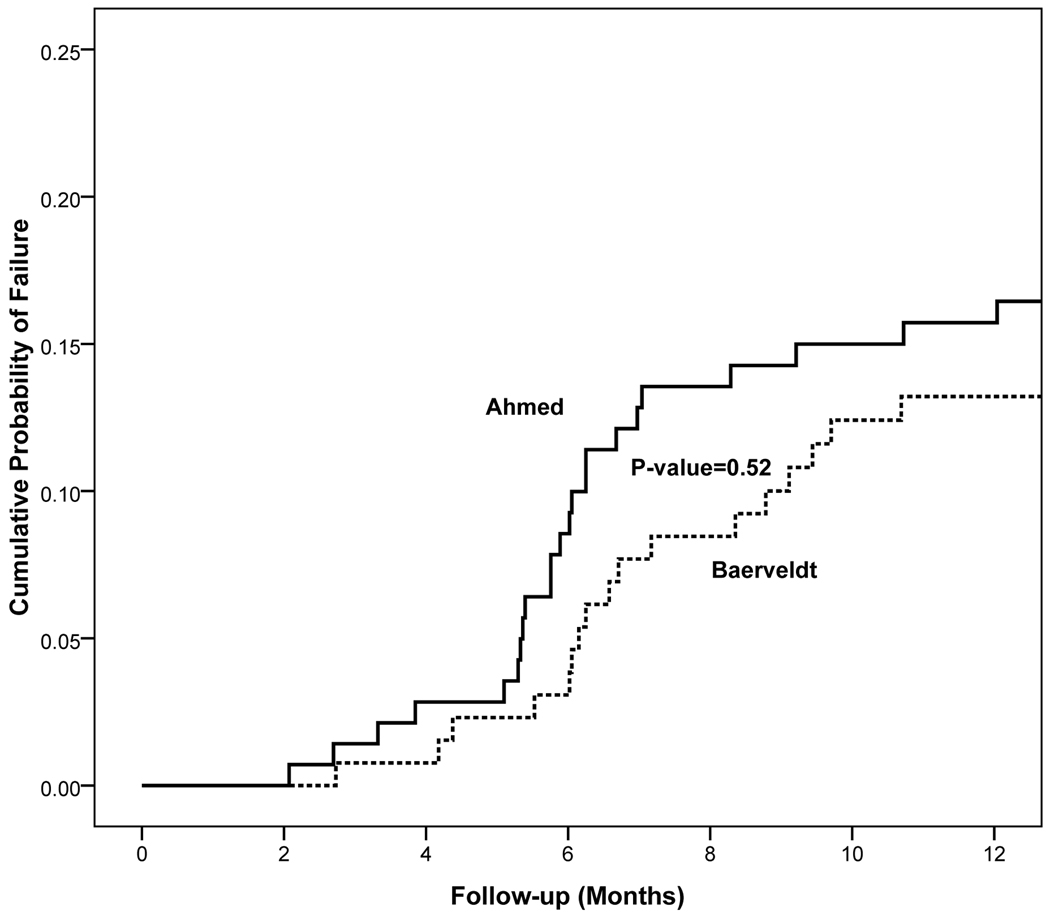
Kaplan-Meier survival analysis was also used to compare failure rates between the two treatment groups (Figure 3). The cumulative probability of failure was 16.4% (standard error = 3.1%) in the AGV group and 14% (standard error = 3.1 %) in the BGI group at 1 year. The reasons for treatment failure are listed in Table 2 (available at http://aaojournal.org). There were 20 patients in the AGV group who had inadequately controlled IOP, including 11 patients who required a reoperation for glaucoma. Failure because of inadequate IOP control occurred in 7 patients in the BGI group, including 1 patient who had additional glaucoma surgery. Persistent hypotony was the cause for treatment failure in 0 patients in the AGV group, and 2 patients in the BGI group (p = 0.23, Fisher exact test). There were 2 (1%) eyes in the AGV group and 6 (5%) in the BGI group that lost light perception during the first year of follow-up (p = 0.16, Fisher’s exact test). All of the eyes that lost light perception vision were in the neovascular glaucoma stratum and vision loss was related to underlying disease rather than glaucoma in all of these cases. Kaplan-Meier analysis of time to failure did not reveal a significant difference in failure for any reason between the two groups (Figure 3; stratified log-rank p=0.56).
Figure 3.
Kaplan-Meier curves showing the cumulative probability of failure from any cause in the Ahmed Baerveldt Comparison (ABC) Study.
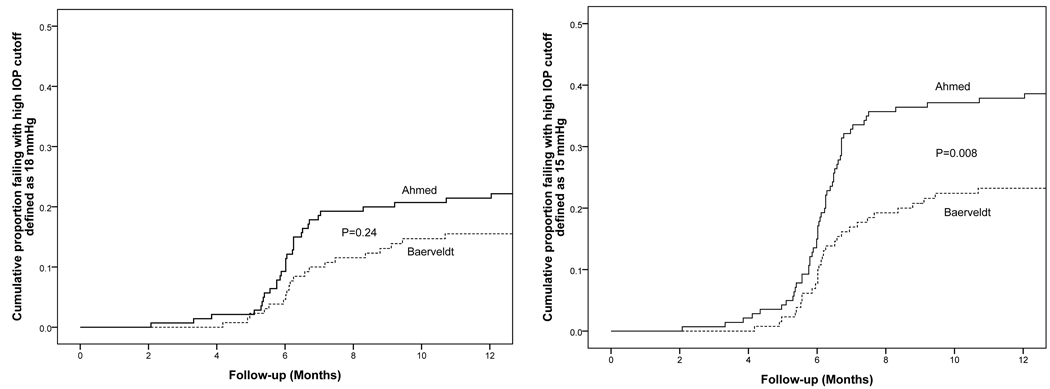
Figure 4 presents the failure rates for the two treatment groups using alternative outcome criteria. Patients with persistent hypotony or reoperation for glaucoma were still classified as treatment failures; however, the upper IOP limit defining success and failure was changed. When inadequate IOP control was defined as IOP greater than 17 mm Hg or not reduced by 20% on two consecutive follow-up visits after 3 months, the cumulative probability of failure at 1 year was 22.2% in the AGV group and 16.3% in the BGI group (p = .24, stratified log rank test). When inadequate IOP control was defined as IOP greater than 14 mm Hg or not reduced by 20% on two consecutive follow-up visits after 3 months, the cumulative probability of failure was 38.6% in the AGV group and 24.0% in the BGI group at 1 year (p = .008, stratified log rank test).
Figure 4.
Kaplan-Meier curves showing the cumulative probability of failure from any cause in the Ahmed Baerveldt Comparison (ABC) Study defining inadequate intraocular pressure (IOP) control as IOP > 17 mm Hg (left) or IOP > 14 mm Hg (right).
Baseline demographic and clinical features were evaluated as possible predictors for treatment failure and are shown in Table 3 (available at http://aaojournal.org). Univariate and multivariate analyses are presented. In the univariate analysis, type of glaucoma (stratum), higher baseline IOP, a prior laser procedure, and fewer than 20 prior surgeries with a particular implant type by the operating surgeon were associated with higher failure rates for any reason. Complicated type of glaucoma (neovascular), a prior laser procedure, and less surgical experience remained significant risk factors for failure in the multivariate analysis.
Reoperation for Glaucoma
Patients in the AGV group required more reoperations for glaucoma than the BGI group (Table 2, available at http://aaojournal.org). Eleven (8%) patients in the AGV group had reoperations for glaucoma whereas one (1%) patient in the BGI group underwent reoperations for glaucoma (p = 0.016, Fisher’s Exact test).
Because the surgeon was not masked to the treatment assignment, a potential bias existed in the decision to reoperate for IOP control. To evaluate for reoperation bias, the IOP levels were compared between treatment groups among patients who failed because of inadequate IOP control. For the cases failing by high IOP at two consecutive study visits (i.e., 6 and 12 months) without reoperation, there were no significant differences (p = 0.36 and p = 0.65 for 6- and 12-months respectively). The 6- and 12- month AGV mean (SD) IOPs were 20.3 mmHg (4.4) and 22.9 mmHg (8.2), respectively, whereas the 6- and 12-month BGI mean (SD) IOPs were 23.7 mmHg (9.3) and 25.8 mmHg (16.0). Among AGV cases reoperated for glaucoma the mean (SD) preoperative IOP immediately prior to surgery was 34.8 mmHg (9.5). The IOP before reoperation for the patient in the BGI group was unavailable.
Visual Acuity
Visual acuity results are shown in Table 4. There was a significant decrease in Snellen VA in both treatment groups during the first year of follow-up. In the AGV group, logMAR Snellen VA (mean ± SD) decreased from 1.07 ± 1.01 at baseline to 1.18 ± 1.07 at the 1-year follow-up visit (p = .017, paired t-test). In the BGI group, logMAR Snellen VA (mean ± SD) decreased from 1.04 ± 1.00 at baseline to 1.20 ± 1.19 at the 1-year follow-up visit (p = .001, paired t-test). There was no significant difference in Snellen VA (p = 0.74, student t-test) between the two groups at 1 year.
Table 4.
Visual Acuity Results in the Ahmed Baerveldt Comparison Study
| Ahmed Group | Baerveldt Group | P-value | |
|---|---|---|---|
| Snellen VA, logMAR mean ± SD | |||
| Baseline | 1.07 ± 1.01 | 1.04 ± 1.00 | .80† |
| 1 year | 1.18 ± 1.07 | 1.23 ± 1.19 | .74† |
| Loss of ≥2 Snellen lines, n (%)* | 40 (30) | 40 (34) | 0.57‡ |
| Glaucoma | 5 (13%) | 7 (18%) | |
| Macular disease | 5 (13%) | 6 (15%) | |
| Cataract | 7 (18%) | 6 (15%) | |
| Other | 12 (30%) | 15 (38%) | |
| Unknown | 11 (28%) | 6 (15%) | |
VA – Visual Acuity
Patients may have more than one reason for decreased vision.
Two sided Student t-test
Chi-square test
Snellen VA was decreased by two or more lines from baseline in 40 (30%) patients in the AGV group and 40 (34%) patients in the BGI group at 1 year, and this difference in rate of vision loss between treatment groups was not statistically significant (p = 0.57, chi-square test). The most frequent causes of vision loss during the first year of the study were glaucoma, macular disease, and cataract. The reason for decreased vision was unknown in 11 (28%) patients in the AGV group and 6 (15%) patients in the BGI group. Other miscellaneous causes for reduced vision in 12 patients in the AGV group included vitreous hemorrhage, corneal epithelial defect, retinal detachment, diabetic retinopathy, band keratopathy, and neovascular membrane. Other causes of vision loss in 15 patients in the BGI group included corneal edema, retinal detachment, diabetic retinopathy, endophthalmitis, and posterior capsular opacification. There were no significant differences in the reasons for visual acuity loss between the two groups. All 8 patients who lost light perception had neovascular glaucoma.
A multivariate linear regression analysis with loss of ≥ 2 Snellen lines of visual acuity at 1 year as the dependent variable and diagnostic stratum, baseline acuity, treatment assignment, and any incidence of complication as independent variables was performed to investigate the reason(s) for decreased visual acuity. The single most important predictor of acuity loss was diagnostic stratum (p < 0.001) and the only other variable to enter the model was baseline acuity (p=0.006; patients with better preop acuity were more likely to lose two or more lines of vision by one year). Cases from the neovascular and high risk strata were, respectively, 5.7 (95% CI: 2.5, 13.3) and 2.2 (95% CI: 0.9, 5.0) more likely to suffer visual acuity loss than cases in the primary/prior surgery stratum. Neither postoperative complication (p=0.10) or treatment assignment (p=0.63) was statistically significant.
Postoperative Interventions
The number and frequency of patients that required postoperative interventions during the first 12 months of follow-up prior to glaucoma reoperation are listed in Table 5 (available at http://aaojournal.org). The most frequently performed postoperative intervention was reformation of the anterior chamber, which occurred in 9 patients (6%) in the AGV group compared to 15 patients (11%) in the BGI group. The total number of interventions higher in the BGI group but this was not statistically significant (p=0.077).
Postoperative Complications
Table 6 lists the early postoperative complications and Table 7 (available at http://aaojournal.org) shows late postoperative complications. Tube occlusion occurred more commonly in the Baerveldt group than the Ahmed group in both the early (p = 0.015) and late (p = 0.059) postoperative periods. Corneal edema (p = 0.035) was also observed more frequently in the BGI group compared with the AGV group in the early postoperative period. The percent of patients with early complications was significantly higher in the BGI group (n = 77, 58%) than in the AGV group (n = 61, 43%, p = 0.016). There was no significant difference between the percent of patients with late postoperative complications in the AGV group (n = 41, 29%) and the BGI group (n = 49, 37%, p = 0.16).
Table 6.
Number (%) of Early (≤ 3 months) Postoperative Complications in the Ahmed Baerveldt Comparison Study
| Complication | Ahmed | Baerveldt | p-value | Total |
|---|---|---|---|---|
| Tube Occlusion | 3 (2%) | 12 (9%) | 0.015 | 15 (5%) |
| Choroidal Effusion | 21 (15%) | 13 (10%) | 0.37 | 34 (123%) |
| Suprachoroidal Hemorrhage |
0 | 2 (2%) | 0.23 | 2 (1%) |
| Endophthalmitis | 0 | 1 (1%) | 0.48 | 1 (0.4%) |
| Cystoid Macular Edema |
8 (6%) | 2 (2%) | 0.11 | 10 (4%) |
| Shallow Anterior Chamber |
27 (19%) | 26 (20%) | 1.00 | 53 (19%) |
| Hypotony Maculopathy |
5 (3%) | 3 (2%) | 0.72 | 8 (3%) |
| Diplopia | 9 (6%) | 7 (5%) | 0.80 | 16 (6%) |
| Corneal Edema | 17 (12%) | 29 (22%) | 0.035 | 46 (17%) |
| Tube - Corneal touch | 7 (5%) | 8 (6%) | 0.79 | 15 (5%) |
| Tube Erosion | 1 (1%) | 1 (1%) | 1.00 | 2 (1%) |
| Hyphema | 13 (9%) | 22 (17%) | 0.072 | 35 (13%) |
| Vitreous Hemorrhage | 2 (1%) | 3 (2%) | 0.675 | 5 (2%) |
|
Total Patients with Early Complications |
61 (43%) | 77 (58%) | 0.016 | 138 (50%) |
Data are presented as number (percentage).
The number of patients experiencing serious complications, defined a priori as complications that required a return to the operating room to manage the complication and/or associated with loss of two or more Snellen vision, was significantly higher in the BGI group (n = 45, 34%) than in the AGV group (n = 29, 20%, p = 0.014). Table 8 provides complete data for serious complications.
Table 8.
Serious Complications Associated with Reoperation or Vision Loss in the Ahmed Baerveldt Comparison Study
| Ahmed Group (n = 143) |
Baerveldt Group (n = 133) |
P-value* | |
|---|---|---|---|
| Reoperation for complications | 7 (5) | 17 (13) | .031 |
| Vision loss ≥ 2 Snellen lines† | 26 (18) | 36 (27) | 0.085 |
|
Total Patients with Serious Complications‡ |
29 (20) | 45 (34) | .014 |
Data are presented as number (percentage).
Fisher’s Exact test
Some patients did not have Snellen visual acuity at 1 year because they missed the 1 year visit.
Patients can have both reoperation for a complication and vision loss.
Reoperation for Complications
Reoperations for complications were performed in 7 (5%) patients in the to the AGV group and 17 (13%) patients in the BGI group (p = 0.031, Fisher’s Exact test). The reasons for reoperations in the AGV group included extension of a retracted tube (2), clearing of an occluded tube (1), repair of a conjunctival wound leak (1), replacement of patch graft (1), implant removal secondary to diplopia (1), and surgical iridectomy for suspected pupillary block resulting in a flat anterior chamber (1). The reasons for reoperations in the BGI group included clearing of an occluded tube (7), conjunctival repair for leak or tube erosion (3), replacement of patch graft (1), pars plana vitrectomy to clear postoperative hemorrhage (1), ligation of tube for over-filtration (1), tube removal secondary to tube-corneal touch (1), implant removal secondary to an exposed plate (1), implant removal secondary to suspected P Acnes endophthalmitis (1), and drainage of suprachoroidal hemorrhage (1).
Cataract Surgery during Follow-Up
There were 92 phakic patients in the study, 47 in the AGV group and 45 in the BGI group. Of these patients, six (13%) in the AGV group had cataract surgery prior to any reoperation for glaucoma and 9 (20%) in the BGI group had cataract surgery prior to glaucoma reoperation (p = 0.41, Fisher’s exact test).
Effect of Surgeon Experience on Treatment Outcome and Complications
As reported in the baseline paper,15 there were differences in surgeon experience in implanting the two types of implants. There were two surgeons (8%) who had performed fewer than 5 Ahmed implantations, though both were experienced BGI surgeons. There were five surgeons (20%) who had performed fewer than 5 BGI procedures but all five were experienced Ahmed surgeons. A pre-planned separate analysis was performed using cases from surgeons who had performed at least 20 of each type of implant prior to the beginning of the study. Table 3 (available at http://aaojournal.org) shows the effect of experience with implantation of a particular device on risk of failure. There was a small effect in the univariate and multivariate analyses demonstrating that failure was perhaps 20% less likely (C.I. 0.6 – 1.0) in the AGV group if the surgeon had placed 20 or more AGVs prior to the study and 30% less likely in the BGI group (0.6 – 0.9) if the surgeon had placed 20 or more BGIs prior to the study.
Table 9 (available at http://aaojournal.org) presents the effect of surgeon experience with a particular type of implant with postoperative complications. Tube - corneal touch occurred more often in cases done by surgeons with fewer than 20 prior cases using the BGI. When both treatment groups were combined, surgical experience with a particular type of implant was significantly related to the occurrence of tube-corneal touch (p = 0.032).
DISCUSSION
The ABC study was designed to compare the outcomes and complications for two aqueous shunts commonly used for refractory glaucoma, the Ahmed glaucoma valve and the Baerveldt glaucoma implant. Both procedures lowered IOP and medication use significantly from baseline. In addition, the failure rates by predetermined criteria were similar for both implants. However, the Ahmed group had a higher rate of reoperation for glaucoma than the Baerveldt group. This is consistent with a greater efficacy of the Baerveldt implant, as indicated by slightly greater pressure reduction, and a tendency for greater glaucoma medication use by the Ahmed group. The average IOP after 1 year of follow-up was 2.2 mmHg lower in the Baerveldt group, a statistically significant difference. During the first postoperative month, IOP was lower in the Ahmed than the Baerveldt group, as might be expected due to the valve mechanism in the Ahmed implant. Surgeons had the option of using tube fenestrations in the Baerveldt group for early IOP pressure control, but this surgical maneuver has variable success.17 The patients in the Baerveldt group required twice as many postoperative interventions and experienced a third more serious postoperative complications, which were also statistically significant. While the Baerveldt implant provided slightly better IOP lowering at 1 year and less need for reoperation for elevated IOP, this improved success came at the price of more serious complications.
There have been numerous retrospective case series reporting the results of the use of a single model implant such as the Ahmed and Baerveldt in refractory glaucoma. Schwartz and colleagues recently published a review of the literature comparing results with different types of aqueous shunts.13 They point out that it is difficult to compare results from single model implant case series since each of these studies involve different groups of patients, surgeons, definitions of success, and follow-up times.13 There are four studies that have directly compared the results of the Ahmed and the Baerveldt implants in retrospective comparative case series.7–11 Tsai and colleagues published early- and intermediate-term results7, 10 in a group of patients who underwent either implantation of the older, AGV-S2 or the BGI (350 mm2 or 250 mm2) by a single surgeon. The two groups differed significantly in age, preoperative IOP, and diagnosis. Similar to the current study, IOP was lower in the Ahmed group at the 1-day and 1-week postoperative visits and no significant difference in overall survival rates between the implants was found. IOP was the same in the two groups at one year, but after one year, the IOP in the Ahmed group got steadily higher and stayed higher out to four years of follow-up. Wang et al9 published a retrospective comparative case series of 41 Asian patients who underwent either an AGV-S2 or BGI 350 mm2 implant by a single surgeon. The patients were followed for an average of 23 months in both groups. The average age in the Ahmed group was 12 years older than the Baerveldt group. They found no statistically significant differences in survival rates or IOP at last follow-up in their small group of patients, although the survival rate was higher in the Baerveldt group (88%) compared to the Ahmed group (77%) and the final IOP was 2.5 mmHg lower in the Ahmed group. To attempt to compensate for the selection bias inherent in retrospective studies, Syed and colleagues8 performed a comparison of patients who received the AGV-S2 (n = 32) and the BGI 350 mm2 (n = 32) matching for age, glaucoma diagnosis, and preoperative IOP. A non-time adjusted survival comparison failed to find any difference in success and the average IOPs were similar in the two groups throughout the approximate 1-year of the study. Survival analysis, which takes into account length of follow-up and drop outs, was not performed. Goulet and associates11 performed a retrospective comparative case series in their institution of 59 patients who received an Ahmed S2 implant and 133 patients who received a Baerveldt 250 mm2 implant. Their study showed a higher success rate and lower IOP for patients who received the Baerveldt implant after an average follow-up of 20 – 23 months. So the current retrospective evidence is inconclusive as to which implant lowers IOP better, has higher success rate, and fewer complications. These studies all suffer from their retrospective design and selection bias of which patients received which implant, although one study8 matched on several potentially confounding variables. In addition, all of the aforementioned studies used the AGV S2 model, which has a polypropylene plate, rather than the AGV FP7 model, which has a silicone plate (as does the BGI 350 mm2). There is evidence that the silicone material in the AGV FP7 model provides better IOP reduction than the AGV S2 model.18, 19 The strength of the randomized prospective trial design used in the current study is that selection bias is eliminated and confounding variables tend to be balanced in the two groups, making conclusions stronger. The comparability of the two treatment groups created by randomization was examined in the baseline paper and no significant differences were found. In addition, the multicentered study design with 25 different surgeons operating upon patients in 4 continents improves the generalizability of the results.
Visual acuity decreased by 2 or more lines of Snellen visual acuity in approximately 32% of patients overall and was not different between the groups. Among patients with complications, 18% of patients in the AGV group and 27% of patients in the BGI group by one year of follow-up. Snellen VA was similar between treatment groups at 1 year and no significant differences in the rates and reasons for vision loss were present in the two groups. These rates of visual acuity loss are high but consistent with those found in the TVT study groups at one year.5 Most of the causes of loss of two or more Snellen lines were related to cataract, age related macular degeneration and glaucoma, which was also found in the TVT study.5,6 It is unclear whether the vision loss was more associated with surgical complications or the underlying severity of disease in the group of patients studied. Of note, all 8 patients who lost light perception vision were in the neovascular glaucoma stratum and there was a higher prevalence of surgical complications in this group as well.
Many surgical complications were reported in the ABC Study but most were transient and did not require intervention. A similar high rate of complications was reported in the TVT Study at one year.5 More patients in the BGI group experienced early postoperative complications than the AGV group in the ABC Study and the complication rate between groups was similar for late complications. However, all surgical complications are not equal in severity, and the rate of serious complications associated with reoperation and/or vision loss was higher in the BGI group.
The specific design features of the Ahmed and Baerveldt implants may explain some differences in clinical outcomes. The Ahmed implant has a restrictive “valve” device designed to prevent hypotony. This is particularly important in the immediate postoperative period before a capsule forms around the end plate, which restricts flow later. The Baerveldt implant does not have a flow restrictor, and hypotony with its resultant complications is much more common20 if flow is not restricted by the surgeon using a suture ligature,13, 21 which either dissolves or is removed once the end plate encapsulates to limit flow. It is therefore not surprising that in the current study, IOP was higher in the Baerveldt group in the period before flow was established 4 to 6 weeks postoperatively. Although there were only two cases of failure due to persistent hypotony by one year, both cases occurred in the BGI group. The second important design difference between the two implants is the size of the drainage plate, which are 184 mm2 for the Ahmed glaucoma valve and 350 mm2 for the Baerveldt implant. Several studies have shown that lower mean IOP can be achieved with larger implant plate sizes of the same general design. Heuer and colleagues12 performed a prospective randomized trial of the single- (134 mm2) versus double-plate (268 mm2) Molteno implant and found both higher success rates and lower IOPs with the larger implant. There were also more postoperative complications associated with the double plate Molteno implant. This same group performed a study randomizing 73 patients to the 350 mm2 and the 500 mm2 Baerveldt implant and found no difference in success or IOP lowering22 at 18 months. A subsequent longer term analysis of the same patients found the 350 mm2 implant to have a slightly higher success rate than the 500 mm2 implant.24 In the current study, the Baerveldt implant, with a larger surface area, provided slightly lower IOPs at 1-year and fewer failures due to inadequate IOP control. Subsequent reports with longer follow-up of the current patients will provide additional information on the relative efficacy of these two implants on long-term IOP control.
One of the potential limitations in the current study is surgical experience with the two treatment arms of the study. Although one would hope that randomization would distribute patients receiving a particular implant to surgeons of differing experiences equally, we were concerned about this as a source of bias. Investigators were required to submit an estimate of the number of surgical procedures that they had performed using each type of implant. If a surgeon had performed fewer than five surgeries using a particular implant, they were required to submit a videotape of themselves performing the procedure to make sure proper surgical techniques were followed. In addition, an analysis of success and complications dividing surgeons into those who had performed fewer than 20 versus 20 or more of a particular procedure was performed. This analysis showed that experience (≥20 prior cases) with a particular implant reduced the risk of failure in the multivariate analysis but not in the univariate analysis. There was no relationship between the total number of complications and surgical experience with a particular implant. It does not appear that relative inexperience with implantation of either the Ahmed or Baerveldt implant affected success rates or complications.
In summary, the ABC study found greater IOP reduction with the Baerveldt Glaucoma Implant after 1 year of follow-up, but fewer early and serious complications were observed with the Ahmed Glaucoma Valve. The efficacy of glaucoma procedures in reducing IOP must be evaluated in light of the adverse events associated with their use. Therefore, this study does not demonstrate clear superiority of one implant over the other. Also, with a significance level set at 0.05, there is always a one in twenty chance that any statistically significant results found in this study could have occurred by chance alone. Additional follow-up is needed to fully evaluate the risk-benefit ratio of both devices in the surgical management of refractory glaucomas. The ABC study is designed to continue follow-up of participants out to five years.
Supplementary Material
Acknowledgments
SUPPORTED BY: NIH P30 EY014801 and unrestricted grants from Research to Prevent Blindness and New World Medical, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES: Mr. Barton has received fees for lecturing from New World Medical, Inc, and Abbott Medical Optics, Inc. Drs. Brandt and Samuelson have received fees for consulting from Abbott Medical Optics.
REFERENCES
- 1.Ramulu P, Corcoran KJ, Corcoran SL, Robin AL. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology. 2007;114:2265–2270. doi: 10.1016/j.ophtha.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen PP, Yamamoto T, Sawada A, et al. Use of antifibrosis agents and glaucoma drainage devices in the American and Japanese Glaucoma Societies. J Glaucoma. 1997;6:192–196. [PubMed] [Google Scholar]
- 3.Joshi AB, Parrish RK, II, Feuer WF. 2002 Survey of the American Glaucoma Society: practice preferences for glaucoma surgery and antifibrotic use. J Glaucoma. 2005;14:172–174. doi: 10.1097/01.ijg.0000151684.12033.4d. [DOI] [PubMed] [Google Scholar]
- 4.Gedde SJ, Schiffman JC, Feuer WJ, et al. Tube Versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy Study after one year of follow-up. Am J Ophthalmol. 2007;143:9–22. doi: 10.1016/j.ajo.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Gedde SJ, Herndon LW, Brandt JD, et al. Tube Versus Trabeculectomy Study Group. Surgical complications in the Tube Versus Trabeculectomy Study during the first year of follow-up. Am J Ophthalmol. 2007;143:23–31. doi: 10.1016/j.ajo.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Gedde SJ, Schiffman JC, Feuer WJ, et al. Tube Versus Trabeculectomy Study Group. Three-year follow-up of the Tube Versus Trabeculectomy Study. Am J Ophthalmol. 2009;148:670–684. doi: 10.1016/j.ajo.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Tsai JC, Johnson CC, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma: a single-surgeon comparison of outcome. Ophthalmology. 2003;110:1814–1821. doi: 10.1016/S0161-6420(03)00574-8. [DOI] [PubMed] [Google Scholar]
- 8.Syed HM, Law SK, Nam SH, et al. Baerveldt-350 implant versus Ahmed valve for refractory glaucoma: a case-controlled comparison. J Glaucoma. 2004;13:38–45. doi: 10.1097/00061198-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Wang JC, See JL, Chew PT. Experience with the use of Baerveldt and Ahmed glaucoma drainage implants in an Asian population. Ophthalmology. 2004;111:1383–1388. doi: 10.1016/j.ophtha.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Tsai JC, Johnson CC, Kammer JA, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma. II: Longer-term outcomes from a single surgeon. Ophthalmology. 2006;113:913–917. doi: 10.1016/j.ophtha.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Goulet RJ, III, Phan AD, Cantor LB, WuDunn D. Efficacy of the Ahmed S2 glaucoma valve compared with the Baerveldt 250-mm2 glaucoma implant. Ophthalmology. 2008;115:1141–1147. doi: 10.1016/j.ophtha.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single-plate versus double-plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology. 1992;99:1512–1519. doi: 10.1016/s0161-6420(92)31772-5. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz KS, Lee RK, Gedde SJ. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol. 2006;17:181–189. doi: 10.1097/01.icu.0000193080.55240.7e. [DOI] [PubMed] [Google Scholar]
- 14.Minckler DS, Francis BA, Hodapp EA, et al. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115:1089–1098. doi: 10.1016/j.ophtha.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Barton K, Gedde SJ, Budenz DL, et al. Ahmed Baerveldt Comparison Study Group. The Ahmed Baerveldt Comparison Study: methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology. doi: 10.1016/j.ophtha.2010.07.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol. 1996;121:349–366. doi: 10.1016/s0002-9394(14)70431-3. [DOI] [PubMed] [Google Scholar]
- 17.Emerick GT, Gedde SJ, Budenz DL. Tube fenestrations in Baerveldt glaucoma implant surgery: 1-year results compared with standard implant surgery. J Glaucoma. 2002;11:340–346. doi: 10.1097/00061198-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Law SK, Nguyen A, Coleman AL, Caprioli J. Comparison of safety and efficacy between silicone and polypropylene Ahmed glaucoma valves in refractory glaucoma. Ophthalmology. 2005;112:1514–1520. doi: 10.1016/j.ophtha.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Ishida K, Netland PA, Costa VP, et al. Comparison of polypropylene and silicone Ahmed glaucoma valves. Ophthalmology. 2006;113:1320–1326. doi: 10.1016/j.ophtha.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Law SK, Kalenak JW, Connor TB, Jr, et al. Retinal complications after aqueous shunt surgical procedures for glaucoma. Arch Ophthalmol. 1996;114:1473–1480. doi: 10.1001/archopht.1996.01100140671004. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen Q, Budenz DL, Parrish RK., II Complications of Baerveldt glaucoma implants. Arch Ophthalmol. 1998;116:571–575. doi: 10.1001/archopht.116.5.571. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd MA, Baerveldt G, Fellenbaum PS, et al. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology. 1994;101:1456–1463. doi: 10.1016/s0161-6420(94)31152-3. discussion 1463-4. [DOI] [PubMed] [Google Scholar]
- 23.Britt MT, LaBree LD, Lloyd MA, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better? Ophthalmology. 1999;106:2312–2318. doi: 10.1016/S0161-6420(99)90532-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.